Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| BI-RADS | Breast Imaging Reporting and Data System |
| BP | Blood pressure |
| CIs | Confidence intervals |
| CVD | Cardiovascular disease |
| HEPA | Health-enhancing physical activity |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| hsCRP | High sensitive C-reactive protein |
| LDL-C | Low-density lipoprotein cholesterol |
| PR | Prevalence ratio |
Appendix A
| Menopausal Stages | p for Trend | ||||
|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | ||
| Never-smokers | |||||
| No. | 44,443 | 13,177 | 5997 | 13,742 | |
| Cases of dense breasts (%) | 47.7 | 45.1 | 34.4 | 13.3 | |
| Age-adjusted PR (95% CI) | reference | 0.96 (0.94–0.99) | 0.84 (0.81–0.87) | 0.48 (0.45–0.51) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.96 (0.94–0.97) | 0.89 (0.86–0.92) | 0.48 (0.45–0.50) | <0.001 |
| Former smokers | |||||
| No. | 1201 | 402 | 146 | 187 | |
| Cases of dense breasts (%) | 42.3 | 41.8 | 28.8 | 17.1 | |
| Age-adjusted PR (95% CI) | reference | 0.99 (0.87–1.13) | 0.73 (0.56–0.95) | 0.62 (0.41–0.93) | 0.005 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.93 (0.83–1.05) | 0.83 (0.65–1.06) | 0.59 (0.42–0.85) | 0.002 |
| Current smokers | |||||
| No. | 838 | 303 | 120 | 270 | |
| Cases of dense breasts (%) | 43.4 | 47.5 | 40.0 | 15.9 | |
| Age-adjusted PR (95% CI) | reference | 1.11 (0.96–1.28) | 1.03 (0.82–1.30) | 0.68 (0.48–0.96) | 0.25 |
| Multivariable-adjusted PR (95% CI) a | reference | 1.07 (0.94–1.22) | 1.09 (0.88–1.36) | 0.74 (0.54–1.01) | 0.158 |
References
- Boyd, N.; Martin, L.; Gunasekara, A.; Melnichouk, O.; Maudsley, G.; Peressotti, C.; Yaffe, M.; Minkin, S. Mammographic density and breast cancer risk: Evaluation of a novel method of measuring breast tissue volumes. Cancer Epidemiol. Prev. Biomark. 2009, 18, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Brandt, K.R.; Ghosh, K.; Scott, C.G.; Maloney, S.D.; Carston, M.J.; Pankratz, V.S.; Sellers, T.A. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol. Prev. Biomark. 2007, 16, 43–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freer, P.E. Mammographic breast density: Impact on breast cancer risk and implications for screening. Radiographics 2015, 35, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.; Martin, L.; Stone, J.; Little, L.; Minkin, S.; Yaffe, M. A longitudinal study of the effects of menopause on mammographic features. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1048–1053. [Google Scholar]
- Martin, L.J.; Boyd, N.F. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: Hypotheses based on epidemiological evidence. Breast Cancer Res. 2008, 10, 201. [Google Scholar] [CrossRef]
- Boyd, N.F.; Lockwood, G.A.; Martin, L.J.; Knight, J.A.; Byng, J.W.; Yaffe, M.J.; Tritchler, D.L. Mammographic densities and breast cancer risk. Breast Dis. 1998, 10, 113–126. [Google Scholar] [CrossRef]
