A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. Plasmids
2.3. Cells and Transfection
2.4. Western Blot Assay
2.5. Cytotoxicity Assay
2.6. Cell-Based MERS-CoV RdRp Activity Assay
2.7. Calculation of Z-Factor and Z′-Factor
2.8. Statistical Analysis
3. Results
3.1. Generation of the Cell-Based MERS-CoV RdRp Activity Reporter Assay System
3.2. Evaluation of MERS-CoV RdRp Expression and Optimization of the Reporter Assay System
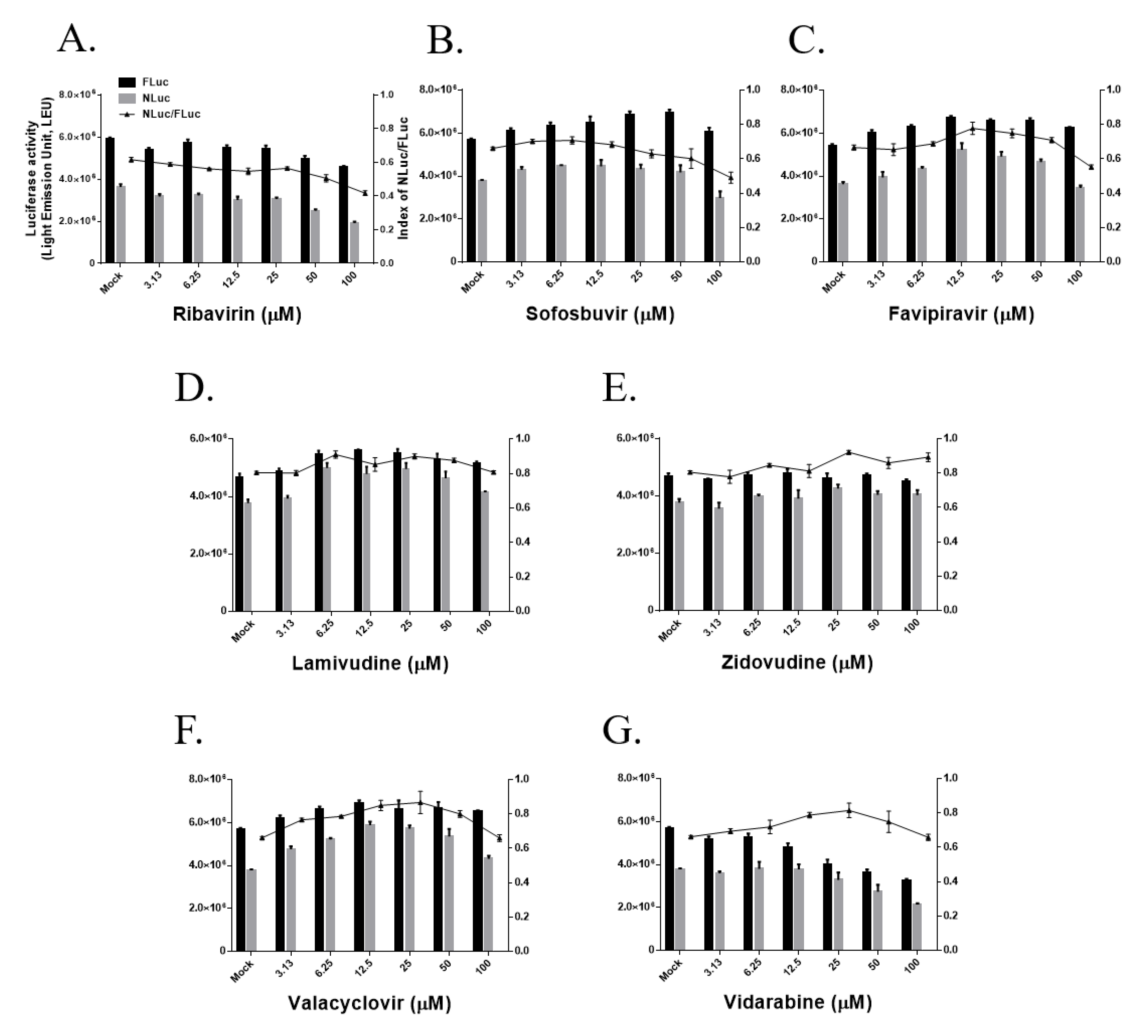
3.3. Effects of Nucleoside/Nucleotide Analogs on MERS-CoV RdRp Activity
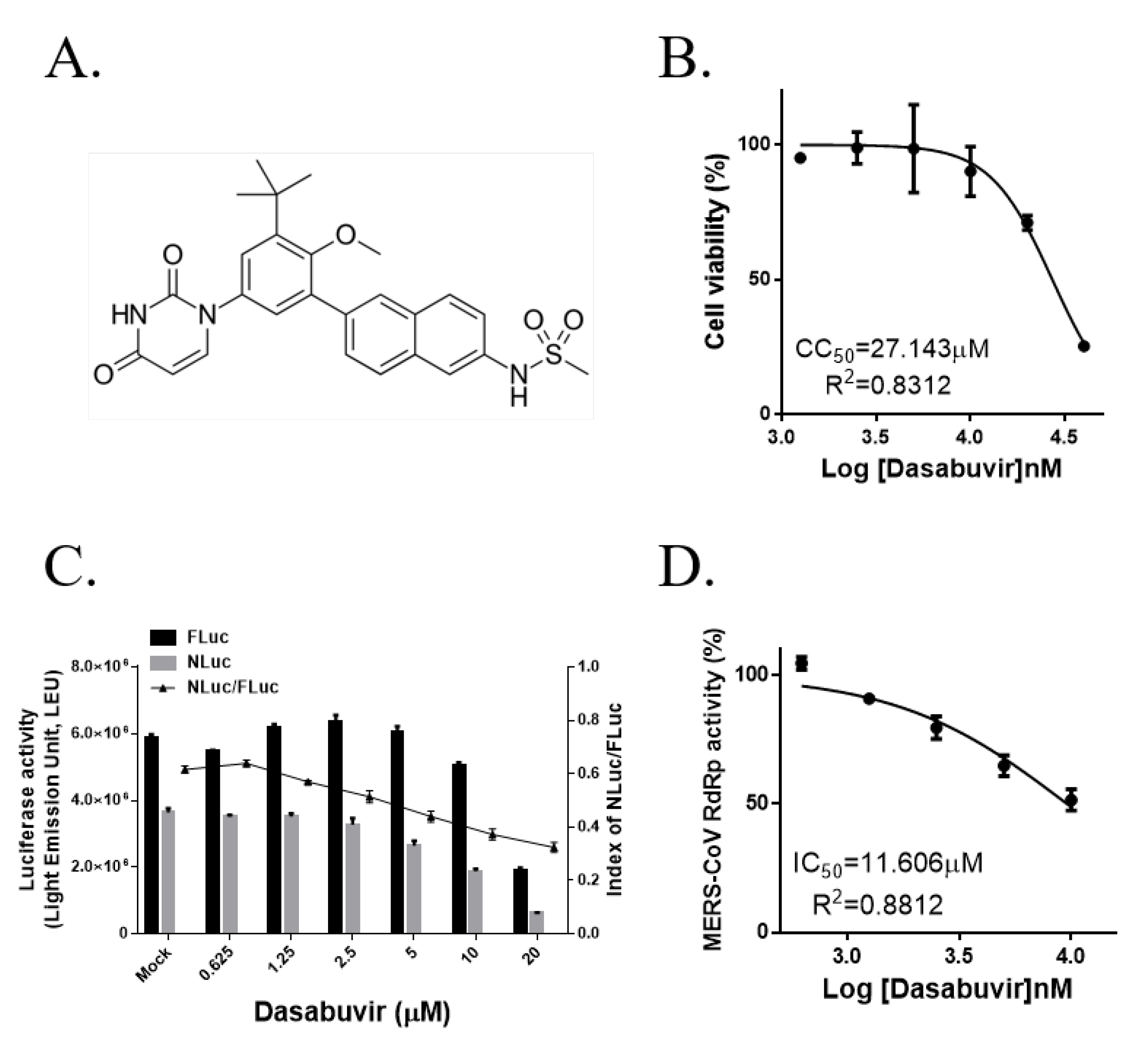
3.4. The Non-Nucleoside HCV RdRp Inhibitor Dasabuvir Partially Inhibits MERS-CoV RdRp Activity
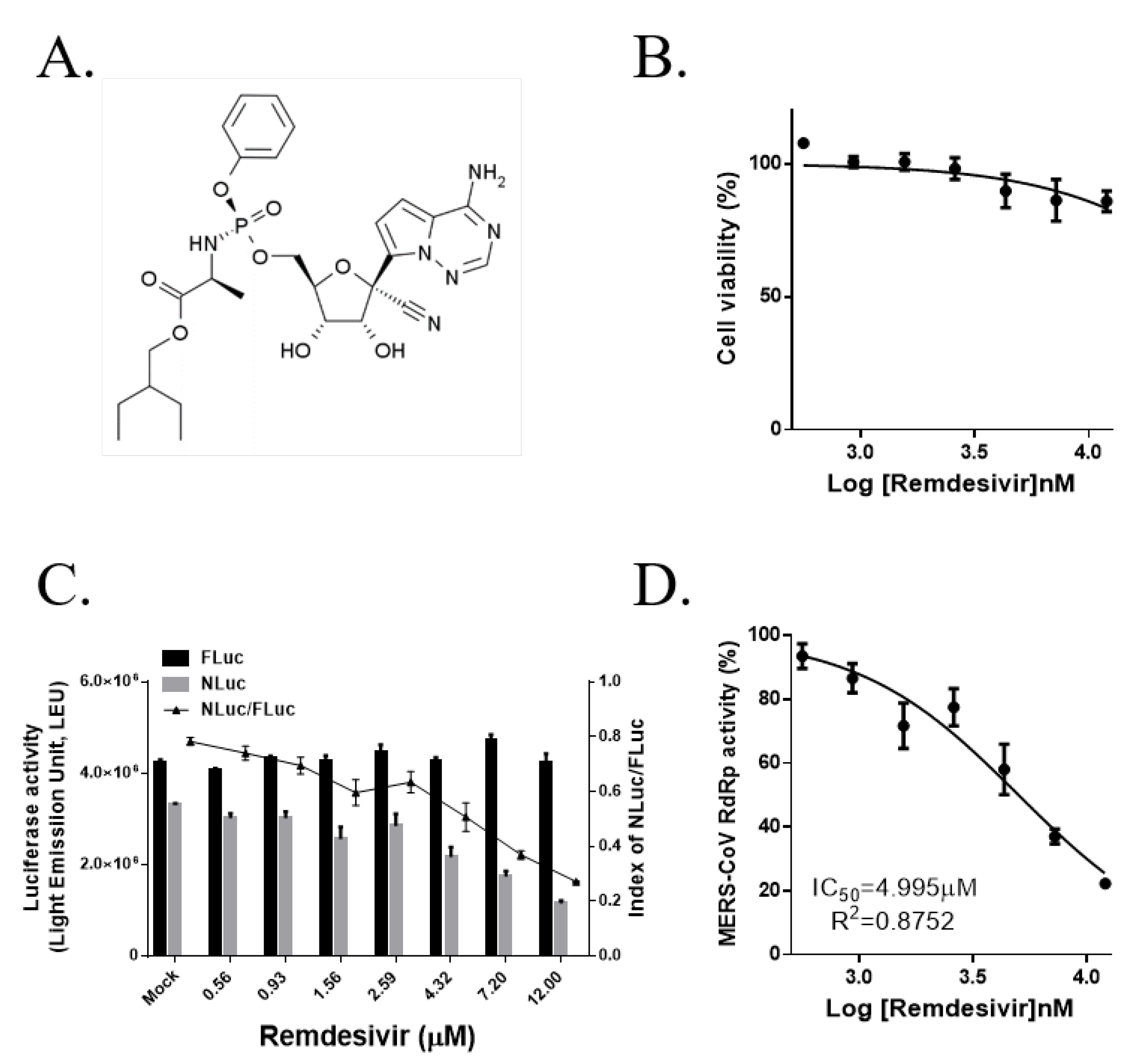
3.5. Remdesivir (GS-5734) Inhibits MERS-CoV RdRp Activity in a Cell-Based Reporter Assay
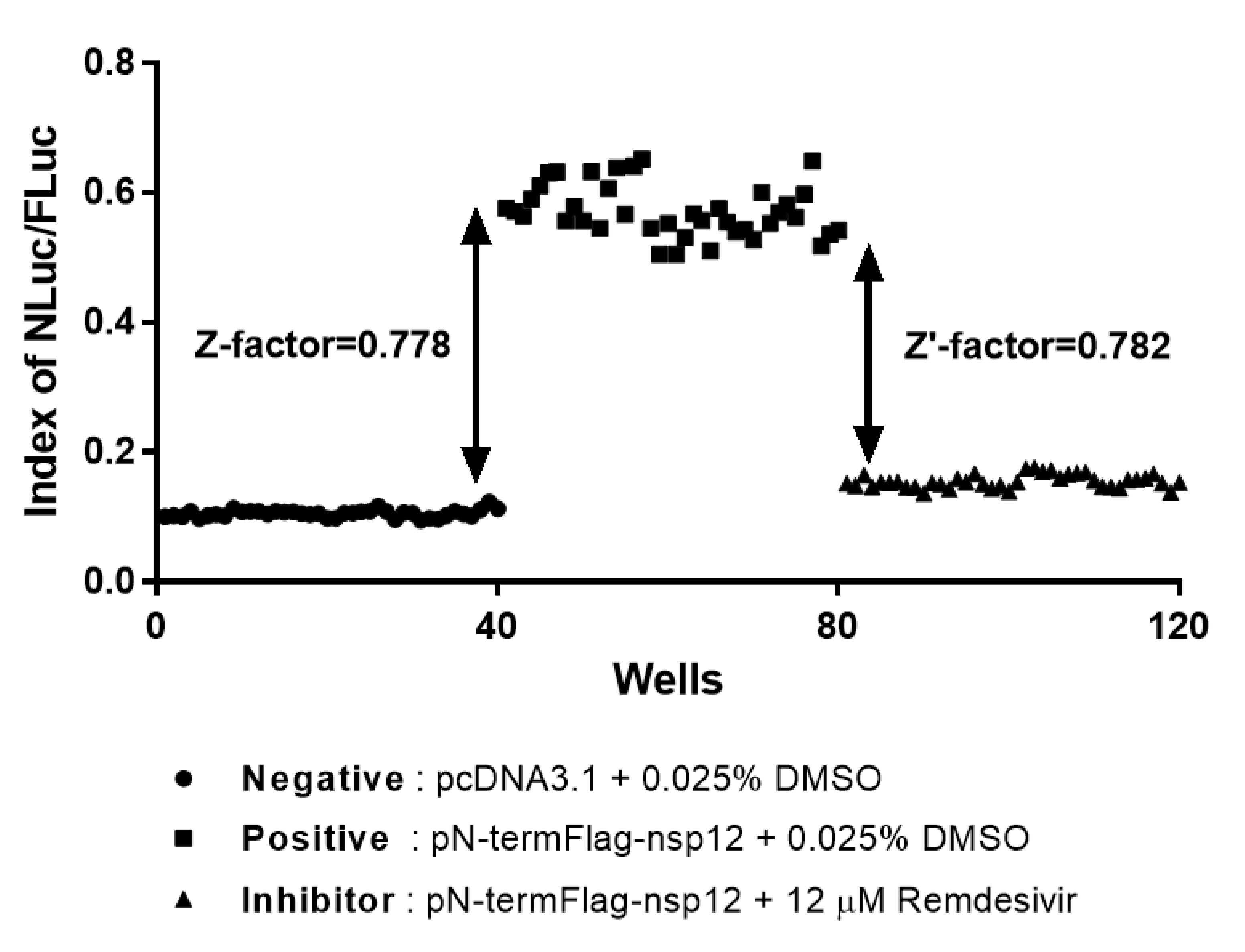
3.6. Reliability and Reproducibility of the Cell-Based MERS-CoV RdRp Activity Reporter Assay System in HTS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3CLpro | 3C-like protease |
| CoV | coronavirus |
| COVID-19 | coronavirus disease 2019 |
| C-term | C-terminal |
| FLuc | firefly luciferase |
| HAE | human airway epithelial |
| HCV | hepatitis C virus |
| HDV | hepatitis delta virus |
| HIV | human immunodeficiency virus |
| HSV | herpes simplex virus |
| HTS | high-throughput screening |
| IRES | internal ribosome entry site |
| MERS | Middle East respiratory syndrome |
| MHV | mouse hepatitis virus |
| NLuc | Nano-glo® luciferase |
| NSP | nonstructural proteins |
| N-term | N-terminal |
| PLpro | papain-like protease |
| RdRp | RNA-dependent RNA polymerase |
| SARI | severe acute respiratory infection |
