The Hidden Burden of Severe Asthma: From Patient Perspective to New Opportunities for Clinicians
Abstract
1. Introduction
2. The Different Faces of Severe Asthma
2.1. The Patient’s Perspective
2.2. The Clinician’s Perspective
2.3. The Funding Agency’s and the Researcher’s Perspective
2.4. The Scientific Societies’ Perspective
2.5. The Perspective of the Medical Educator
3. What Makes Asthma a Severe Disease?
3.1. Comorbidities
3.2. Tolerance and Resistance to β2-Agonists
3.3. Aging
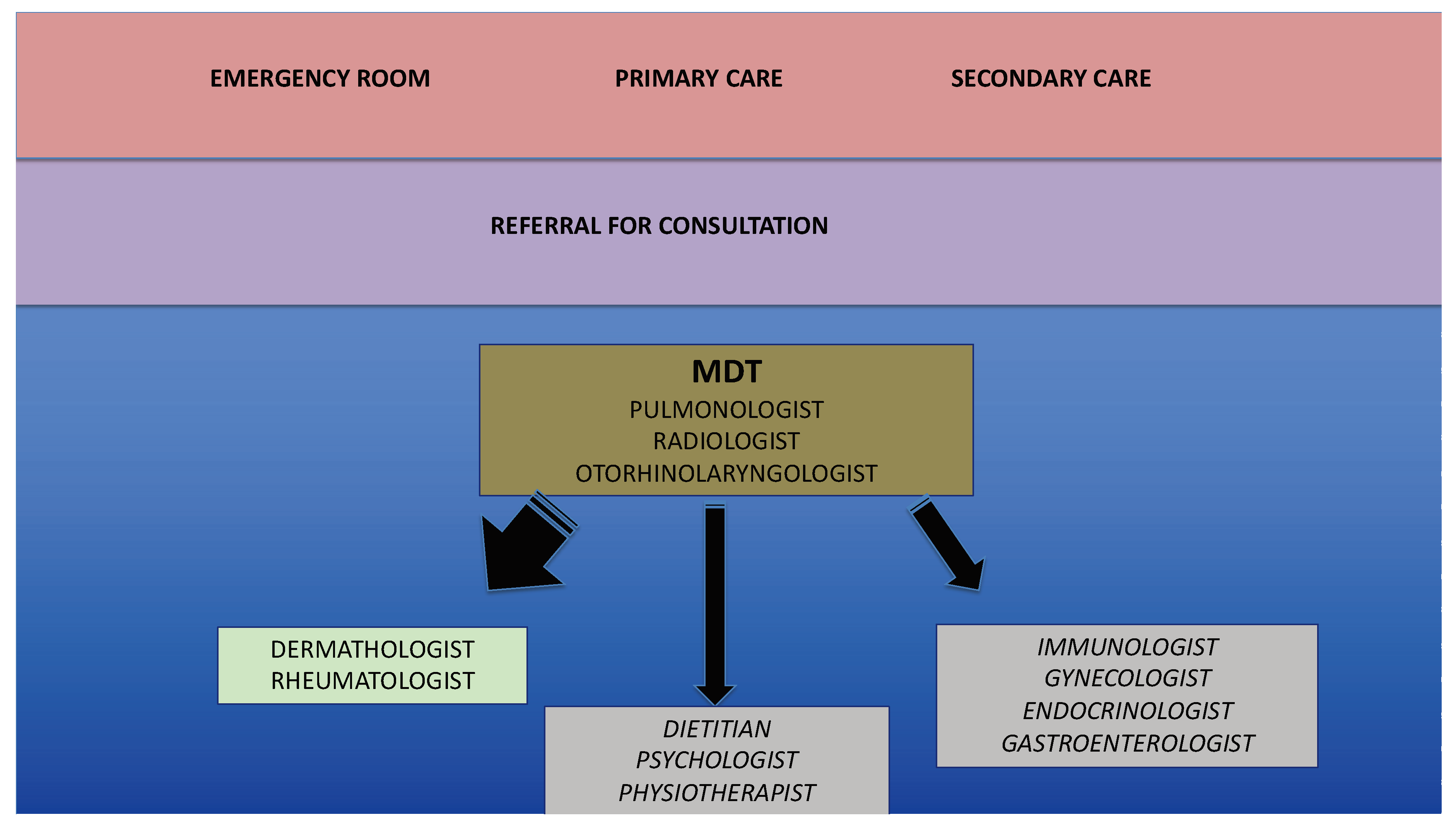
4. What Are the Most Appropriate Tools to Assess/Monitor Severe Asthma?
4.1. Second Level Functional Assessment
4.2. Imaging
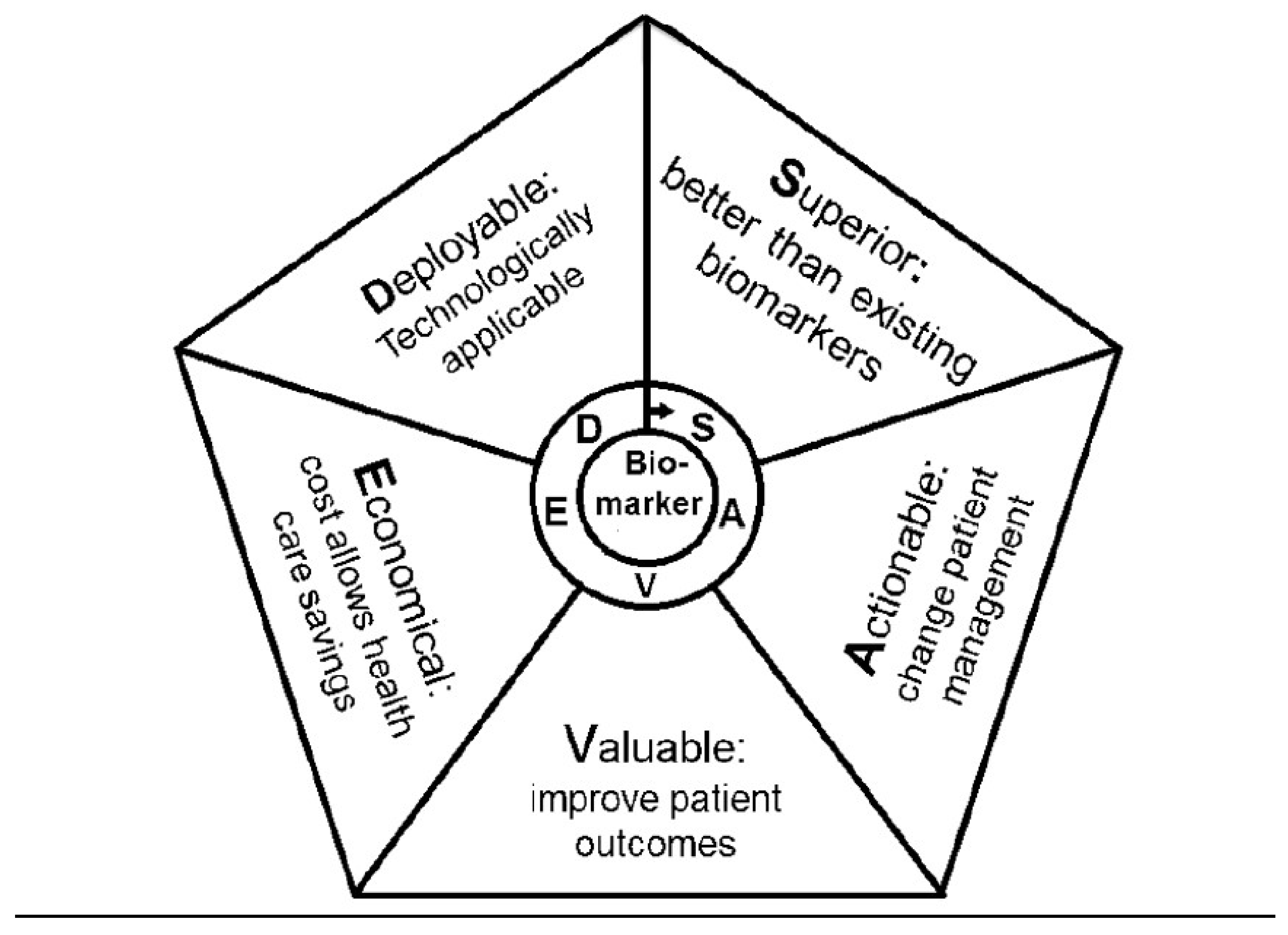
4.3. Biomarkers
4.4. Expert Systems and Artificial Intelligence
5. The Masked Facades of Severe Asthma
5.1. COPD and ACO
5.2. Eosinophilic Disorders
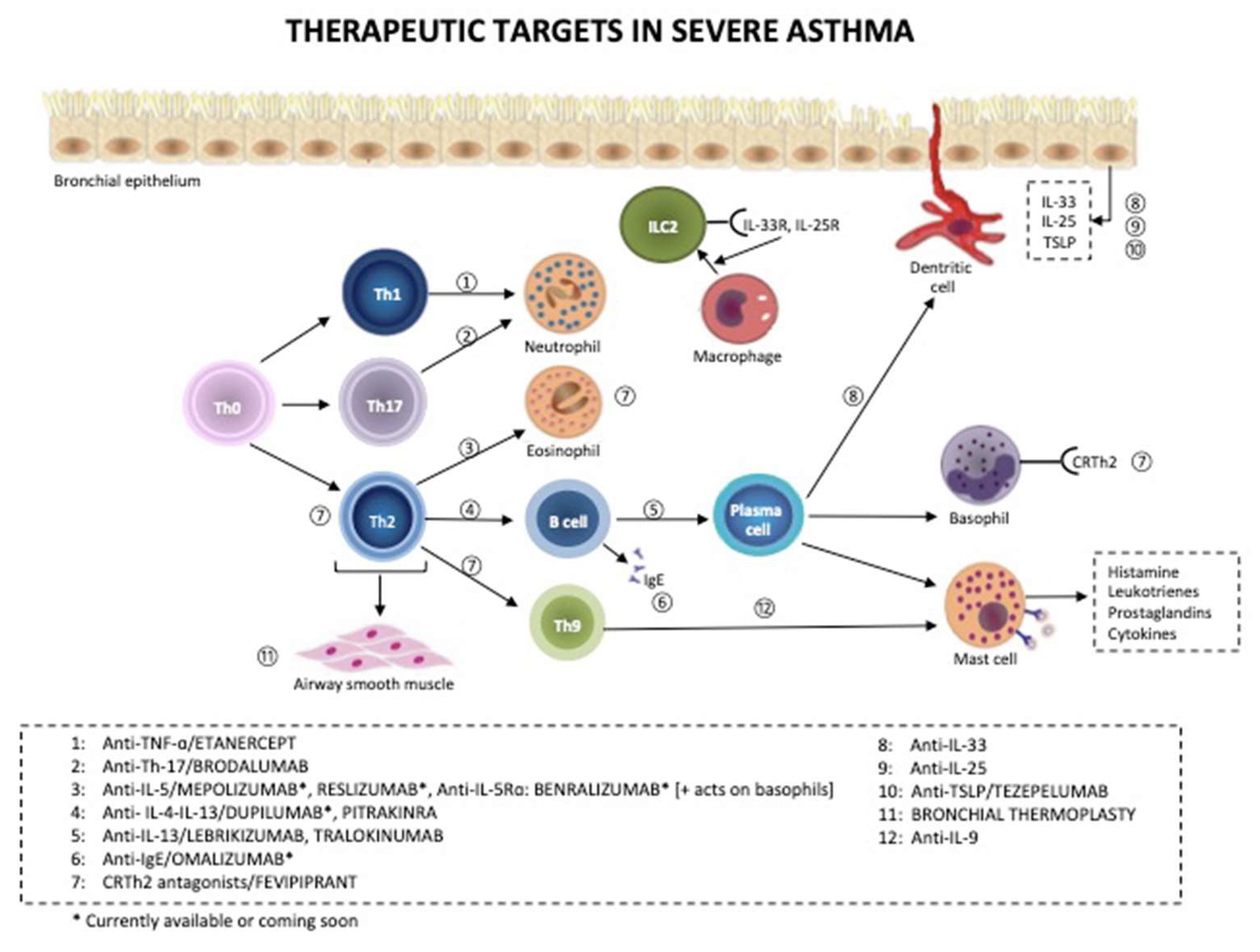
6. Challenges in the Treatment of Severe Asthma
6.1. Current Algorithm (as Proposed by GINA)
6.2. Drugs for COPD (Do They Also Work in Severe Asthma?)
6.3. Vitamin D
6.4. Current Treatment
6.5. Future Directions
6.6. Non Pharmacological Treatments
7. Severe Asthma in Acute or Difficult to Treat Settings
7.1. Acute Settings