- Vachon, C.M.; Sellers, T.A.; Vierkant, R.A.; Wu, F.F.; Brandt, K.R. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol. Prev. Biomark. 2002, 11, 1382–1388. [Google Scholar]
- Lundstrom, E.; Christow, A.; Kersemaekers, W.; Svane, G.; Azavedo, E.; Soderqvist, G.; Mol-Arts, M.; Barkfeldt, J.; von Schoultz, B. Effects of tibolone and continuous combined hormone replacement therapy on mammographic breast density. Am. J. Obstet. Gynecol. 2002, 186, 717–722. [Google Scholar] [CrossRef]
- Persson, I.; Thurfjell, E.; Holmberg, L. Effect of estrogen and estrogen-progestin replacement regimens on mammographic breast parenchymal density. J. Clin. Oncol. 1997, 15, 3201–3207. [Google Scholar] [CrossRef]
- Cuzick, J.; Warwick, J.; Pinney, E.; Warren, R.M.; Duffy, S.W. Tamoxifen and breast density in women at increased risk of breast cancer. J. Natl. Cancer Inst. 2004, 96, 621–628. [Google Scholar] [CrossRef]
- Checka, C.M.; Chun, J.E.; Schnabel, F.R.; Lee, J.; Toth, H. The relationship of mammographic density and age: Implications for breast cancer screening. AJR Am. J. Roentgenol. 2012, 198, W292–W295. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid changes during the menopause transition in relation to age and weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Kozakowski, J.; Gietka-Czernel, M.; Leszczynska, D.; Majos, A. Obesity in menopause—Our negligence or an unfortunate inevitability? Prz. Menopauzalny 2017, 16, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Kuni, C.C.; Anderson, K.; Anderson, V.E.; Sellers, T.A. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 2000, 11, 653–662. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose-response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar] [CrossRef]
- Premenopausal Breast Cancer Collaborative, G.; Schoemaker, M.J.; Nichols, H.B.; Wright, L.B.; Brook, M.N.; Jones, M.E.; O'Brien, K.M.; Adami, H.O.; Baglietto, L.; Bernstein, L.; et al. Association of Body Mass Index and Age With Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018, 4, e181771. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S.; Choi, Y.; Zhang, Y.; Cho, J.; Kwon, M.J.; Hyun, Y.Y.; Lee, K.B.; Kim, H.; Jung, H.S.; et al. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann. Intern. Med. 2016, 164, 305–312. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Malavolti, M.; Mussi, C.; Poli, M.; Fantuzzi, A.L.; Salvioli, G.; Battistini, N.; Bedogni, G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann. Hum. Biol. 2003, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Lee, S.; Park, H.S.; Kim, S.M.; Kwon, H.S.; Kim, D.Y.; Kim, D.J.; Cho, G.J.; Han, J.H.; Kim, S.R.; Park, C.Y.; et al. Cut-off points of waist circumference for defining abdominal obesity in the Korean population. Korean J. Obes. 2006, 15, 9. [Google Scholar]
