Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Endoscopic Procedure
2.3. Histologic Assessment
2.4. Data Collection and Follow-up
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics and Clinicopathologic Features of the T1 CRC Treated with ER
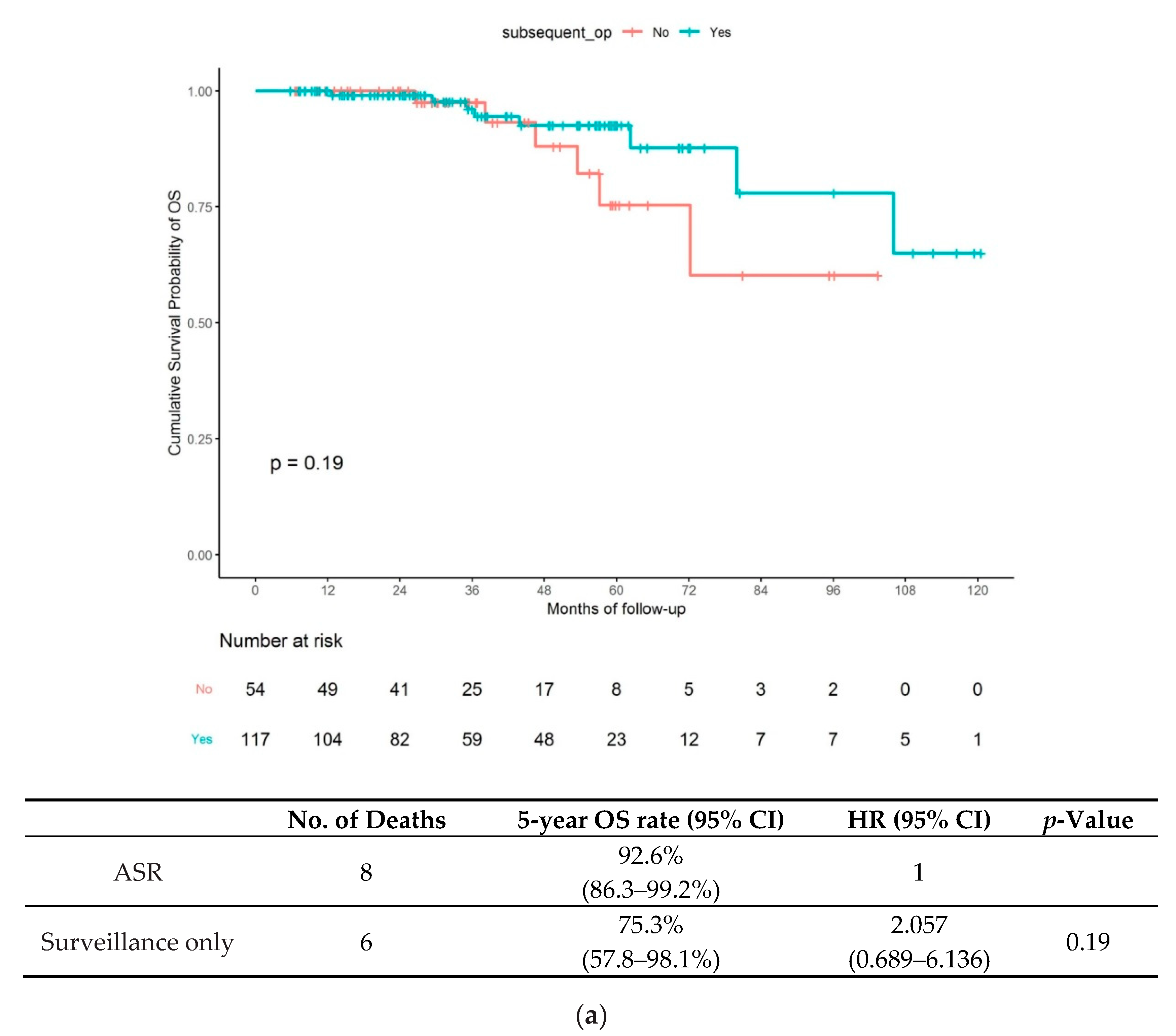
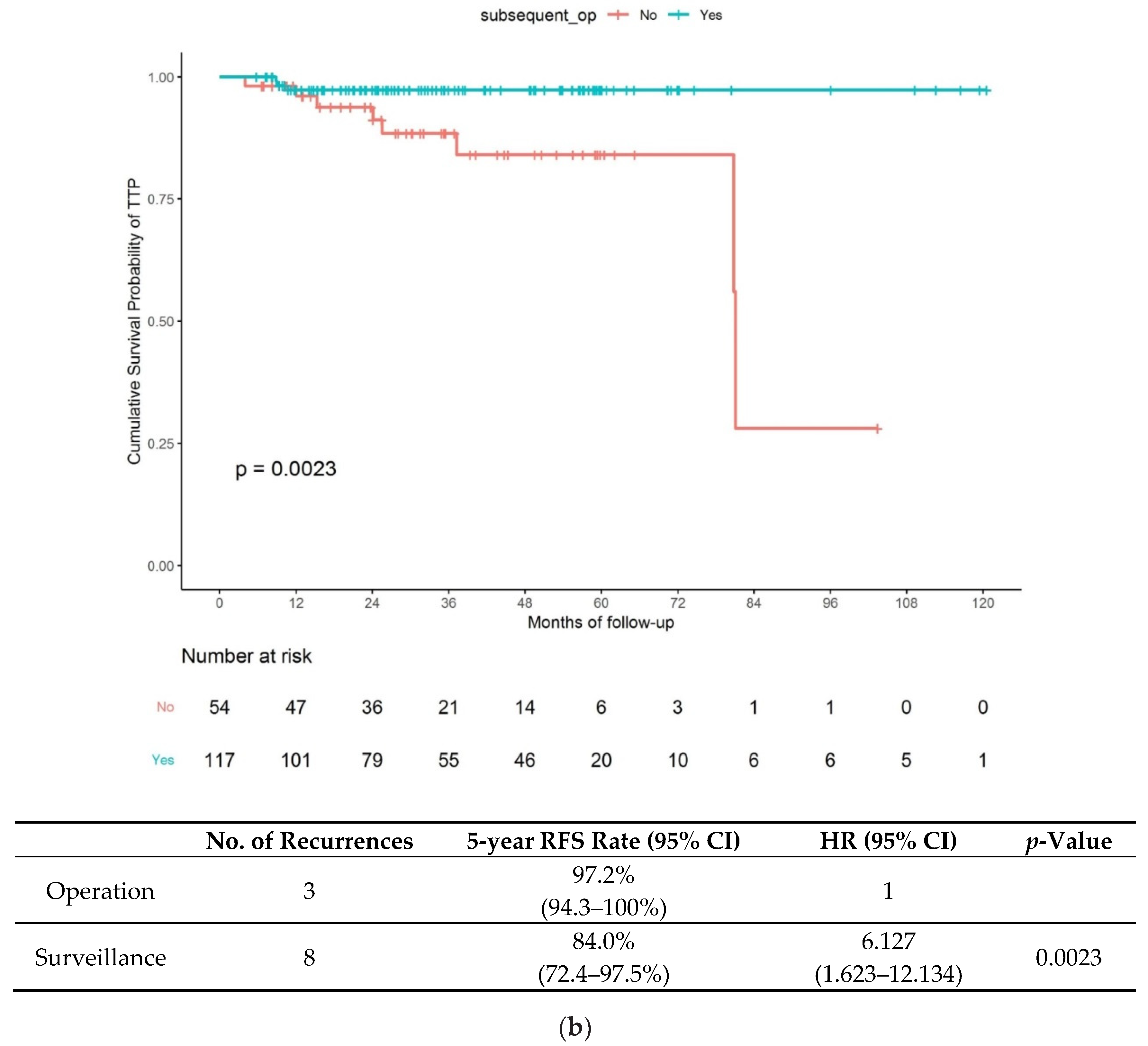
3.2. Long-Term Outcome of Patients in the C-ER and NC-ER Groups
- (1)
- Long-term outcomes in the C-ER group:
- (2)
- Long-term outcomes in the NC-ER group
3.3. Characteristics of Recurrent T1 CRC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muto, T.; Oya, M. Recent advances in diagnosis and treatment of colorectal T1 carcinoma. Dis. Colon Rectum 2003, 46, S89–S93. [Google Scholar] [PubMed]
- Shida, H.; Ban, K.; Matsumoto, M.; Masuda, K.; Imanari, T.; Machida, T.; Yamamoto, T.; Inoue, T. Asymptomatic colorectal cancer detected by screening. Dis. Colon Rectum 1996, 39, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Iida, S.; Hasegawa, H.; Okabayashi, K.; Moritani, K.; Mukai, M.; Kitagawa, Y. Risk factors for postoperative recurrence in patients with pathologically T1 colorectal cancer. World J. Surg. 2012, 36, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.; Nojima, M.; Nosho, K.; Omori, S.; Kusumi, T.; Okuda, H.; Tsukagoshi, H.; Fujita, M.; Yamamoto, H.; Hosokawa, M. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin. Gastroenterol. Hepatol. 2014, 12, 292–302. [Google Scholar] [CrossRef]
- Yoda, Y.; Ikematsu, H.; Matsuda, T.; Yamaguchi, Y.; Hotta, K.; Kobayashi, N.; Fujii, T.; Oono, Y.; Sakamoto, T.; Nakajima, T. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013, 45, 718–724. [Google Scholar] [CrossRef]
- Dy, G.W.; Gore, J.L.; Forouzanfar, M.H.; Naghavi, M.; Fitzmaurice, C. Global Burden of Urologic Cancers, 1990–2013. Eur. Urol. 2017, 71, 437–446. [Google Scholar] [CrossRef]
- Cooper, H.S.; Deppisch, L.M.; Gourley, W.K.; Kahn, E.I.; Lev, R.; Manley, P.N.; Pascal, R.R.; Qizilbash, A.H.; Rickert, R.R.; Silverman, J.F. Endoscopically removed malignant colorectal polyps: Clinicopathologic correlations. Gastroenterology 1995, 108, 1657–1665. [Google Scholar] [CrossRef]
- Minamoto, T.; Mai, M.; Ogino, T.; Sawaguchi, K.; Ohta, T.; Fujimoto, T.; Takahashi, Y. Early invasive colorectal carcinomas metastatic to the lymph node with attention to their nonpolypoid development. Am. J. Gastroenterol. 1993, 88, 1035–1039. [Google Scholar]