7.2. Pregnancy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, K.F.; Godard, P.; Adelroth, E.; Ayres, J.; Barnes, N.; Barnes, P.; Bel, E.; Burney, P.; Chanez, P.; Connett, G.; et al. Difficult/therapy-resistant asthma: The need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. Eur. Respir. J. 1999, 13, 1198–1208. [Google Scholar] [PubMed]
- American Thoracic Society. Proceedings of the ATS workshop on refractory asthma: Current understanding, recommendations, and unanswered questions. Am. J. Respir. Crit Care Med. 2000, 162, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Chanez, P.; Wenzel, S.E.; Anderson, G.P.; Anto, J.M.; Bel, E.H.; Boulet, L.P.; Brightling, C.E.; Busse, W.W.; Castro, M.; Dahlen, B.; et al. Severe asthma in adults: What are the important questions? J. Allergy Clin. Immunol. 2007, 119, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention. 2019. Available online: http://www.ginasthma.org/ (accessed on 31 May 2019).
- Gibeon, D.; Heaney, L.G.; Brightling, C.E.; Niven, R.; Mansur, A.H.; Chaudhuri, R.; Bucknall, C.E.; Menzies-Gow, A.N.; British Thoracic Society Difficult Asthma Network. Dedicated severe asthma services improve health-care use and quality of life. Chest 2015, 148, 870–876. [Google Scholar] [CrossRef]
- Burke, H.; Davis, J.; Evans, S.; Flower, L.; Tan, A.; Kurukulaaratchy, R.J. A multidisciplinary team case management approach reduces the burden of frequent asthma admissions. ERJ Open Res. 2016, 2, 00039-2016. [Google Scholar] [CrossRef]