| SARS | severe acute respiratory syndrome |
| UTR | untranslated region |
References
- Wang, Y.; Sun, J.; Zhu, A.; Zhao, J.; Zhao, J. Current understanding of middle east respiratory syndrome coronavirus infection in human and animal models. J. Thorac. Dis. 2018, 10, S2260–S2271. [Google Scholar] [CrossRef] [PubMed]
- Mehand, M.S.; Al Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Genet. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Durai, P.; Batool, M.; Shah, M.; Choi, S. Middle East respiratory syndrome coronavirus: Transmission, virology and therapeutic targeting to aid in outbreak control. Exp. Mol. Med. 2015, 47, e181. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Wang, L.; Zhang, N.; Deng, X.; Su, M.; Su, Y.; Hu, L.; He, C.; Yu, F.; Jiang, S.; et al. Development of Small-Molecule MERS-CoV Inhibitors. Viruses 2018, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.O.; Singh, K.; Kassim, A.; Coleman, C.; Elliott, R.; Weiss, S.R.; Frieman, M.B.; Sarafianos, S.G. Evaluation of SSYA10-001 as a Replication Inhibitor of Severe Acute Respiratory Syndrome, Mouse Hepatitis, and Middle East Respiratory Syndrome Coronaviruses. Antimicrob. Agents Chemother. 2014, 58, 4894–4898. [Google Scholar] [CrossRef]
- Eltahla, A.A.; Luciani, F.; White, P.; Lloyd, A.R.; Bull, R.A. Inhibitors of the Hepatitis C Virus Polymerase; Mode of Action and Resistance. Viruses 2015, 7, 5206–5224. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Dash, S.; Aydin, Y.; Stephens, C.M. Hepatitis C Virus NS5B RNA-Dependent RNA Polymerase Inhibitor. In Viral Polymerases; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 211–235. [Google Scholar]