- American College of Radiology; BI-RADS Committee. ACR BI-RADS® Atlas: Breast Imaging Reporting and Data System, 5th ed.; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Kang, E.Y.; Shin, J.H.; Kang, S.G.; Hwang, Y.N.; Cha, E.S.; Song, S.W. Relationship between Mammographic Dense Breast and Other Risk Factors of Breast Cancer in Korean Women. J. Korean Acad. Fam. Med. 2007, 28, 937–942. [Google Scholar]
- Mathiesen, U.L.; Franzen, L.E.; Aselius, H.; Resjo, M.; Jacobsson, L.; Foberg, U.; Fryden, A.; Bodemar, G. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig. Liver Dis. 2002, 34, 516–522. [Google Scholar] [CrossRef]
- Boyd, N.F.; Lockwood, G.A.; Byng, J.W.; Little, L.E.; Yaffe, M.J.; Tritchler, D.L. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. Br. J. Cancer 1998, 78, 1233–1238. [Google Scholar] [CrossRef]
- Burton, A.; Maskarinec, G.; Perez-Gomez, B.; Vachon, C.; Miao, H.; Lajous, M.; Lopez-Ridaura, R.; Rice, M.; Pereira, A.; Garmendia, M.L.; et al. Mammographic density and ageing: A collaborative pooled analysis of cross-sectional data from 22 countries worldwide. PLoS Med. 2017, 14, e1002335. [Google Scholar] [CrossRef]
- Su, H.I.; Freeman, E.W. Hormone changes associated with the menopausal transition. Minerva Ginecol. 2009, 61, 483–489. [Google Scholar]
- Sowers, M.R.; Zheng, H.; McConnell, D.; Nan, B.; Harlow, S.D.; Randolph, J.F., Jr. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Gold, E.B.; Conroy, S.M.; Crandall, C.J.; Greendale, G.A.; Oestreicher, N.; Quesenberry, C.P., Jr.; Habel, L.A. Active, but not passive cigarette smoking was inversely associated with mammographic density. Cancer Causes Control. 2010, 21, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tanko, L.B.; Christiansen, C. An update on the antiestrogenic effect of smoking: A literature review with implications for researchers and practitioners. Menopause 2004, 11, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, K.K.; Lynge, E.; Vejborg, I.; Tjonneland, A.; von Euler-Chelpin, M.; Andersen, Z.J. Cigarette smoking and mammographic density in the Danish Diet, Cancer and Health cohort. Cancer Causes Control 2016, 27, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, G.; Pagano, I.; Lurie, G.; Kolonel, L.N. A longitudinal investigation of mammographic density: The multiethnic cohort. Cancer Epidemiol. Prev. Biomark. 2006, 15, 732–739. [Google Scholar] [CrossRef]
- Engmann, N.J.; Scott, C.; Jensen, M.R.; Winham, S.J.; Ma, L.; Brandt, K.R.; Mahmoudzadeh, A. Longitudinal Changes in Volumetric Breast Density in Healthy Women across the Menopausal Transition. Cancer Epidemiol. Prev. Biomark. 2019, 28, 1324–1330. [Google Scholar] [CrossRef]
