Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen?
Abstract
1. Endometriosis
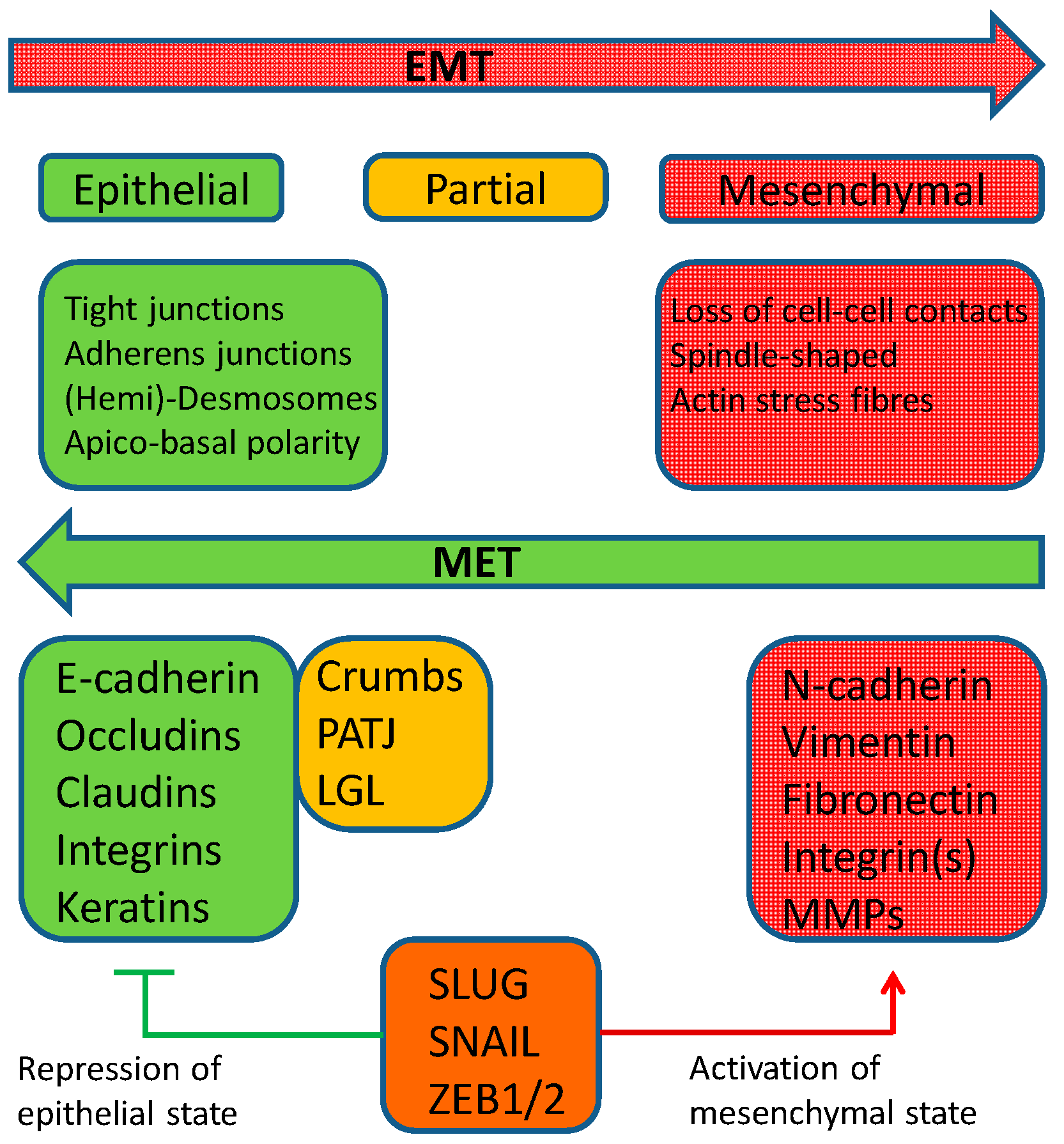
2. Epithelial–Mesenchymal Transition
3. Mesenchymal–Epithelial Transition (MET)
4. Materials and Methods
5. The Role of EMT in Endometriosis
5.1. Epithelial Markers
5.1.1. E-Cadherin (Cadherin-1) in Endometriosis
5.1.2. β-Catenin in Endometriosis
5.1.3. Cell–Cell Contacts (Claudins) in Endometriosis
5.2. Mesenchymal EMT Markers
5.2.1. Snail and Slug (Snail2) in Endometriosis
5.2.2. ZEB1 in Endometriosis
5.2.3. Twist in Endometriosis
5.2.4. Vimentin in Endometriosis
5.2.5. N-Cadherin (Cadherin-2) in Endometriosis
6. EMT in Endometriosis—When Does It Happen?
6.1. Summary of the Epithelial EMT Markers in Endometriosis
6.2. Summary of the Mesenchymal EMT Markers in Endometriosis
6.3. Perspectives and Clinical Relevance of EMT in Endometriosis
7. Conclusions
- EMT in endometriosis is not involved as a main factor in the dissemination of endometrial cells;
- Only a partial EMT takes place after implantation with no significant loss of cell–cell contacts and no loss of the epithelial phenotype;
- The partial EMT results from the different microenvironments at the distinct integration sites of the endometrial implants;
- This is a new type of EMT which we term type IV.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clement, P.B. The pathology of endometriosis. Adv. Anat. Pathol. 2007, 14, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Coxon, L.; Horne, A.W.; Vincent, K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 53–67. [Google Scholar] [CrossRef]
- Guo, S.-W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Blumenkrantz, M.J.; Gallagher, N.A.; Bashore, R.; Tenckhoff, H. Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstet. Gynecol. 1981, 57, 667–670. [Google Scholar] [PubMed]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Liu, D.T.; Hitchcock, A. Endometriosis: Its association with retrograde menstruation, dysmenorrhea and tubal pathology. BJOG Int. J. Obstet. Gynaecol. 1986, 93, 859–862. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ide, P.; Vandenbroucke, W.; Brosens, I.A. New aspects of the pathophysiology of endometriosis and associated fertility. J. Reprod. Med. 1980, 24, 257–260. [Google Scholar]
- Bartosik, D.; Jacobs, S.L.; Kelly, L.J. Endometrial tissue in peritoneal fluid. Fertil. Steril. 1986, 46, 796–800. [Google Scholar] [CrossRef]
- Kruitwagen, R.F.; Poels, L.G.; Willemsen, W.N.; De Ronde, I.J.; Jap, P.H.; Rolland, R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil. Steril. 1991, 55, 297–303. [Google Scholar] [CrossRef]
- Dorien, F.O.; Roskams, T.; Van den Eynde, K.; Vanhie, A.; Peterse, D.P.; Meuleman, C.; Tomassetti, C.; Peeraer, K.; D’Hooghe, T.M.; Fassbender, A.; et al. The presence of endometrial cells in peritoneal fluid of women with and without endometriosis. Reprod. Sci. 2017, 24, 242–251. [Google Scholar] [CrossRef]
- Bokor, A.; Debrock, S.; Drijkoningen, M.; Goossens, W.; Fülöp, V.; D’Hooghe, T. Quantity and quality of retrograde menstruation: A case control study. Reprod. Biol. Endocrinol. 2009, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Shih, A.; Renteira, S.M.; Seckin, T.; Blau, B.; Simpfendorfer, K.; Lee, A.; Metz, C.N.; Gregersen, P.K. Analysis of menstrual effluent: Diagnostic potential for endometriosis. Mol. Med. 2018, 24. [Google Scholar] [CrossRef]
- Viganó, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef]
- Ballard, K.D.; Seaman, H.E.; de Vries, C.S.; Wright, J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1382–1391. [Google Scholar] [CrossRef]
- Eisenberg, V.; Weil, C.; Chodick, G.; Shalev, V. Epidemiology of endometriosis: A large population-based database study from a healthcare provider with 2 million members. BJOG Int. J. Obstet. Gynaecol. 2017, 125, 55–62. [Google Scholar] [CrossRef]