- Watanabe, T.; Muro, K.; Ajioka, Y.; Hashiguchi, Y.; Ito, Y.; Saito, Y.; Hamaguchi, T.; Ishida, H.; Ishiguro, M.; Ishihara, S. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2018, 23, 1–34. [Google Scholar] [CrossRef]
- Benizri, E.I.; Bereder, J.M.; Rahili, A.; Bernard, J.L.; Vanbiervliet, G.; Filippi, J.; Hébuterne, X.; Benchimol, D. Additional colectomy after colonoscopic polypectomy for T1 colon cancer: A fine balance between oncologic benefit and operative risk. Int. J. Colorectal Dis. 2012, 27, 1473–1478. [Google Scholar] [CrossRef]
- Iguchi, K.; Mushiake, H.; Aoyama, T.; Suwa, H.; Yukawa, N.; Ota, M.; Rino, Y.; Kunisaki, C.; Endo, I.; Masuda, M. Additional Surgical Resection After Endoscopic Resection for Patients with High-risk T1 Colorectal Cancer. In Vivo 2019, 33, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Baek, D.H.; Song, G.A.; Kim, G.H.; Lee, B.E. Long-term outcomes of endoscopically resected laterally spreading tumors with a positive histological lateral margin. Surg. Endosc. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Baron, J.A.; Lieberman, D.A.; Schatzkin, A.; Lanza, E.; Winawer, S.J.; Zauber, A.G.; Jiang, R.; Ahnen, D.J.; Bond, J.H.; et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009, 136, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, R.; Burgart, L.J.; Nivatvongs, S.; Larson, D.R. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis. Colon Rectum 2002, 45, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Shimazaki, H.; Aida, S.; Hase, K.; Matsukuma, S.; Kanai, T.; Kurihara, H.; Ozawa, K.; et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004, 127, 385–394. [Google Scholar] [CrossRef]
- Ikematsu, H.; Yoda, Y.; Matsuda, T.; Yamaguchi, Y.; Hotta, K.; Kobayashi, N.; Fujii, T.; Oono, Y.; Sakamoto, T.; Nakajima, T.; et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013, 144, 551–559. [Google Scholar] [CrossRef]
- Kitajima, K.; Fujimori, T.; Fujii, S.; Takeda, J.; Ohkura, Y.; Kawamata, H.; Kumamoto, T.; Ishiguro, S.; Kato, Y.; Shimoda, T.; et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: A Japanese collaborative study. J. Gastroenterol. 2004, 39, 534–543. [Google Scholar] [CrossRef]
- Bhangu, A.; Brown, G.; Nicholls, R.J.; Wong, J.; Darzi, A.; Tekkis, P. Survival outcome of local excision versus radical resection of colon or rectal carcinoma: A Surveillance, Epidemiology, and End Results (SEER) population-based study. Ann. Surg. 2013, 258, 563–569. [Google Scholar] [CrossRef]