- Hart, V.; Reeves, K.W.; Sturgeon, S.R.; Reich, N.G.; Sievert, L.L.; Kerlikowske, K.; Ma, L.; Shepherd, J.; Tice, J.A.; Mahmoudzadeh, A.P.; et al. The effect of change in body mass index on volumetric measures of mammographic density. Cancer Epidemiol. Prev. Biomark. 2015, 24, 1724–1730. [Google Scholar] [CrossRef]
- Shieh, Y.; Scott, C.G.; Jensen, M.R.; Norman, A.D.; Bertrand, K.A.; Pankratz, V.S.; Brandt, K.R.; Visscher, D.W.; Shepherd, J.A.; Tamimi, R.M.; et al. Body mass index, mammographic density, and breast cancer risk by estrogen receptor subtype. Breast Cancer Res. 2019, 21, 48. [Google Scholar] [CrossRef]
- Hudson, S.; Vik Hjerkind, K.; Vinnicombe, S.; Allen, S.; Trewin, C.; Ursin, G.; Dos-Santos-Silva, I.; De Stavola, B.L. Adjusting for BMI in analyses of volumetric mammographic density and breast cancer risk. Breast Cancer Res. 2018, 20, 156. [Google Scholar] [CrossRef]
- Baglietto, L.; Krishnan, K.; Stone, J.; Apicella, C.; Southey, M.C.; English, D.R.; Hopper, J.L.; Giles, G.G. Associations of mammographic dense and nondense areas and body mass index with risk of breast cancer. Am. J. Epidemiol. 2014, 179, 475–483. [Google Scholar] [CrossRef]
- Soguel, L.; Durocher, F.; Tchernof, A.; Diorio, C. Adiposity, breast density, and breast cancer risk: Epidemiological and biological considerations. Eur. J. Cancer Prev. 2017, 26, 511–520. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lobie, P.E. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes. Rev. 2000, 1, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Boyd, N.F.; Martin, L.J.; Bronskill, M.; Yaffe, M.J.; Duric, N.; Minkin, S. Breast tissue composition and susceptibility to breast cancer. J. Natl. Cancer Inst. 2010, 102, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Sartor, H.; Lang, K.; Rosso, A.; Borgquist, S.; Zackrisson, S.; Timberg, P. Measuring mammographic density: Comparing a fully automated volumetric assessment versus European radiologists’ qualitative classification. Eur. Radiol. 2016, 26, 4354–4360. [Google Scholar] [CrossRef]
| Characteristic | Dense Breasts | p-Value | |
|---|---|---|---|
| Absent | Present | ||
| Number | 50,080 | 32,597 | |
| Age (years) a | 45.8 (8.0) | 41.6 (5.0) | <0.001 |
| Height (m) a | 159.6 (5.3) | 160.7 (5.0) | <0.001 |
| BMI (kg/m2) a | 23.2 (3.2) | 20.9 (2.4) | <0.001 |
| BMI category (kg/m2) | <0.001 | ||
| <18.5 (underweight) | 3.0 | 13.0 | |
| 18.5–22.9 (normal weight) | 51.0 | 70.2 | |
| 23–24.9 (overweight) | 21.9 | 10.8 | |
| ≥25 (obesity) | 24.1 | 6.1 | |
| Body-fat percentage (n = 82,633) | 32.5 (5.7) | 28.0 (5.4) | <0.001 |
| Waist circumference (cm) (n = 82,659) | 78.7 (8.3) | 72.5 (6.5) | <0.001 |
| Fatty liver on ultrasound (%) (n = 82,267) | 23.8 | 5.9 | <0.001 |
| Smoking status (n = 80,826) | 0.369 | ||
| Never smokers, (%) | 95.6 | 95.8 | |
| Former smokers, (%) | 2.5 | 2.3 | |
| Current smokers, (%) | 1.9 | 1.9 | |
| Alcohol intake ≥10 g/day, (%) c (n = 77,407) | 12.3 | 12.2 | 0.796 |