- Louis, G.M.B.; Hediger, M.L.; Peterson, C.M.; Croughan, M.; Sundaram, R.; Stanford, J.; Chen, Z.; Fujimoto, V.Y.; Varner, M.W.; Trumble, A.; et al. Incidence of endometriosis by study population and diagnostic method: The ENDO study. Fertil. Steril. 2011, 96, 360–365. [Google Scholar] [CrossRef]
- Coutinho, L.M.; Ferreira, M.C.; Rocha, A.L.L.; Carneiro, M.M.; Reis, F.M. New biomarkers in endometriosis. Adv. Clin. Chem. 2019, 89, 59–77. [Google Scholar] [CrossRef]
- Audebert, A.; Petousis, S.; Margioula-Siarkou, C.; Ravanos, K.; Prapas, N.; Prapas, Y. Anatomic distribution of endometriosis: A reappraisal based on series of 1101 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 36–40. [Google Scholar] [CrossRef]
- Scioscia, M.; Bruni, F.; Ceccaroni, M.; Steinkasserer, M.; Stepniewska, A.; Minelli, L. Distribution of endometriotic lesions in endometriosis stage IV supports the menstrual reflux theory and requires specific preoperative assessment and therapy. Acta Obstet. Gynecol. Scand. 2010, 90, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Olive, D.L.; Haney, A.F. Endometriosis: Pathogenetic implications of the anatomic distribution. Obstet. Gynecol. 1986, 67, 335–338. [Google Scholar]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Zheng, W.; Li, N.; Wang, J.; Ulukus, E.C.; Ulukus, M.; Arici, A.; Liang, S.X. Initial endometriosis showing direct morphologic evidence of metaplasia in the pathogenesis of ovarian endometriosis. Int. J. Gynecol. Pathol. 2005, 24, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Kudipudi, P.K.; Horne, F.; Meinhold-Heerlein, I. Endometriosis in MRKH cases as a proof for the coelomic metaplasia hypothesis? Reproduction 2019, 158, R41–R47. [Google Scholar] [CrossRef]
- Koninckx, P.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Gargett, C.; Schwab, K.E.; Brosens, J.J.; Puttemans, P.; Benagiano, G.; Brosens, I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 2014, 20, 591–598. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Herbertz, M.; Beil, D.; Huppert, P.; Mall, G.; Kissler, S.; Noe, M.; Wildt, L. Uterine peristaltic activity and the development of endometriosis. Ann. N. Y. Acad. Sci. 2004, 1034, 338–355. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; Viceconte, R.; Bulzomi, P.; D’Armiento, M.; D’Avino, A.; Baldi, A. Embryologic origin of endometriosis: Analysis of 101 human female fetuses. J. Cell. Physiol. 2012, 227, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gargett, C.E.; Guo, S.-W.; Puttemans, P.; Gordts, S.; Brosens, J.J.; Benagiano, G. Origins and progression of adolescent endometriosis. Reprod. Sci. 2016, 23, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Trisolini, R.; Cancellieri, A.; Regnard, J.F. Thoracic endometriosis: Current knowledge. Ann. Thorac. Surg. 2006, 81, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. Biomed. Online 2020, 40, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, K.; Bena, J.F.; McGill, K.M.; Minger, J.; Falcone, T. Surgical treatment of endometriosis. Obstet. Gynecol. 2008, 111, 1285–1292. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Ma, L.; McKinnon, B.; Mueller, M.D. Recurrence patterns after surgery in patients with different endometriosis subtypes: A long-term hospital-based cohort study. J. Clin. Med. 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basis science and new insights based on gene expression. J. Cell. Physiol. 2018, 234, 19384–19392. [Google Scholar] [CrossRef] [PubMed]
- Young, V.J.; Brown, J.K.; Saunders, P.T.; Horne, A.W. The role of the peritoneum in the pathogenesis of endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lang, J.H. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Med. Sci. Monit. 2011, 17, 92–99. [Google Scholar]
