Movement Velocity as A Measure of Exercise Intensity in Persons with Multiple Sclerosis: A Validity Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Leg Press Testing Procedure
2.4. Bench Press Testing Procedure
2.5. Statistical Analyses
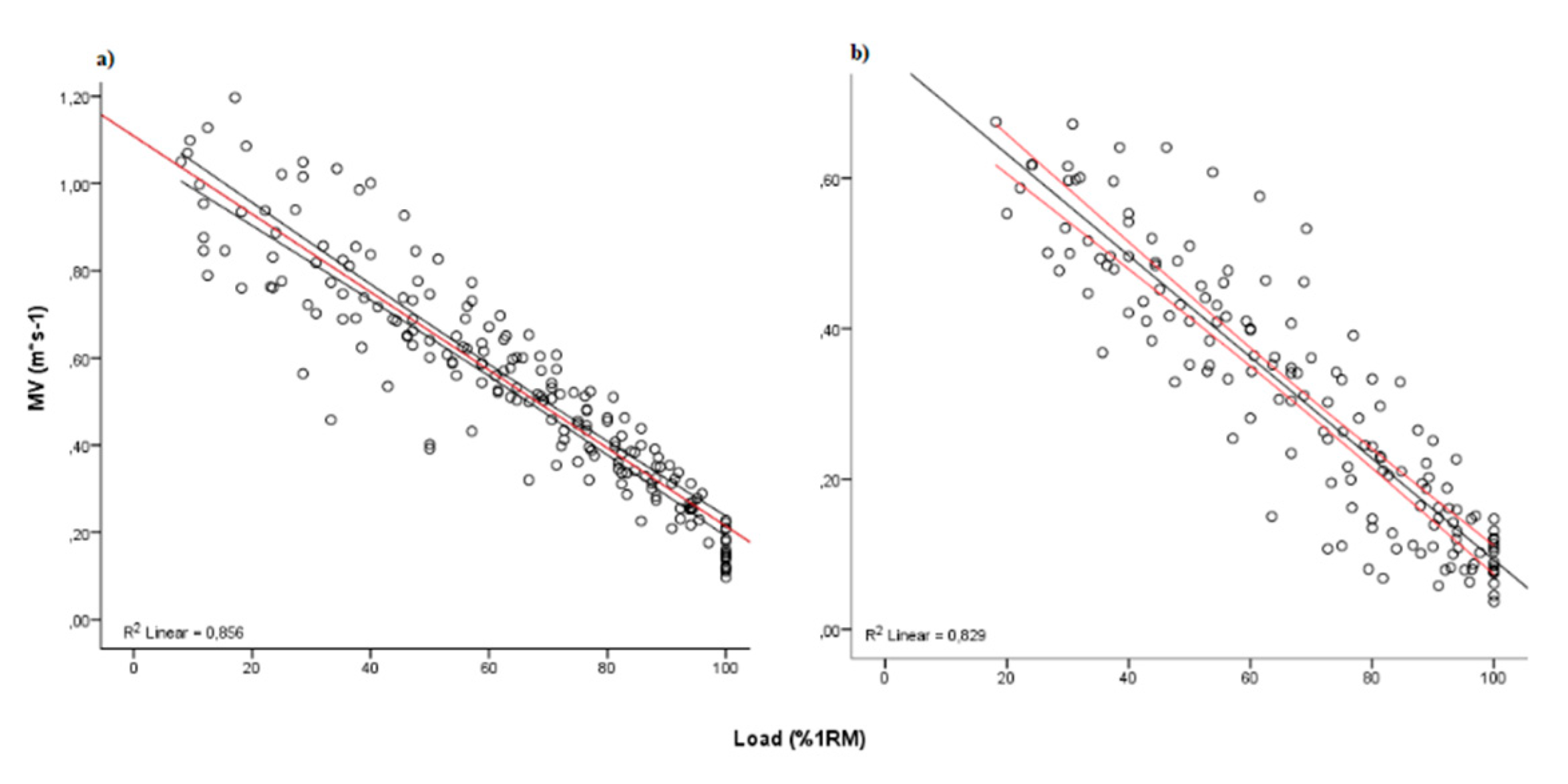
3. Results
4. Discussion
5. Limitations and Strengths of the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Anacker, S.L.; Di Fabio, R.P. Influence of sensory inputs on standing balance in community-dwelling elders with a recent history of falling. Phys. Ther. 1992, 72, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; Kovacs, L.; Ketelaer, P.; Carton, H.; Decramer, M. Respiratory muscle weakness and respiratory muscle training in severely disabled multiple sclerosis patients. Arch. Phys. Med. Rehabil. 2000, 81, 747–751. [Google Scholar] [CrossRef]
- De Ruiter, C.J.; Jongen, P.J.H.; Van der Woude, L.H.V.; De Haan, A. Contractile speed and fatigue of adductor pollicis muscle in multiple sclerosis. Muscle Nerve 2001, 24, 1173–1180. [Google Scholar] [CrossRef]
- Stuifbergen, A.K. Health-Promoting behaviors and quality of life among individuals with multiple sclerosis. Sch. Inq. Nurs. Pract. 1995, 9, 31–50. [Google Scholar]
- Kjølhede, T.; Vissing, K.; Dalgas, U. Multiple sclerosis and progressive resistance training: A systematic review. Mult. Scler. J. 2012, 18, 1215–1228. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Hansen, H.J.; Knudsen, C.; Overgaard, K.; Ingemann-Hansen, T. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009, 10, 668–674. [Google Scholar] [CrossRef]
- Dalgas, U.; Stenager, E.; Jakobsen, J.; Petersen, T.; Hansen, H.J.; Knudsen, C.; Overgaard, K.; Ingemann-Hansen, T. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult. Scler. J. 2010, 16, 480–490. [Google Scholar] [CrossRef]
- Zhanneta, K.; Irina, S.; Tatyana, B.; Olena, R.; Olena, L.; Anna, I. The applying of the concept of individualization in sport. J. Phys. Educ. Sport 2015, 15, 172–177. [Google Scholar]
- Moss, B.M.; Refsnes, P.E.; Abildgaard, A.; Nicolaysen, K.; Jensen, J. Effects of maximal effort strength training with different loads on dynamic strength, cross-sectional area, load-power and load-velocity relationships. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.F.; Dodd, K.J.; Prasad, D.; Denisenko, S. Progressive resistance exercise for people with multiple sclerosis. Disabil. Rehabil. 2006, 28, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Ratamess, N.; Alvar, B.; Evetoch, T. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687. [Google Scholar]
- Niewiadomski, W.; Laskowska, D.; Gąsiorowska, A.; Cybulski, G.; Strasz, A.; Langfort, J. Determination and prediction of one repetition maximum (1RM): Safety considerations. J. Hum. Kinet. 2008, 19, 109–120. [Google Scholar] [CrossRef]