| HEPA, (%) (n = 82,441) | 14.9 | 12.1 | <0.001 |
| Higher education, (%) d (n = 80,664) | 70.8 | 82.2 | <0.001 |
| Hypertension, (%) (n = 82,671) | 10.3 | 3.0 | <0.001 |
| Diabetes mellitus, (%) (n = 82,670) | 4.6 | 1.1 | <0.001 |
| Medication for hyperlipidemia, (%) | 5.1 | 1.0 | <0.001 |
| Family history of breast cancer (%) | 3.3 | 3.3 | 0.661 |
| Early menarche (%) e (n = 82,274) | 4.5 | 4.3 | 0.049 |
| Parity number (%) (n = 79,130) | <0.001 | ||
| 0 | 7.8 | 13.8 | |
| 1–2 | 78.1 | 79.7 | |
| ≥3 | 14.1 | 6.6 | |
| Female hormone medication (%) | 1.6 | 1.7 | 0.382 |
| Systolic BP (mmHg) a (n = 82,671) | 107.2 (12.8) | 102.1 (10.3) | <0.001 |
| Diastolic BP (mmHg) a (n = 82,671) | 68.1 (9.3) | 65.3 (8.1) | <0.001 |
| Glucose (mg/dL) a | 95.0 (15.1) | 91.4 (9.8) | <0.001 |
| Total cholesterol (mg/dL) a | 194.7 (34.7) | 186.2 (30.9) | <0.001 |
| LDL-C (mg/dL) a (n = 82,504) | 125.4 (33.4) | 113.9 (28.6) | <0.001 |
| HDL-C (mg/dL) a | 64.8 (16.0) | 70.0 (15.6) | <0.001 |
| Triglycerides (mg/dL) b | 81 (60–113) | 69 (54–91) | <0.001 |
| hsCRP (mg/L) b (n = 57,460) | 0.4 (0.3–0.8) | 0.3 (0.2–0.5) | <0.001 |
| HOMA-IR b (n = 81,649) | 1.32 (0.88–1.98) | 1.11 (0.76–1.58) | <0.001 |
| Total calorie intake (kcal/day) b (n = 47,947) | 1131 (812–1496) | 1092 (789–1444) | <0.001 |
| Menopausal Stages | p for Trend | ||||
|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | ||
| Overall population | |||||
| No. | 46,532 | 13,896 | 6287 | 15,962 | |
| Cases of dense breasts (%) | 47.5 | 45.0 | 34.4 | 13.1 | |
| Age-adjusted PR (95% CI) | reference | 0.97 (0.95–0.99) | 0.84 (0.81–0.87) | 0.48 (0.46–0.51) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.96 (0.94–0.98) | 0.89 (0.86–0.92) | 0.48 (0.46–0.51) | <0.001 |
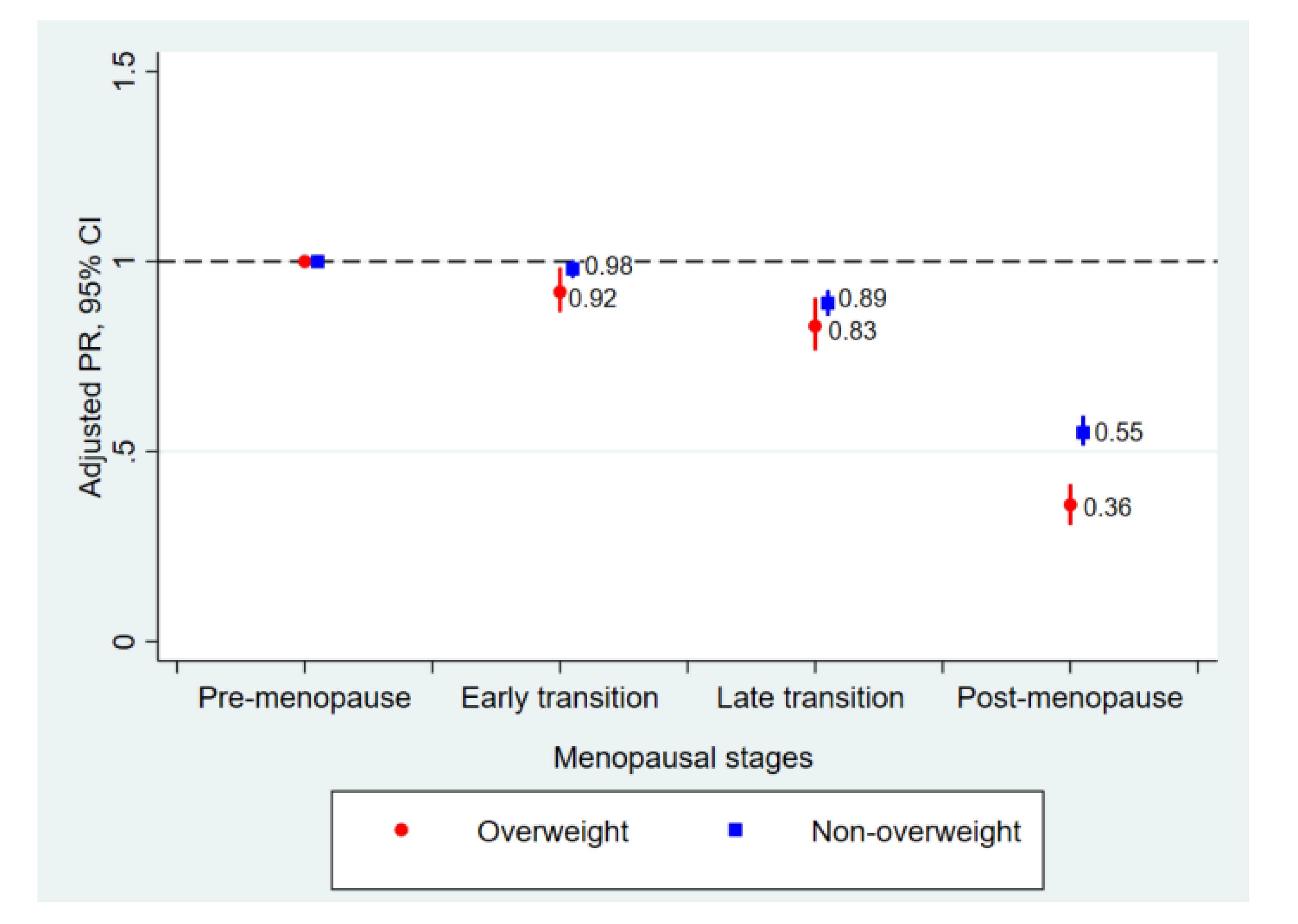
| Non-overweight (BMI < 23 kg/m2) | |||||
| No. | 32,772 | 9660 | 3690 | 8005 | |
| Cases of dense breasts (%) | 56.6 | 54.7 | 45.0 | 20.1 | |
| Age-adjusted PR (95% CI) | reference | 0.98 (0.96–1.00) | 0.89 (0.86–0.92) | 0.54 (0.51–0.57) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.98 (0.96–1.00) | 0.89 (0.86–0.92) | 0.55 (0.52–0.59) | <0.001 |
| Overweight (BMI ≥ 23 kg/m2) | |||||
| No. | 13,760 | 4236 | 2597 | 7957 | |
| Cases of dense breasts (%) | 25.8 | 23.0 | 19.3 | 6.1 | |
| Age-adjusted PR (95% CI) | reference | 0.90 (0.85–0.96) | 0.83 (0.76–0.90) | 0.35 (0.30–0.40) | <0.001 |
| Multivariable-adjusted PR (95% CI) a | reference | 0.92 (0.87–0.98) | 0.83 (0.77–0.90) | 0.36 (0.31–0.41) | <0.001 |
| Subgroup | Menopausal Stages | p for Trend | p for Interaction | |||
|---|---|---|---|---|---|---|
| Pre-Menopause | Early Transition | Late Transition | Post-Menopause | |||
| Waist circumference | 0.001 | |||||
| <85 cm (n = 70,752) | reference | 0.98 (0.96–1.00) | 0.88 (0.85–0.91) | 0.53 (0.51–0.56) | <0.001 | |
| ≥85 cm (n = 11,907) | reference | 0.84 (0.74–0.95) | 0.84 (0.72–0.97) | 0.31 (0.24–0.41) | <0.001 | |