- McDonald, V.M.; Vertigan, A.E.; Gibson, P.G. How to set up a severe asthma service. Respirology 2011, 16, 900–911. [Google Scholar] [CrossRef]
- Zeiger, R.S.; Chipps, B.E.; Haselkorn, T.; Rasouliyan, L.; Simons, F.E.; Fish, J.E. Comparison of asthma exacerbations in pediatric and adult patients with severe or difficult-to-treat asthma. J. Allergy Clin. Immunol. 2009, 124, 1106–1108. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Schadt, E.E. Systems biology of asthma and allergic diseases: A multiscale approach. J. Allergy Clin. Immunol. 2015, 135, 31–42. [Google Scholar] [CrossRef]
- Oksel, C.; Custovic, A. Development of allergic sensitization and its relevance to paediatric asthma. Curr. Opin Allergy Clin. Immunol. 2018, 18, 109–116. [Google Scholar] [CrossRef]
- O’Byrne, P.M.; Pedersen, S.; Lamm, C.J.; Tan, W.C.; Busse, W.W.; Group, S.I. Severe exacerbations and decline in lung function in asthma. Am. J. Respir. Crit Care Med. 2009, 179, 19–24. [Google Scholar] [CrossRef]
- Altman, M.C.; Gill, M.A.; Whalen, E.; Babineau, D.C.; Shao, B.; Liu, A.H.; Jepson, B.; Gruchalla, R.S.; O’Connor, G.T.; Pongracic, J.A.; et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 2019, 20, 637–651. [Google Scholar] [CrossRef]