- Lau, J.Y.N.; Tam, R.C.; Liang, T.J.; Hong, Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology 2002, 35, 1002–1009. [Google Scholar] [CrossRef]
- Fung, A.; Jin, Z.; Dyatkina, N.; Wang, G.; Beigelman, L.; Deval, J. Efficiency of Incorporation and Chain Termination Determines the Inhibition Potency of 2′-Modified Nucleotide Analogs against Hepatitis C Virus Polymerase. Antimicrob. Agents Chemother. 2014, 58, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Gentile, I.; Buonomo, A.R.; Borgia, G. Dasabuvir: A Non-Nucleoside Inhibitor of NS5B for the Treatment of Hepatitis C Virus Infection. Rev. Recent Clin. Trials 2014, 9, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Falzarano, D.; De Wit, E.; Martellaro, C.; Callison, J.; Munster, V.J.; Feldmann, H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013, 3, srep01686. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Tseng, C.-K.; Chen, K.-J.; Huang, K.-J.; Lin, C.-K.; Lin, Y.-T. A cell-based reporter assay for inhibitor screening of hepatitis C virus RNA-dependent RNA polymerase. Anal. Biochem. 2010, 403, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Chung, T.D.Y.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gohara, D.; Ha, C.S.; Kumar, S.; Ghosh, B.; Arnold, J.J.; Wisniewski, T.J.; Cameron, C.E. Production of “Authentic” Poliovirus RNA-Dependent RNA Polymerase (3Dpol) by Ubiquitin–Protease-Mediated Cleavage in Escherichia coli. Protein Expr. Purif. 1999, 17, 128–138. [Google Scholar] [CrossRef]
- Thompson, A.A.; Peersen, O.B. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 2004, 23, 3462–3471. [Google Scholar] [CrossRef]