- Jørgensen, M.L.K.; Dalgas, U.; Wens, I.; Hvid, L.G. Muscle strength and power in persons with multiple sclerosis—A systematic review and meta-analysis. J. Neurol. Sci. 2017, 376, 225–241. [Google Scholar] [CrossRef]
- Christogianni, A.; Bibb, R.; Davis, S.L.; Jay, O.; Barnett, M.; Evangelou, N.; Filingeri, D. Temperature sensitivity in multiple sclerosis: An overview of its impact on sensory and cognitive symptoms. Temperature 2018, 5, 208–223. [Google Scholar] [CrossRef]
- Cheung, J.; Rancourt, A.; Di Poce, S.; Levine, A.; Hoang, J.; Ismail, F.; Boulias, C.; Phadke, C.P. Patient-identified factors that influence spasticity in people with stroke and multiple sclerosis receiving botulinum toxin injection treatments. Physiother. Canada 2015, 67, 157–166. [Google Scholar] [CrossRef]
- Wens, I.; Dalgas, U.; Vandenabeele, F.; Grevendonk, L.; Verboven, K.; Hansen, D.; Eijnde, B.O. High intensity exercise in multiple sclerosis: Effects on muscle contractile characteristics and exercise capacity, a randomised controlled trial. PLoS ONE 2015, 10, e0133697. [Google Scholar] [CrossRef]
- Gail, S.; Künzell, S. Reliability of a 5-Repetition Maximum Strength Test in Recreational Athletes. Dtsch. Z. Sportmed. 2014, 65, 314–317. [Google Scholar] [CrossRef]
- Abdul-Hameed, U.; Rangra, P.; Shareef, M.Y.; Hussain, M.E. Reliability of 1-repetition maximum estimation for upper and lower body muscular strength measurement in untrained middle aged type 2 diabetic patients. Asian J. Sports Med. 2012, 3, 267–273. [Google Scholar] [CrossRef]
- Sánchez-Medina, L.; Gonzalez-Badillo, J.J.; Perez, C.E.; Pallarés, J.G. Velocity-and power-load relationships of the bench pull vs. bench press exercises. Int. J. Sports Med. 2014, 35, 209–216. [Google Scholar] [CrossRef]
- Conceição, F.; Fernandes, J.; Lewis, M.; Gonzaléz-Badillo, J.J.; Jimenéz-Reyes, P. Movement velocity as a measure of exercise intensity in three lower limb exercises. J. Sports Sci. 2016, 34, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Pardo, P.J.; González-Hernández, J.M.; García-Ramos, A.; Lopez-Vivancos, A.; Jiménez-Reyes, P. Movement velocity can be used to estimate the relative load during the bench press and leg press exercises in older women. PeerJ 2019, 7, e7533. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Konečný, L.; Pospíšil, P.; Vank, P.; Mífková, L.; Pochmonová, J.; Havelková, A.; Siegelová, J.; Dobšák, P. Combination of aerobic and resistant training in multiple sclerosis. Scr. Medica Fac. Medicae Univ. Brun. Masaryk. 2010, 1, 78560422. [Google Scholar]