- Yamashita, K.; Oka, S.; Tanaka, S.; Nagata, S.; Kuwai, T.; Furudoi, A.; Tamura, T.; Kunihiro, M.; Okanobu, H.; Nakadoi, K.; et al. Long-term prognosis after treatment for T1 carcinoma of laterally spreading tumors: A multicenter retrospective study. Int. J. Colorectal Dis. 2019, 34, 481–490. [Google Scholar] [CrossRef]
- Antonelli, G.; Vanella, G.; Orlando, D.; Angeletti, S.; Di Giulio, E. Recurrence and cancer-specific mortality after endoscopic resection of low- and high-risk pT1 colorectal cancers: A meta-analysis. Gastrointest. Endosc. 2019, 90, 559–569. [Google Scholar] [CrossRef]
- Choi, P.W.; Yu, C.S.; Jang, S.J.; Jung, S.H.; Kim, H.C.; Kim, J.C. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J. Surg. 2008, 32, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Rickert, A.; Aliyev, R.; Belle, S.; Post, S.; Kienle, P.; Kähler, G. Oncologic colorectal resection after endoscopic treatment of malignant polyps: Does endoscopy have an adverse effect on oncologic and surgical outcomes? Gastrointest. Endosc. 2014, 79, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.H.; Nielsen, H.; Pedersen, L.; Harling, H.; Laurberg, S. Seasonal variation in short-term mortality after surgery for colorectal cancer? Colorectal Dis. 2010, 12, e31–e36. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.C.; Mitra, N.; Sonnad, S.; Armstrong, K.; Wirtalla, C.; Kelz, R.R.; Mahmoud, N.N. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann. Surg. 2008, 248, 675–686. [Google Scholar] [CrossRef]
- Hassan, C.; Zullo, A.; Risio, M.; Rossini, F.P.; Morini, S. Histologic risk factors and clinical outcome in colorectal malignant polyp: A pooled-data analysis. Dis. Colon Rectum 2005, 48, 1588–1596. [Google Scholar] [CrossRef]
- Akatsu, T.; Aiura, K.; Shimazu, M.; Ueda, M.; Wakabayashi, G.; Tanabe, M.; Kawachi, S.; Hayashida, T.; Kameyama, K.; Sakamoto, M.; et al. Endoscopic ultrasonography of nonfunctioning pancreatic islet cell tumors with histologic correlation. Hepatogastroenterology 2004, 51, 1590–1594. [Google Scholar]
- Nakadoi, K.; Tanaka, S.; Kanao, H.; Terasaki, M.; Takata, S.; Oka, S.; Yoshida, S.; Arihiro, K.; Chayama, K. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J. Gastroenterol. Hepatol. 2012, 27, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.L.; Teerenstra, S.; de Wilt, J.H.; Cunningham, C.; Nagtegaal, I.D. Predicting lymph node metastasis in pT1 colorectal cancer: A systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013, 45, 827–834. [Google Scholar] [CrossRef]