- Subissi, L.; Posthuma, C.C.; Collet, A.; Zevenhoven-Dobbe, J.C.; Gorbalenya, A.E.; Decroly, E.; Snijder, E.J.; Canard, B.; Imbert, I. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc. Natl. Acad. Sci. USA 2014, 111, E3900–E3909. [Google Scholar] [CrossRef]
- Imbert, I.; Guillemot, J.-C.; Bourhis, J.-M.; Bussetta, C.; Coutard, B.; Egloff, M.-P.; Ferron, F.; E Gorbalenya, A.; Canard, B. A second, non-canonical RNA-dependent RNA polymerase in SARS Coronavirus. EMBO J. 2006, 25, 4933–4942. [Google Scholar] [CrossRef]
- Turner, T.L.; Kopp, B.T.; Paul, G.; Landgrave, L.C.; Hayes, N.; Thompson, R. Respiratory syncytial virus: Current and emerging treatment options. Clin. Outcomes Res. 2014, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- James, J.S. Ribavirin approved for hepatitis C combination treatment. AIDS Treat. News 1998, 7. [Google Scholar]
- Johnson, S.; Henschke, N.; Maayan, N.; Mills, I.; Buckley, B.; Kakourou, A.; Marshall, R. Ribavirin for treating Crimean Congo haemorrhagic fever. Cochrane Database Syst. Rev. 2018, 6, CD012713. [Google Scholar] [CrossRef] [PubMed]
- Stedman, C.A.M. Sofosbuvir, a NS5B polymerase inhibitor in the treatment of hepatitis C: A review of its clinical potential. Ther. Adv. Gastroenterol. 2013, 7, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Baranovich, T.; Wong, S.-S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (Favipiravir) Induces Lethal Mutagenesis in Influenza A H1N1 Viruses In Vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef]
- Anderson, P.; Rower, J.E.; Anderson, P.L. Zidovudine and Lamivudine for HIV Infection. Clin. Med. Rev. Ther. 2010, 2, 115–127. [Google Scholar] [CrossRef]