- Senna, G.; Guerriero, M.; Paggiaro, P.L.; Blasi, F.; Caminati, M.; Heffler, E.; Latorre, M.; Canonica, G.W.; Sani. SANI-Severe Asthma Network in Italy: A way forward to monitor severe asthma. Clin. Mol. Allergy 2017, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Blasi, F.; Latorre, M.; Menzella, F.; Paggiaro, P.; Pelaia, G.; Senna, G.; Canonica, G.W.; Network, S. The Severe Asthma Network in Italy: Findings and Perspectives. J. Allergy Clin. Immunol. Pract. 2019, 7, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Colombo, G.L.; Bruno, G.M.; Di Matteo, S.; Martinotti, C.; Blasi, F.; Bucca, C.; Crimi, N.; Paggiaro, P.; Pelaia, G.; et al. Shadow cost of oral corticosteroids-related adverse events: A pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ. J. 2019, 12, 100007. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Bagnasco, D.; Canonica, G.W. Strategies to reduce corticosteroid-related adverse events in asthma. Curr. Opin Allergy Clin. Immunol. 2019, 19, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Viegi, G.; Centanni, S.; Blasi, F. Uneven distribution of professors and instructors in medical disciplines dealing with the four main chronic non-communicable diseases: The case of the Italian Universities. Multidiscip. Respir. Med. 2017, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma (GINA). DIFFICULT-TO-TREAT & SEVERE ASTHMA in Adolescent and Adult Patients: Diagnosis and Management. 2019. Available online: http://www.ginasthma.org/ (accessed on 31 May 2019).