- Benagiano, G.; Brosens, J.J.; Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 2013, 20, 386–402. [Google Scholar] [CrossRef]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-α regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor κB in human endometriotic epithelial cells. Mol. Pharmacol. 2008, 73, 1394–1404. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis†. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.-M. Epithelial-to-mesenchymal transition in the female reproductive tract: From normal functioning to disease pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S. The role of mesenchymal–epithelial transition in endometrial function. Hum. Reprod. Update 2018, 25, 114–133. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal–epithelial transition in development and reprogramming. Nat. Cell. Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Dongre, A.A.; Weinberg, R. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef]
- Diepenbruck, M.; Christofori, G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr. Opin. Cell Biol. 2016, 43, 7–13. [Google Scholar] [CrossRef]
- Savagner, P. Epithelial–mesenchymal transitions. Curr. Top. Dev. Biol. 2015, 112, 273–300. [Google Scholar] [CrossRef]
- Micalizzi, U.S.; Haber, D.A.; Maheswaran, S. Cancer metastasis through the prism of epithelial-to-mesenchymal transition in circulating tumor cells. Mol. Oncol. 2017, 11, 770–780. [Google Scholar] [CrossRef]
- Maheswaran, S.; Haber, D.A. Transition loses its invasive edge. Nature 2015, 527, 452–453. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.; Pappalardo, E.; Jorgens, D.M.; Coutinho, K.; Tsai, W.-T.; Aziz, K.; Auer, M.; Tran, P.T.; Bader, J.S.; Ewald, A.J. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J. Cell Biol. 2014, 204, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, U.S.; Maheswaran, S.; Haber, D.A. A conduit to metastasis: Circulating tumor cell biology. Genes Dev. 2017, 31, 1827–1840. [Google Scholar] [CrossRef]
- Tayoun, T.; Faugeroux, V.; Oulhen, M.; Aberlenc, A.; Pawlikowska, P.; Farace, F. CTC-derived models: A window into the seeding capacity of circulating tumor cells (CTCs). Cells 2019, 8, 1145. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.M.; Fang, C.-M.; Chuah, L.-H.; Leong, C.O.; Ngai, S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. 2018, 121, 11–22. [Google Scholar] [CrossRef]
- Hugo, H.; Kokkinos, M.I.; Blick, T.; Ackland, L.; Thompson, E.W.; Newgreen, D.F. Defining the e-cadherin repressor interactome in epithelial-mesenchymal transition: The PMC42 model as a case study. Cells Tissues Organs 2011, 193, 23–40. [Google Scholar] [CrossRef]
- Estrada, O.M.M.; Cullerés, A.; Soriano, F.; Peinado, H.; Bolós, V.; Martínez, F.O.; Reina, M.; Cano, A.; Fabre, M.; Vilaró, S. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells1. Biochem. J. 2006, 394, 449–457. [Google Scholar] [CrossRef]
- Savagner, P.; Yamada, K.M.; Thiery, J.P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor–induced epithelial–mesenchymal transition. J. Cell Biol. 1997, 137, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Guaita, S.; Puig, I.; Garrido, M.; Dominguez, D.; Batlle, E.; Sancho, E.; Dedhar, S.; Baulida, J.; Franci, C.; De Herreros, A.G. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression andZEB1 expression. J. Biol. Chem. 2002, 277, 39209–39216. [Google Scholar] [CrossRef]