- Mou, S.; Soetikno, R.; Shimoda, T.; Rouse, R.; Kaltenbach, T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: A systematic review and meta-analysis. Surg. Endosc. 2013, 27, 2692–2703. [Google Scholar] [CrossRef]
- Miyachi, H.; Kudo, S.E.; Ichimasa, K.; Hisayuki, T.; Oikawa, H.; Matsudaira, S.; Kouyama, Y.; Kimura, Y.J.; Misawa, M.; Mori, Y.; et al. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J. Gastroenterol. Hepatol. 2016, 31, 1126–1132. [Google Scholar] [CrossRef]
- Kim, B.; Kim, E.H.; Park, S.J.; Cheon, J.H.; Kim, T.I.; Kim, W.H.; Kim, H.; Hong, S.P. The risk of lymph node metastasis makes it unsafe to expand the conventional indications for endoscopic treatment of T1 colorectal cancer: A retrospective study of 428 patients. Medicine 2016, 95, e4373. [Google Scholar] [CrossRef] [PubMed]
- Butte, J.M.; Tang, P.; Gonen, M.; Shia, J.; Schattner, M.; Nash, G.M.; Temple, L.K.; Weiser, M.R. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis. Colon Rectum 2012, 55, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Han, K.S.; Hyun, J.H.; Lee, S.J.; Kim, B.; Hong, C.W.; Kim, B.C.; Sohn, D.K.; Chang, H.J.; Kim, M.J.; et al. Risk of recurrence after endoscopic resection of early colorectal cancer with positive margins. Endoscopy 2018, 50, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Bouvier, A.M.; Jooste, V.; Cottet, V.; Romain, G.; Faivre, J.; Manfredi, S.; Lepage, C. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut 2019, 68, 111–117. [Google Scholar] [CrossRef]
- Choi, D.H.; Sohn, D.K.; Chang, H.J.; Lim, S.B.; Choi, H.S.; Jeong, S.Y. Indications for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinomas: A prospective cohort study. Dis. Colon Rectum 2009, 52, 438–445. [Google Scholar] [CrossRef]

| Variable | C-ER, N (%) (Overall N = 49) | NC-ER, N (%) (Overall, N = 171) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 64.5 ± 8.8 | 63.9 ± 10.0 | 0.688 |
| Sex, n (%) | >0.999 | ||
| Male | 34 (69.4) | 120 (70.2) | |
| Female | 15 (30.6) | 51 (29.8) | |
| Location, n (%) | 0.175 | ||
| Colon | 47 (95.9) | 151 (88.3) | |
| Rectum | 2 (4.1) | 20 (11.7) | |
| Size, mm, mean ± SD | 13.2 ± 6.4 | 15.1 ± 7.1 | 0.085 |
| Macroscopic type, n (%) | 0.190 | ||
| Sessile | 17 (34.7) | 87 (50.9) | |
| Flat | 9 (18.4) | 24 (14.0) | |
| LST-G | 6 (12.2) | 12 (7.0) | |
| LST-NG | 17 (34.7) | 48 (28.1) | |
| Resection method, n (%) | 0.195 | ||
| ESD | 39 (79.6) | 152 (88.9) | |
| EMR | 10 (20.4) | 19 (11.1) | |
| Adverse events | 0.444 | ||
| Acute bleeding, n (%) | 1 (2.1) | 11 (6.6) | |
| Delayed bleeding, n (%) | 0 (0.0) | 2 (1.2) | |