- Cazzola, M.; Calzetta, L.; Bettoncelli, G.; Novelli, L.; Cricelli, C.; Rogliani, P. Asthma and comorbid medical illness. Eur. Respir. J. 2011, 38, 42–49. [Google Scholar] [CrossRef]
- Tay, T.R.; Radhakrishna, N.; Hore-Lacy, F.; Smith, C.; Hoy, R.; Dabscheck, E.; Hew, M. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology 2016, 21, 1384–1390. [Google Scholar] [CrossRef]
- Clark, V.L.; Gibson, P.G.; Genn, G.; Hiles, S.A.; Pavord, I.D.; McDonald, V.M. Multidimensional assessment of severe asthma: A systematic review and meta-analysis. Respirology 2017, 22, 1262–1275. [Google Scholar] [CrossRef]
- Radhakrishna, N.; Tay, T.R.; Hore-Lacy, F.; Stirling, R.; Hoy, R.; Dabscheck, E.; Hew, M. Validated questionnaires heighten detection of difficult asthma comorbidities. J. Asthma 2017, 54, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Molecular mechanisms of beta2-adrenergic receptor function, response, and regulation. J. Allergy Clin. Immunol. 2006, 117, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Rogliani, P.; Matera, M.G. beta2-agonist therapy in lung disease. Am. J. Respir. Crit. Care Med. 2013, 187, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; Robitaille, C.; Deschesnes, F.; Villeneuve, H.; Boulay, M.E. Comparative Clinical, Physiological, and Inflammatory Characteristics of Elderly Subjects With or Without Asthma and Young Subjects With Asthma. Chest 2017, 152, 1203–1213. [Google Scholar] [CrossRef]
- Battaglia, S.; Sandrini, M.C.; Catalano, F.; Arcoleo, G.; Giardini, G.; Vergani, C.; Bellia, V. Effects of aging on sensation of dyspnea and health-related quality of life in elderly asthmatics. Aging Clin. Exp. Res. 2005, 17, 287–292. [Google Scholar] [CrossRef]
- Benfante, A.; Scichilone, N. The geriatric asthma: Pitfalls and challenges. Asthma Res. Pract. 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Park, H.W.; Song, W.J.; Kim, S.H.; Park, H.K.; Kim, S.H.; Kwon, Y.E.; Kwon, H.S.; Kim, T.B.; Chang, Y.S.; Cho, Y.S.; et al. Classification and implementation of asthma phenotypes in elderly patients. Ann. Allergy Asthma Immunol. 2015, 114, 18–22. [Google Scholar] [CrossRef]
- Enright, P.L.; McClelland, R.L.; Newman, A.B.; Gottlieb, D.J.; Lebowitz, M.D. Underdiagnosis and undertreatment of asthma in the elderly. Cardiovascular Health Study Research Group. Chest 1999, 116, 603–613. [Google Scholar] [CrossRef]
- Skloot, G.S.; Busse, P.J.; Braman, S.S.; Kovacs, E.J.; Dixon, A.E.; Vaz Fragoso, C.A.; Scichilone, N.; Prakash, Y.S.; Pabelick, C.M.; Mathur, S.K.; et al. An Official American Thoracic Society Workshop Report: Evaluation and Management of Asthma in the Elderly. Ann. Am. Thorac. Soc. 2016, 13, 2064–2077. [Google Scholar] [CrossRef]
- Scichilone, N.; Marchese, R.; Catalano, F.; Togias, A.; Vignola, A.M.; Bellia, V. The bronchodilatory effect of deep inspiration diminishes with aging. Respir. Med. 2004, 98, 838–843. [Google Scholar] [CrossRef]
- Sorkness, R.L.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Chung, K.F.; Curran-Everett, D.; Erzurum, S.C.; Gaston, B.M.; Israel, E.; et al. Lung function in adults with stable but severe asthma: Air trapping and incomplete reversal of obstruction with bronchodilation. J. Appl. Physiol. 2008, 104, 394–403. [Google Scholar] [CrossRef]
- Van Veen, I.H.; Sterk, P.J.; Schot, R.; Gauw, S.A.; Rabe, K.F.; Bel, E.H. Alveolar nitric oxide versus measures of peripheral airway dysfunction in severe asthma. Eur. Respir. J. 2006, 27, 951–956. [Google Scholar] [CrossRef]