- Brabletz, T. To differentiate or not—Routes towards metastasis. Nat. Rev. Cancer 2012, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Jolly, M.K.; Boareto, M.; Parsana, P.; Mooney, S.M.; Pienta, K.J.; Levine, H.; Ben-Jacob, E. OVOL guides the epithelial-hybrid-mesenchymal transition. Oncotarget 2015, 6, 15436–15448. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, D.; Chen, Y.; Min, Z.; Quan, Y. GRHL2 inhibits colorectal cancer progression and metastasis via oppressing epithelial-mesenchymal transition. Cancer Biol. Ther. 2019, 20, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.; Shao, J.; Picon, H.M.; Bristow, C.; Ge, Z.; Peoples, M.; Robinson, F.; Jeter-Jones, S.L.; Schlosberg, C.; Grzeskowiak, C.L.; et al. A functional genomic screen in vivo identifies CEACAM5 as a clinically relevant driver of breast cancer metastasis. NPJ Breast Cancer 2018, 4. [Google Scholar] [CrossRef]
- Qi, S.; Yan, L.; Liu, Z.; Mu, Y.-L.; Li, M.; Zhao, X.; Chen, Z.-J.; Zhang, H. Melatonin inhibits 17β-estradiol-induced migration, invasion and epithelial-mesenchymal transition in normal and endometriotic endometrial epithelial cells. Reprod. Biol. Endocrinol. 2018, 16, 62. [Google Scholar] [CrossRef]
- Wu, R.-F.; Chen, Z.-X.; Zhou, W.-D.; Li, Y.-Z.; Huang, Z.-X.; Lin, D.-C.; Ren, L.-L.; Chen, Q.-X.; Chen, Q.-H. High expression of ZEB1 in endometriosis and its role in 17β-estradiol-induced epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2018, 11, 4744–4758. [Google Scholar]
- Li, J.; Ma, J.; Fei, X.; Zhang, T.; Zhou, J.; Lin, J. Roles of cell migration and invasion mediated by Twist in endometriosis. J. Obstet. Gynaecol. Res. 2019, 45, 1488–1496. [Google Scholar] [CrossRef]
- Van Der Linden, P.J.; De Goeij, A.F.A.; Dunselman, G.; Van Der Linden, E.P.; Ramaekers, F.C.; Evers, J.L. Expression of integrins and E-cadherin in cells from menstrual effluent, endometrium, peritoneal fluid, peritoneum, and endometriosis. Fertil. Steril. 1994, 61, 85–90. [Google Scholar] [CrossRef]
- Ota, H.; Tanaka, T. Integrin adhesion molecules in the endometrial glandular epithelium in patients with endometriosis or adenomyosis. J. Obstet. Gynaecol. Res. 1997, 23, 485–491. [Google Scholar] [CrossRef]
- Gaetje, R.; Kotzian, S.; Herrmann, G.; Baumann, R.; Starzinski-Powitz, A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 1997, 150, 461–467. [Google Scholar] [PubMed]
- Béliard, A.; Donnez, J.; Nisolle, M.; Foidart, J.-M. Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil. Steril. 1997, 67, 266–272. [Google Scholar] [CrossRef]
- Scotti, S.; Regidor, P.-A.; Schindler, A.; Winterhager, E. Reduced proliferation and cell adhesion in endometriosis. Mol. Hum. Reprod. 2000, 6, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Yamashita, Y.; Takehara, M.; Terai, Y.; Kumagai, K.; Ueki, K.; Kanda, K.; Hung, Y.C.; Ueki, M. Gene expression of adhesion molecules and matrix metalloproteinases in endometriosis. Gynecol. Endocrinol. 2002, 16, 391–402. [Google Scholar] [CrossRef]
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Daraï, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet. Gynecol. Scand. 2002, 81, 195–203. [Google Scholar] [CrossRef]
- Shaco-Levy, R.; Sharabi, S.; Benharroch, D.; Piura, B.; Sion-Vardy, N. Matrix metalloproteinases 2 and 9, E-cadherin, and β-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 139, 226–232. [Google Scholar] [CrossRef]
- Bartley, J.; Jülicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2013, 289, 871–881. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Xiong, W.; Zhang, L.; Liu, H.; Du, Y.; Li, N. Hypoxia-inducible factor Iα-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum. Reprod. 2016, 31, 1327–1338. [Google Scholar] [CrossRef]
- Cai, X.; Shen, M.; Liu, X.; Guo, S.-W. Reduced expression of eukaryotic translation initiation factor 3 subunit e and its possible involvement in the epithelial-mesenchymal transition in endometriosis. Reprod. Sci. 2017, 25, 102–109. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Liu, H.; Li, N.; Du, Y.; He, H.; Zhang, Z.; Liu, Y. E2-mediated EMT by activation of β-catenin/Snail signalling during the development of ovarian endometriosis. J. Cell. Mol. Med. 2019, 23, 8035–8045. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Dai, H.; Zhao, J.; Liu, T.; Zhang, G. Silencing of forkhead box M1 reverses transforming growth factor-β1-induced invasion and epithelial-mesenchymal transition of endometriotic epithelial cells. Gynecol. Obstet. Investig. 2019, 84, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Grund, S.; Grümmer, R. Direct cell–cell interactions in the endometrium and in endometrial pathophysiology. Int. J. Mol. Sci. 2018, 19, 2227. [Google Scholar] [CrossRef] [PubMed]