| Perforation, n (%) | 1 (2.0) | 3 (1.8) | |
| Pathology | |||
| Well or moderately differentiated | 49 (100) | 171 (100) | |
| Poorly differentiated/mucinous/signet ring cell carcinoma | 0 (0.0) | 0 (0.0) | |
| Submucosal invasion depth | 599.7 ± 273.4 | 2261.3 ± 1270.9 | 0.001 |
| Lymphovascular invasion | 0 (0) | 11 (6.4) | 0.009 |
| Margin positivity | 0 (0) | 54 (31.6) | <0.001 |
| Recurrence | 0 (0) | 11 (6.4) | 0.129 |
| Death | 0 (0) | 14 (8.2) | 0.043 |
| Cancer-related death | 0 (0) | 1 (0.6) | >0.999 |
| Recurrence N (%) (Overall N = 8) | No recurrence N (%) (Overall N =46) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 72.5 ± 8.9 | 65.2 ± 11.0 | 0.062 |
| Sex, n (%) | >0.999 | ||
| Male | 6 (75.0) | 35 (76.1) | |
| Female | 2 (25.0) | 11 (23.9) | |
| Location, n (%) | 0.577 | ||
| Colon | 8 (100) | 39 (84.8) | |
| Rectum | 0 (0) | 7 (15.2) | |
| Size (mm), mean ± SD | 19.3 ± 9.3 | 15.0 ± 7.4 | 0.253 |
| Macroscopic type, n (%) | 0.896 | ||
| Sessile | 4 (50.0) | 17 (37.0) | |
| Flat | 1 (12.5) | 8 (17.4) | |
| LST-G | 0 (0) | 6 (13.0) | |
| LST-NG | 3 (37.5) | 15 (32.6) | |
| Resection method, n (%) | 0.588 | ||
| ESD | 6 (75.0) | 40 (87.0) | |
| EMR | 2 (25.0) | 6 (13.0) | |
| Pathology | |||
| Well and moderately differentiated | 8 (100) | 46 (100) | |
| Submucosal invasion depth | 1977 ± 1443 | 1481 ± 770 | 0.372 |
| Lymphovascular invasion | >0.999 | ||
| Positive | 0 (0) | 5 (10.6) | |
| Negative | 8 (100) | 41 (89.4) | |
| Margin | 0.275 | ||
| Positive | 2 (28.6) | 7 (14.9) | |
| Negative | 6 (71.4) | 39 (85.1) |
| No. of Patients | No. of Events | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Age (years) | ||||||||
| ≥65 | 36 | 7 | 3.328 | 0.386–28.726 | 0.245 | |||
| <65 | 18 | 1 | 1 | |||||
| Sex | ||||||||
| Male | 41 | 6 | 1 | |||||
| Female | 13 | 2 | 1.279 | 0.232–7.037 | 0.777 | |||
| Size (mm) | ||||||||
| ≥15 | 28 | 5 | 0.832 | 0.167–4.139 | ||||
| <15 | 26 | 3 | 1 | 0.822 | ||||
| Submucosal invasion depth | ||||||||
| ≥2500 | 10 | 3 | 5.383 | 1.079–26.858 | 0.040 | 7.298 | 1.253–42.500 | 0.027 |
| <2500 | 44 | 5 | ||||||
| Lymphovascular invasion | 0.999 | |||||||
| Positive | 5 | 0 | 1 | |||||
| Negative | 49 | 8 | 0 | 0.000–Inf | ||||
| Margin | 0.055 | 0.046 | ||||||
| Positive | 7 | 2 | 5.390 | 0.965–30.096 | 7.189 | 1.033–50.029 | ||
| Negative | 47 | 6 | 1 | 1 | ||||