- García-Ramos, A.; Pestana-Melero, F.L.; Pérez-Castilla, A.; Rojas, F.J.; Haff, G.G. Differences in the load-velocity profile between 4 bench-press variants. Int. J. Sports Physiol. Perform. 2018, 13, 326–331. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Pestana-Melero, F.L.; Haff, G.G.; Rojas, F.J.; Pérez-Castilla, A.; García-Ramos, A. Reliability of the load-velocity relationship obtained through linear and polynomial regression models to predict the 1-repetition maximum load. J. Appl. Biomech. 2018, 34, 184–190. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- González-Badillo, J.J.; Sánchez-Medina, L. Movement velocity as a measure of loading intensity in resistance training. Int. J. Sports Med. 2010, 31, 347–352. [Google Scholar] [CrossRef]
- Loturco, I.; Kobal, R.; Moraes, J.E.; Kitamura, K.; Cal Abad, C.C.; Pereira, L.A.; Nakamura, F.Y. Predicting the Maximum Dynamic Strength in Bench Press: The High Precision of the Bar Velocity Approach. J. Strength Cond. Res. 2017, 31, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.F.T.; Lamb, K.L.; Twist, C. A comparison of load-velocity and load-power relationships between well-trained young and middle-aged males during three popular resistance exercises. J. Strength Cond. Res. 2018, 32, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Thoumie, P.; Mevellec, E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2002, 73, 313–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramari, C.; Hvid, L.G.; De David, A.C.; Dalgas, U. The importance of lower-extremity muscle strength for lower-limb functional capacity in multiple sclerosis: Systematic review. Ann. Phys. Rehabil. Med. 2020, 63, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, M.S.; Pollock, M.L. Prescription of resistance training for health and disease. Med. Sci. Sports Exerc. 1999, 31, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Peacock, C.A.; Sanders, G.J.; Wilson, K.A.; Fickes-Ryan, E.J.; Corbett, D.B.; Von Carlowitz, K.P.; Ridgel, A.L. Introducing a multifaceted exercise intervention particular to older adults diagnosed with parkinson’s disease: A preliminary study. Aging Clin. Exp. Res. 2014, 26, 403–409. [Google Scholar] [CrossRef]
- Torrejón, A.; Balsalobre-Fernández, C.; Haff, G.G.; García-Ramos, A. The load-velocity profile differs more between men and women than between individuals with different strength levels. Sport Biomech. 2018, 18, 245–255. [Google Scholar] [CrossRef]
- Ploutz-Snyder, L.L.; Giamis, E.L. Orientation and Familiarization to 1RM Strength Testing in Old and Young Women. J. Strength Cond. Res. 2001, 15, 519–523. [Google Scholar]
- Kent-Braun, J.A.; Ng, A.V.; Castro, M.; Weiner, M.W.; Gelinas, D.; Dudley, G.A.; Miller, R.G. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J. Appl. Physiol. 1997, 83, 1998–2004. [Google Scholar] [CrossRef]
- Schwid, S.R.; Thornton, C.A.; Pandya, S.; Manzur, K.L.; Sanjak, M.; Petrie, M.D.; McDermott, M.P.; Goodman, A.D. Quantitative assessment of motor fatigue and strength in MS. Neurology 1999, 53, 743–750. [Google Scholar] [CrossRef]
- Garner, D.J.P.; Widrick, J.J. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 2003, 27, 456–464. [Google Scholar] [CrossRef] [PubMed]