- Gaetje, R.; Holtrich, U.; Engels, K.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Differential expression of claudins in human endometrium and endometriosis. Gynecol. Endocrinol. 2008, 24, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Y.; Li, X.; Weng, Z.-P.; Wang, B. Altered expression of claudin-3 and claudin-4 in ectopic endometrium of women with endometriosis. Fertil. Steril. 2009, 91, 1692–1699. [Google Scholar] [CrossRef]
- Horné, F.; Dietze, R.; Berkes, E.; Oehmke, F.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Impaired localization of claudin-11 in endometriotic epithelial cells compared to endometrial cells. Reprod. Sci. 2018, 26, 1181–1192. [Google Scholar] [CrossRef]
- Hoerscher, A.; Horné, F.; Dietze, R.; Berkes, E.; Oehmke, F.; Tinneberg, H.-R.; Meinhold-Heerlein, I.; Konrad, L. Localization of claudin-2 and claudin-3 in eutopic and ectopic endometrium is highly similar. Arch. Gynecol. Obstet. 2020, 301, 1003–1011. [Google Scholar] [CrossRef]
- Furuya, M.; Masuda, H.; Hara, K.; Uchida, H.; Sato, K.; Sato, S.; Asada, H.; Maruyama, T.; Yoshimura, Y.; Katabuchi, H.; et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet. Gynecol. Scand. 2017, 96, 1128–1135. [Google Scholar] [CrossRef]
- Konrad, L.; Gronbach, J.; Horné, F.; Mecha, E.O.; Berkes, E.; Frank, M.; Gattenlöhner, S.; Omwandho, C.O.; Oehmke, F.; Tinneberg, H.R. Similar characteristics of the endometrial and endometriotic epithelium. Reprod. Sci. 2019, 26, 49–59. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Balendran, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Küssel, L.; Reischer, T.; Wenzl, R.; Streubel, B.; et al. Enhanced expression of the stemness-related factors OCT4, SOX15 and TWIST1 in ectopic endometrium of endometriosis patients. Reprod. Biol. Endocrinol. 2016, 14, 81. [Google Scholar] [CrossRef]
- Nisolle, M.; Casanas-Roux, F.; Donnez, J. Coexpression of cytokeratin and vimentin in eutopic endometrium and endometriosis throughout the menstrual cycle: Evaluation by a computerized method. Fertil. Steril. 1995, 64, 69–75. [Google Scholar] [CrossRef]
- Song, I.O.; Hong, S.R.; Huh, Y.; Yoo, K.J.; Koong, M.K.; Jun, J.Y.; Kang, I.S. Expression of vimentin and cytokeratin in eutopic and ectopic endometrium of women with adenomyosis and ovarian endometrioma. Am. J. Reprod. Immunol. 1998, 40, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schmalhofer, O.; Brabletz, S.; Brabletz, T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009, 28, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Van Patten, K.; Parkash, V.; Jain, D. Cadherin expression in gastrointestinal tract endometriosis: Possible role in deep tissue invasion and development of malignancy. Mod. Pathol. 2009, 23, 38–44. [Google Scholar] [CrossRef]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.; Armstrong, A.J.; Hanash, S.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Adachi, S.; Kase, H.; Motoyama, T.; Inoue, I.; Enomoto, T. Different mutation profiles between epithelium and stroma in endometriosis and normal endometrium. Hum. Reprod. 2019, 34, 1899–1905. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamaawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal expansion and diversification of cancer-associated mutations in endometriosis and normal endometrium. Cell. Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Kennedy, S.H.; Barlow, D.H. Endometriotic disease: The role of peritoneal fluid. Hum. Reprod. Update 1999, 4, 741–751. [Google Scholar] [CrossRef]