| Patient | Sex/Age (years) | Location | Size, mm | Resection Method | Resection Type | Initial Histology | Submucosal Invasion Depth | Lympho-Vascular Invasion | Margin Status at ER | Operation | Time to Recurrence, Month | Recurrence Type | Recurrence Treatment | Death | Death Cause |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/73 | RC | 25 | ESD | En bloc | Mode-diff | 1450 | – | – | Yes | 9.1 | Distant meta(liver meta) | Chemotherapy | Yes | Cerebrovascular disease |
| 2 | Male/72 | Rectum | 35 | ESD | Piecemeal | Well-diff | 2750 | – | VM | No | 4.1 | Local recurrence | Op. refuse | Yes | Pneumonia |
| 3 | Male/65 | LC | 15 | ESD | En bloc | Mode-diff | 3000 | – | VM | Yes | 9.1 | Distant meta(lung and bone meta) | Chemotherapy | Yes | Colon cancer |
| 4 | Male/71 | RC | 30 | ESD | Piecemeal | Well-diff | 440 | – | VM | No | 25.9 | Local recurrence | Endoscopic resection | No | |
| 5 | Male/66 | LC | 25 | EMR | Piecemeal | Mode-diff | 1000 | – | VM | Yes | 10.8 | Local recurrence | Operation | No | |
| 6 | Female/58 | LC | 25 | ESD | En bloc | Well-diff | 1250 | – | – | No | 37.8 | Local recurrence | Operation | No | |
| 7 | Male/66 | LC | 25 | ESD | En bloc | Mode-diff | 1475 | – | – | No | 82.2 | Distant meta(liver meta) | Chemotherapy | No | |
| 8 | Male/75 | LC | 20 | EMR | En bloc | Mode-diff | 2250 | – | – | No | 81.9 | Local recurrence | Op. refuse | No | |
| 9 | Female/81 | LC | 14 | EMR | En bloc | Mode-diff | 2500 | – | – | No | 24.8 | Local recurrence | Operation | No | |
| 10 | Male/87 | LC | 15 | ESD | En bloc | Mode-diff | 4150 | – | – | No | 12.0 | Local recurrence | Operation | No | |
| 11 | Male/70 | LC | 10 | EMR | En bloc | Mode-diff | 1000 | – | – | No | 15.0 | Local recurrence | Operation | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.Y.; Baek, D.H.; Lee, M.W.; Kim, G.H.; Park, D.Y.; Song, G.A. Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection. J. Clin. Med. 2020, 9, 2451. https://doi.org/10.3390/jcm9082451
Park EY, Baek DH, Lee MW, Kim GH, Park DY, Song GA. Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection. Journal of Clinical Medicine. 2020; 9(8):2451. https://doi.org/10.3390/jcm9082451
Chicago/Turabian StylePark, Eun Young, Dong Hoon Baek, Moon Won Lee, Gwang Ha Kim, Do Youn Park, and Geun Am Song. 2020. "Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection" Journal of Clinical Medicine 9, no. 8: 2451. https://doi.org/10.3390/jcm9082451
APA StylePark, E. Y., Baek, D. H., Lee, M. W., Kim, G. H., Park, D. Y., & Song, G. A. (2020). Long-Term Outcomes of T1 Colorectal Cancer after Endoscopic Resection. Journal of Clinical Medicine, 9(8), 2451. https://doi.org/10.3390/jcm9082451