- De Haan, A.; De Ruiter, C.J.; Van Der Woude, L.H.V.; Jongen, P.J.H. Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve 2000, 23, 1534–1541. [Google Scholar] [CrossRef]
- Ng, A.V.; Miller, R.G.; Gelinas, D.; Kent-Braun, J.A. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004, 29, 843–852. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Mean ± SD (n = 18) |
|---|---|
| Age (Years) | 44.88 ± 10.62 |
| Sex (Men:Women) | 10:9 |
| EDSS | 3.12 ± 1.73 |
| Weight (kg) | 67.19 ± 10.63 |
| Height (m) | 1.66 ± 0.07 |
| BMI (kg·m−2) | 23.28 ± 5.79 |
| Bench Press | Leg Press | |
|---|---|---|
| %1RM | MPV ± SD | MPV ± SD |
| 20 | 0.90 ± 0.16 | 0.68 ± 0.10 |
| 30 | 0.82 ± 0.15 | 0.62 ± 0.09 |
| 40 | 0.73 ± 0.13 | 0.55 ± 0.08 |
| 50 | 0.64 ± 0.11 | 0.48 ± 0.07 |
| 60 | 0.55 ± 0.09 | 0.42 ± 0.06 |
| 70 | 0.46 ± 0.08 | 0.35 ± 0.05 |
| 80 | 0.37 ± 0.06 | 0.29 ± 0.05 |
| 90 | 0.29 ± 0.05 | 0.22 ± 0.04 |
| 100 | 0.20 ± 0.04 | 0.15 ± 0.04 |
| 95% CI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean %1RM | SD %1RM | Mean Equation %1RM | SD Equation %1RM | Statistic | df | p | Mean Difference | SE Difference | Lower | Upper | Cohen’s d | |
| Leg Press | 68.1 | 23.61 | 68.1 | 21.7 | 0.00522 | 153 | 0.996 | 0.004 | 0.754 | −1.485 | 1.493 | 0.000 |
| Bench Press | 63.7 | 25.8 | 63.5 | 23.7 | 0.32 | 194 | 0.749 | 0.229 | 0.714 | −1.179 | 1.636 | 0.023 |
| Leg Press (n = 156) | Bench Press (n = 195) | |||||
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| Estimate | Lower | Upper | Estimate | Lower | Upper | |
| Bias | −0.004 | −1.493 | 1.485 | 0.229 | −1.179 | 1.636 |
| Lower limit of agreement | −18.335 | −20.885 | −15.785 | −19.305 | −21.715 | −16.895 |
| Upper limit of agreement | 18.327 | 15.777 | 20.877 | 19.762 | 17.353 | 22.172 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreu-Caravaca, L.; Ramos-Campo, D.J.; Abellán-Aynés, O.; Rubio-Arias, J.Á. Movement Velocity as A Measure of Exercise Intensity in Persons with Multiple Sclerosis: A Validity Study. J. Clin. Med. 2020, 9, 2458. https://doi.org/10.3390/jcm9082458
Andreu-Caravaca L, Ramos-Campo DJ, Abellán-Aynés O, Rubio-Arias JÁ. Movement Velocity as A Measure of Exercise Intensity in Persons with Multiple Sclerosis: A Validity Study. Journal of Clinical Medicine. 2020; 9(8):2458. https://doi.org/10.3390/jcm9082458
Chicago/Turabian StyleAndreu-Caravaca, Luis, Domingo Jesús Ramos-Campo, Oriol Abellán-Aynés, and Jacobo Ángel Rubio-Arias. 2020. "Movement Velocity as A Measure of Exercise Intensity in Persons with Multiple Sclerosis: A Validity Study" Journal of Clinical Medicine 9, no. 8: 2458. https://doi.org/10.3390/jcm9082458
APA StyleAndreu-Caravaca, L., Ramos-Campo, D. J., Abellán-Aynés, O., & Rubio-Arias, J. Á. (2020). Movement Velocity as A Measure of Exercise Intensity in Persons with Multiple Sclerosis: A Validity Study. Journal of Clinical Medicine, 9(8), 2458. https://doi.org/10.3390/jcm9082458