- Koninckx, P.; Barlow, D.; Kennedy, S. Implantation versus infiltration: The Sampson versus the endometriotic disease theory. Gynecol. Obstet. Investig. 1999, 47, 3–10. [Google Scholar] [CrossRef]
- Vercellini, P.; Frontino, G.; De Giorgi, O.; Aimi, G.; Zaina, B.; Crosignani, P.G. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: A pilot study. Fertil. Steril. 2003, 80, 305–309. [Google Scholar] [CrossRef]
- Koks, C.A.; Dunselman, G.A.; De Goeij, A.F.; Arends, J.W.; Evers, J. Evaluation of a menstrual cup to collect shed endometrium for in vitro studies. Fertil. Steril. 1997, 68, 560–564. [Google Scholar] [CrossRef]
- Bobek, V.; Kolostova, K.; Kucera, E. Circulating endometrial cells in peripheral blood. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, E.; Kiss, I.; Souckova, H.; Tomes, P.; Spicka, J.; Matkowski, R.; Jedryka, M.; Ferrero, S.; Bobek, V.; Kolostova, K. Circulating endometrial cells: A new source of information on endometriosis dynamics. J. Clin. Med. 2019, 8, 1938. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.J.; Redfern, C.P.; Hirst, B.H.; Thomas, E.J. Characterization of human purified epithelial and stromal cells from endometrium and endometriosis in tissue culture. Fertil. Steril. 1992, 57, 990–997. [Google Scholar] [CrossRef]
- Malik, S.; Day, K.; Perrault, I.; Charnock-Jones, D.S.; Smith, S. Menstrual effluent in endometriosis shows no difference in volume, VEGF-A, MMP2 and MMP9 or sFLT. Reprod. Biomed. Online 2006, 12, 174–181. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Gil, C.L.; Ballesteros, A.; Santamaria, X. Endometrial stromal cells circulate in the bloodstream of women with endometriosis: A pilot study. Int. J. Mol. Sci. 2019, 20, 3740. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, H.-L.; Tang, Z.-W.; Neoh, K.H.; Ouyang, D.-F.; Cui, H.; Cheng, H.-Y.; Ma, R.-Q.; Ye, X.; Han, R.P.; et al. Evaluation of circulating endometrial cells as a biomarker for endometriosis. Chin. Med. J. 2017, 130, 2339–2345. [Google Scholar] [CrossRef]
- Demir, A.; Groothuis, P.; Nap, A.; Punyadeera, C.; Goeij, A.; Evers, J.; Dunselman, G.A.J. Menstrual effluent induces epithelial-mesenchymal transitions in mesothelial cells. Hum. Reprod. 2004, 19, 21–29. [Google Scholar] [CrossRef]
- Demir, A.Y.; Groothuis, P.G.; Dunselman, G.A.J.; Schurgers, L.; Evers, J.; De Goeij, A.F.P.M. Molecular characterization of soluble factors from human menstrual effluent that induce epithelial to mesenchymal transitions in mesothelial cells. Cell Tissue Res. 2005, 322, 299–311. [Google Scholar] [CrossRef]
- Albertsen, H.M.; Ward, K. Genes linked to endometriosis by GWAS are integral to cytoskeleton regulation and suggests that mesothelial barrier homeostasis is a factor in the pathogenesis of endometriosis. Reprod. Sci. 2017, 24, 803–811. [Google Scholar] [CrossRef]
- Nap, A.W.; Groothuis, P.G.; Demir, A.Y.; Maas, J.W.; Dunselman, G.A.; De Goeij, A.F.; Evers, J. Tissue integrity is essential for ectopic implantation of human endometrium in the chicken chorioallantoic membrane. Hum. Reprod. 2003, 18, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Feider, C.L.; Woody, S.; Ledet, S.; Zhang, J.; Sebastian, K.; Breen, M.T.; Eberlin, L.S. Molecular imaging of endometriosis tissues using desorption electrospray ionization mass spectrometry. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W. Cancer-associated mutations in endometriosis: Shedding light on the pathogenesis and pathophysiology. Hum. Reprod. Update 2020, 26, 423–449. [Google Scholar] [CrossRef] [PubMed]

| Eutopic Endometrium | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| [69] | 16 | 8 | 8 | 100% | n.d. | 16 | n.d. | 8 | n.d. | −16.70% | n.d. |