| Body-fat percentage | <0.001 | |||||
| <30% (n = 37,730) | reference | 0.97 (0.95–0.99) | 0.94 (0.90–0.98) | 0.63 (0.59–0.67) | <0.001 | |
| ≥30% (n = 44,903) | reference | 0.97 (0.93–1.00) | 0.83 (0.79–0.88) | 0.43 (0.40–0.48) | <0.001 | |
| Fatty liver on ultrasound | <0.001 | |||||
| No (n = 68,481) | reference | 0.97 (0.95–0.98) | 0.90 (0.87–0.93) | 0.52 (0.49–0.54) | <0.001 | |
| Yes (n = 13,786) | reference | 0. 87 (0.78–0.96) | 0.85 (0.75–0.97) | 0.38 (0.31–0.46) | <0.001 | |
| Smoking status | 0.258 | |||||
| Never smokers (n = 79,359) | reference | 0.96 (0.94–0.97) | 0.89 (0.86–0.92) | 0.48 (0.45–0.50) | <0.001 | |
| Former smokers (n = 1936) | reference | 0.93 (0.83–1.05) | 0.83 (0.65–1.06) | 0.59 (0.42–0.85) | 0.002 | |
| Current smokers (n = 1531) | reference | 1.07 (0.94–1.22) | 1.09 (0.88–1.36) | 0.74 (0.54–1.01) | 0.158 | |
| Alcohol intake | 0.347 | |||||
| <10 g /day (n = 67,913) | reference | 0.95 (0.93–0.97) | 0.89 (0.86–0.93) | 0.49 (0.46–0.52) | <0.001 | |
| ≥10 g/day (n = 9494) | reference | 0.98 (0.93–1.03) | 0.85 (0.77–0.94) | 0.50 (0.43–0.58) | <0.001 | |
| HEPA | 0.200 | |||||
| No (n = 71,072) | reference | 0.95 (0.93–0.97) | 0.88 (0.85–0.92) | 0.48 (0.45–0.51) | <0.001 | |
| Yes (n = 11,369) | reference | 1.01 (0.96–1.07) | 0.95 (0.86–1.05) | 0.52 (0.46–0.59) | <0.001 | |
| HOMA-IR | <0.001 | |||||
| <2.5 (n = 72,436) | reference | 0.96 (0.94–0.98) | 0.89 (0.86–0.92) | 0.50 (0.48–0.53) | <0.001 | |
| ≥2.5 (n = 9213) | reference | 0.91 (0.83–0.99) | 0.86 (0.75–0.98) | 0.34 (0.27–0.43) | <0.001 | |
| HsCRP | 0.354 | |||||
| <1.0 mg/L (n = 47,548) | reference | 0.96 (0.94–0.98) | 0.92 (0.88–0.95) | 0.50 (0.47–0.54) | <0.001 | |
| ≥1.0 mg/L (n = 9912) | reference | 1.01 (0.94–1.08) | 0.89 (0.80–1.00) | 0.48 (0.39–0.59) | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.Y.; Chang, Y.; Ahn, J.; Yun, J.-S.; Park, Y.L.; Park, C.H.; Shin, H.; Ryu, S. Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. J. Clin. Med. 2020, 9, 2434. https://doi.org/10.3390/jcm9082434
Kim EY, Chang Y, Ahn J, Yun J-S, Park YL, Park CH, Shin H, Ryu S. Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. Journal of Clinical Medicine. 2020; 9(8):2434. https://doi.org/10.3390/jcm9082434
Chicago/Turabian StyleKim, Eun Young, Yoosoo Chang, Jiin Ahn, Ji-Sup Yun, Yong Lai Park, Chan Heun Park, Hocheol Shin, and Seungho Ryu. 2020. "Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women" Journal of Clinical Medicine 9, no. 8: 2434. https://doi.org/10.3390/jcm9082434
APA StyleKim, E. Y., Chang, Y., Ahn, J., Yun, J.-S., Park, Y. L., Park, C. H., Shin, H., & Ryu, S. (2020). Menopausal Transition, Body Mass Index, and Prevalence of Mammographic Dense Breasts in Middle-Aged Women. Journal of Clinical Medicine, 9(8), 2434. https://doi.org/10.3390/jcm9082434