- Whitley, R.; Alford, C.A.; Hirsch, M.S.; Schooley, R.T.; Luby, J.P.; Aoki, F.Y.; Hanley, D.; Nahmias, A.J.; Soong, S.-J.; The NIAID Collaborative Antiviral Study Group. Vidarabine versus Acyclovir Therapy in Herpes Simplex Encephalitis. N. Engl. J. Med. 1986, 314, 144–149. [Google Scholar] [CrossRef]
- Akaberi, D.; Bergfors, A.; Kjellin, M.; Kameli, N.; Lidemalm, L.; Kolli, B.; Shafer, R.W.; Palanisamy, N.; Lennerstrand, J. Baseline dasabuvir resistance in Hepatitis C virus from the genotypes 1, 2 and 3 and modeling of the NS5B-dasabuvir complex by the in silico approach. Infect. Ecol. Epidemiol. 2018, 8, 1528117. [Google Scholar] [CrossRef]
- Sui, Y.; Wu, Z. Alternative Statistical Parameter for High-Throughput Screening Assay Quality Assessment. J. Biomol. Screen. 2007, 12, 229–234. [Google Scholar] [CrossRef]
- Velthuis, A.J.T.; Arnold, J.J.; Cameron, C.E.; Worm, S.H.E.V.D.; Snijder, E.J. The RNA polymerase activity of SARS-coronavirus nsp12 is primer dependent. Nucleic Acids Res. 2009, 38, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.-G.; Choi, J.-K.; Taylor, D.R.; Oh, J.-W. Biochemical characterization of a recombinant SARS coronavirus nsp12 RNA-dependent RNA polymerase capable of copying viral RNA templates. Arch. Virol. 2012, 157, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Hoofnagle, J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Natural 2005, 436, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.E.; Castro, C. The mechanism of action of ribavirin: Lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr. Opin. Infect. Dis. 2001, 14, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chan, J.F.-W.; Azhar, E.I.; Hui, D.S.; Yuen, K.-Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Prasad, B.V.L.S.; Selvarajan, R. RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- ElFiky, A.A.; Mahdy, S.M.; Elshemey, W.M. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J. Med. Virol. 2017, 89, 1040–1047. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Natural 2016, 531, 381–385. [Google Scholar] [CrossRef]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H.; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef]


| Compound Name | Max Dose (μM) | MERS-CoV RdRp Activity (%) |
|---|---|---|
| Remdesivir | 12 | 22.3 ± 0.3 |
| Dasabuvir | 10 | 51.5 ± 4.2 |
| Ribavirin | 100 | 60.3 ± 2.9 |
| Sofosbuvir | 100 | 62.4 ± 5.3 |
| Favipiravir | 100 | 85.8 ± 2.4 |
| Lamivudine | 100 | 98.3 ± 1.2 |
| Zidovudine | 100 | 110.8 ± 3.7 |
| Vidarabine | 100 | 89.9 ± 2.9 |
| Valacyclovir | 100 | 90.2 ± 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.S.; Kim, G.-W.; Kwon, S.; Jin, Y.-H. A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity. J. Clin. Med. 2020, 9, 2399. https://doi.org/10.3390/jcm9082399
Min JS, Kim G-W, Kwon S, Jin Y-H. A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity. Journal of Clinical Medicine. 2020; 9(8):2399. https://doi.org/10.3390/jcm9082399
Chicago/Turabian StyleMin, Jung Sun, Geon-Woo Kim, Sunoh Kwon, and Young-Hee Jin. 2020. "A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity" Journal of Clinical Medicine 9, no. 8: 2399. https://doi.org/10.3390/jcm9082399
APA StyleMin, J. S., Kim, G.-W., Kwon, S., & Jin, Y.-H. (2020). A Cell-Based Reporter Assay for Screening Inhibitors of MERS Coronavirus RNA-Dependent RNA Polymerase Activity. Journal of Clinical Medicine, 9(8), 2399. https://doi.org/10.3390/jcm9082399