| [70] | 19 | n.sp. | n.sp. | 2.2 P/2.8 S | n.d. | 10 | n.sp. | n.sp. | n.sp. | 2.7 P/3.2 S | n.d. |
| [71] | 26 | n.sp. | n.sp. | 99% | n.d. | 9 | n.sp. | n.sp. | n.sp. | 33% | n.d. |
| [72] | 18 | n.d. | 18 | 100% | n.d. | 18 | n.d. | 18 | n.d. | 89% | n.d. |
| [73] | 49 | 25 | 24 | 2.24 a/1.83 b | (n.s.) | 21 | n.d. | 21 | n.d. | (0.52 c) | (<0.001 a,b/c) |
| [74] | 7 | n.sp. | n.sp. | (1.71 a) | n.d. | 11 | 11 | n.d. | n.d. | (0.82 b) | (0.0025 a/b) |
| [75] | 15 | 15 | n.d. | 92% a | n.d. | 23 | n.d. | 23 | n.d. | 50% b | <0.01 a/b |
| [76] | 10 | n.sp. | n.sp | 91% | n.d. | 15 | n.sp. | n.sp. | n.sp. | 85% | n.s. |
| [40] | 32 | 14 | 18 | 11.2 a | n.s. | 199 | 55 | 30 red, 46 black | 68 | 3.3 b/2.9 red c/19.4 black d/20.3 e | 0.03 a/d |
| 0.005 a/e | |||||||||||
| 0.0001 b,c/d | |||||||||||
| 0.0001 b,c/e | |||||||||||
| [77] | 12 | 12 | n.d. | 2.58 | n.d. | 25 | 9 | 9 | 9 | 2.76 all three | n.s. |
| [78] | 41 | 20 | 21 | 8.35 a/7.24 b | n.s. | 21 | 21 | n.d. | n.d. | 5.14 c | 0.0325 a/c |
| 0.0089 b/c | |||||||||||
| <0.01 a,b/c | |||||||||||
| [79] | 40 | 40 | n.d. | (~0.35) | n.d. | 40 | 40 | n.d. | n.d. | (~0.05) | ≤0.05 |
| [67] | 23 | 12 | 11 | (~38 a/~20 b) | ≤0.05 a/b | 11 | 11 | n.d. | n.d. | (~18 **) | <0.01 a/c |
| [68] | 60 | 30 | 30 | (1.83 a/0.73 b) | (0.001 a/b) | 30 | 30 | n.d. | n.d. | (0.27 c) | ≤0.05 a,b/c |
| [80] | 42 | 21 | 21 | 6.38 a/7.43 b | n.s. | 21 | 21 | n.d. | n.d. | 3.86 c | ≤0.05 a/c |
| <0.01 b/c | |||||||||||
| [81] | 110 | 50 | 60 | (~7.3 a/7.2 b) | n.s. | 65 | 65 | n.d. | n.d. | (~5.0 c) | ≤0.05 a,b/c |
| Eutopic Endometrium | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| [73] | 49 | 25 | 24 | 1.8 a/1.58 b | (n.s.) | 21 | n.d. | 21 | n.d. | (0.76 c) | (≤0.05 b/c) |
| (<0.001 a/c) | |||||||||||
| [74] | 7 | n.sp. | n.sp. | (1.71 a) | n.d. | 11 | 11 | n.d. | n.d. | (0.91 b) | (0.0095 a/b) |
| [76] | 10 | n.sp. | n.sp | 91% | n.d. | 15 | n.sp. | n.sp. | n.sp. | 72% | 0.002 |
| [80] | 42 | 21 | 21 | 14.3%/23.8% | n.s. | 21 | 21 | n.d. | n.d. | 61.9% | n.d. ≤0.05 b/c |
| 2.57 a/3.62 b | 7.14 | <0.01 a/c | |||||||||
| Eutopic Endometrium (Phases) | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| Claudin-1 | |||||||||||
| [83] | 22 | 13 | 9 | (2.05 a both) | n.d. | 17 | n.d. | 17 | n.d. | (1.47 b) | (0.0187 a/b) |
| Claudin-2 | |||||||||||
| [86] | 26 P, S | 12 | 14 | 165 P/178 S 176/189 | n.s. n.s. | 19 | 6 | 6 | 7 | 179/220/209 | n.s. |
| Claudin-3 | |||||||||||
| [83] | 22 | 13 | 9 | (2.27 both) | n.d. | (20) | n.d. | (20) | n.d. | (2.0) | n.s. |
| [84] | 62 P, S | 35 | 27 | 0.53 P/0.6 S0.57 a/0.59 b | n.s. n.s. | 35 | 35 | n.d. | n.d. | 0.17 c | ≤0.05 a/c ≤0.05 b/c |
| [86] | 51 P, S | 19 | 32 | 268 P/253 S261/270 | n.s. n.s. | 62 | 20 | 17 | 25 | 266/263/286 | n.s. |
| Claudin-4 | |||||||||||
| [83] | 20 | n.sp. | n.sp. | (2.3 a all) | n.d. | 20 | n.d. | 20 | n.d. | (1.4) | (0.0031 b) |
| [84] | 62 P, S | 35 | 27 | 0.6 P/0.85 S 0.74 a/0.7 b | n.s. | 35 | 35 | n.d. | n.d. | 0.20 c | ≤0.05 a/c ≤0.05 b/c |
| Claudin-5 | |||||||||||
| [83] | 21 | n.sp. | n.sp. | (1.9 all) | n.d. | 19 | n.d. | 19 | n.d. | (1.42) | (n.s.) |
| Claudin-7 | |||||||||||
| [83] | 19 | n.sp | n.sp | (2.21 all) | n.d. | 21 | n.d. | 21 | n.d. | (1.81) | (n.s.) |
| [85] | 49 P, S | 18 | 31 | 232 P/249 S 241/237 | n.s. n.s. | 62 | 20 | 17 | 25 | 235/278/257 | n.s. |
| Claudin-11 | |||||||||||
| [85] | 49 P, S | 18 | 31 | 185 P/187 S 186/169 | n.s. | 62 | 20 | 17 | 25 | 125/182/159 | n.s. |
| Eutopic Endometrium (Phases) | Ectopic Endometrium | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| [77] | 12 | 12 | n.d. | 1.58 a | n.d. | 24 | n.sp. | n.sp. | n.sp. | 1.96 b | 0.027 a/b |
| [87] | 10 | 10 | n.d. | 100% p | n.d. | 7 | 6 lesions | n.d. | 5 lesions | 100% p | n.d. |
| [79] | 40 | 40 | n.d. | (~0.04) | n.d. | 40 | 40 | n.d. | n.d. | (~0.52) | <0.001 |
| [80] | 42 | 21 | 21 | 19% a/19% b 3.43 d/2.71 e | n.s. | 21 | 21 | n.d. | n.d. | 57.1% c 5.86 f | ≤0.05 a,b/c ≤0.01 e/f |
| Eutopic Endometrium | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| ZEB1 | |||||||||||
| [87] | 10 | 10 | n.d. | 0% a s | n.d. | 7 | 6 lesions | n.d. | 5 lesions | 45% b s | 0.0039 a/b |
| [67] | 23 | 12 | 11 | ~(25 a/200) | <0.001 | 11 | 11 | n.d. | n.d. | (~125 b) | ≤0.05 a/b |
| [88] | 50 | 20 | 30 | 104/71 84 a both | n.s. | 75 | 30 lesions | 21 lesions | 27 lesions | 145/138/202 b | <0.001 a/b |
| Twist | |||||||||||
| [77] | 13 | 13 | n.d. | 1.31 a | n.d. | 26 | 9 | 9 | 9 | 2.42 b all | <0.001 a/b |
| [89] | 119 | 50 | 69 | (~17% with EM) | n.d. | 86 | n.sp. | n.sp. | n.sp. | (~50%) | <0.001 |
| [90] | 119 | 50 | 69 | (~3.0/~1.8 a) | 0.001 | 90 | n.sp. | n.sp. | n.sp. | (~3.0 b) | <0.001 a/b |
| [68] | 60 | 30 Infertile | 30 | (0.17 a/0.47 b) | n.s. | 30 | 30 | n.d. | n.d. | (1.67 c) | (<0.001 a,b/c) |
| Eutopic Endometrium (Phases) | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | with EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| [91] | 29 P, S | 29 | n.d. | 188 a P, 140 ES 93 b LS | <0.01 a/b | 31 | n.d. | 31 black | n.d. | 63 P, 80 ES, 55 LS | n.s. |
| [92] | 20 | n.d. | 20 | 342 a P, 252 b S | <0.01 a/b | 20 | 20 | n.d. | n.d. | 185 c P, 130 d S | ≤0.05 c/d |
| [40] | 32 | 14 | 18 | 10.5 a | n.d. | 199 | 55 | 30 red 46 black | 68 | 1.5 b/34.3 red c/63.4 black d/20.5 e | <0.0001 a/c,d,e <0.0001 b/c,d,e |
| [78] | 41 | 20 | 21 | 10% a/23.8% b | n.s. | 21 | 21 | n.d. | n.d. | 61.9% c | 0.0278 b/c 0.0009 a/c |
| [79] | 40 | 40 | n.d. | (~0.23) | n.d. | 40 | 40 | n.d. | n.d. | (~0.44) | <0.01 |
| [67] | 23 | 12 | 11 | (~20 a/~90) | 0.01 | 11 | 11 | n.d. | n.d. | (~95 b) | <0.01 a/b |
| [88] | 50 | 20 | 30 | 65/76 72 a both | n.s. | 78 | 30 | 27 | 21 | 15 b/46 c/94 | <0.01 a/b <0.001 b/c |
| Eutopic Endometrium | Ectopic Endometrium (Entities) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refs | All (n) | Without EM | With EM | Scores | p-Value | All (n) | Ov. EM | PE | DIE | Scores | p-Value |
| [94] | 26 | 26 | n.d. | 4.2 a P/1.5 b S | <0.01 a/b | 34 | n.d. | 13 | 21 | 1.7 c/2.9 d | ≤0.05 a/c |
| [68] | 60 | 30 | 30 | 10%/30% 0.1 a/0.3 b | n.d. | 30 | 30 | n.d. | n.d. | 60% 0.77 c | ≤0.05 b/c <0.001 a/c |
| [78] | 40 | 20 | 21 | 5% a/9.5% b 1.75 a/1.38 b | n.s. ˃0.05 a/b | 21 | 21 | n.d. | n.d. | 38.1% 3.67 c | 0.02 a/c <0.05 a,b/c |
| [77] | 13 | 13 | n.d. | (1.85 a) | n.d. | 27 | 9 | 9 | 9 | (2.54 b) | (0.005 a/b) |
| [40] | 32 | 14 | 18 | 0/0 a | n.s. | 199 | 55 b | 30 c red, 46 d black | 68 e | 1.2 b/3.9 c/1.7 d/1.3 e | 0.0001 a/c 0.0006 c/d 0.006 a/e |
| [75] | 15 | 15 | n.d. | 100%/93% | n.d. | 23 | n.d. | 23 | n.d. | 100%/89% | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horné, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen? J. Clin. Med. 2020, 9, 1915. https://doi.org/10.3390/jcm9061915
Konrad L, Dietze R, Riaz MA, Scheiner-Bobis G, Behnke J, Horné F, Hoerscher A, Reising C, Meinhold-Heerlein I. Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen? Journal of Clinical Medicine. 2020; 9(6):1915. https://doi.org/10.3390/jcm9061915
Chicago/Turabian StyleKonrad, Lutz, Raimund Dietze, Muhammad A. Riaz, Georgios Scheiner-Bobis, Judith Behnke, Fabian Horné, Alena Hoerscher, Christoph Reising, and Ivo Meinhold-Heerlein. 2020. "Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen?" Journal of Clinical Medicine 9, no. 6: 1915. https://doi.org/10.3390/jcm9061915
APA StyleKonrad, L., Dietze, R., Riaz, M. A., Scheiner-Bobis, G., Behnke, J., Horné, F., Hoerscher, A., Reising, C., & Meinhold-Heerlein, I. (2020). Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen? Journal of Clinical Medicine, 9(6), 1915. https://doi.org/10.3390/jcm9061915