- Walker, C.; Gupta, S.; Raj, V.; Siddiqui, S.; Brightling, C.E. Imaging advances in asthma. Expert Opin Med. Diagn. 2011, 5, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Kruger, S.J.; Cadman, R.V.; Mummy, D.G.; Evans, M.D.; Nagle, S.K.; Denlinger, L.C.; Jarjour, N.N.; Sorkness, R.L.; Fain, S.B. Regional Heterogeneity of Lobar Ventilation in Asthma Using Hyperpolarized Helium-3 MRI. Acad. Radiol. 2018, 25, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Aysola, R.S.; Hoffman, E.A.; Gierada, D.; Wenzel, S.; Cook-Granroth, J.; Tarsi, J.; Zheng, J.; Schechtman, K.B.; Ramkumar, T.P.; Cochran, R.; et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest 2008, 134, 1183–1191. [Google Scholar] [CrossRef]
- James, A.L.; Elliot, J.G.; Jones, R.L.; Carroll, M.L.; Mauad, T.; Bai, T.R.; Abramson, M.J.; McKay, K.O.; Green, F.H. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am. J. Respir. Crit. Care Med. 2012, 185, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Scichilone, N.; Mudge, B.; Diemer, F.B.; Permutt, S.; Togias, A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am. J. Respir. Crit. Care Med. 2001, 163, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Brown, R.; Mitzner, W.; Yarmus, L.; Li, X. Super-achromatic monolithic microprobe for ultrahigh-resolution endoscopic optical coherence tomography at 800 nm. Nat. Commun. 2017, 8, 1531. [Google Scholar] [CrossRef]
- Hollander, Z.; DeMarco, M.L.; Sadatsafavi, M.; McManus, B.M.; Ng, R.T.; Sin, D.D. Biomarker Development in COPD: Moving From P Values to Products to Impact Patient Care. Chest 2017, 151, 455–467. [Google Scholar] [CrossRef]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Popov, T.A. Human exhaled breath analysis. Ann. Allergy Asthma Immunol. 2011, 106, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Braido, F.; Santus, P.; Corsico, A.G.; Di Marco, F.; Melioli, G.; Scichilone, N.; Solidoro, P. Chronic obstructive lung disease “expert system”: Validation of a predictive tool for assisting diagnosis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Reddel, H.K.; ten Hacken, N.H.; van den Berge, M. Asthma and chronic obstructive pulmonary disease: Similarities and differences. Clin. Chest Med. 2014, 35, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; Hanania, N.A. The many faces of asthma-chronic obstructive pulmonary disease overlap. Curr. Opin. Pulm. Med. 2019, 25, 1–10. [Google Scholar] [CrossRef]
- Toledo-Pons, N.; van Boven, J.F.M.; Roman-Rodriguez, M.; Perez, N.; Valera Felices, J.L.; Soriano, J.B.; Cosio, B.G. ACO: Time to move from the description of different phenotypes to the treatable traits. PLoS ONE 2019, 14, e0210915. [Google Scholar] [CrossRef]
- Hastie, A.T.; Martinez, F.J.; Curtis, J.L.; Doerschuk, C.M.; Hansel, N.N.; Christenson, S.; Putcha, N.; Ortega, V.E.; Li, X.; Barr, R.G.; et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: An analysis of the SPIROMICS cohort. Lancet Respir. Med. 2017, 5, 956–967. [Google Scholar] [CrossRef]
- Roche, N.; Chapman, K.R.; Vogelmeier, C.F.; Herth, F.J.F.; Thach, C.; Fogel, R.; Olsson, P.; Patalano, F.; Banerji, D.; Wedzicha, J.A. Blood Eosinophils and Response to Maintenance Chronic Obstructive Pulmonary Disease Treatment. Data from the FLAME Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 1189–1197. [Google Scholar] [CrossRef]
- Rogliani, P.; Ora, J.; Puxeddu, E.; Cazzola, M. Airflow obstruction: Is it asthma or is it COPD? Int. J. Chron. Obstruct. Pulm. Dis. 2016, 11, 3007–3013. [Google Scholar] [CrossRef]
- Valent, P.; Gleich, G.J.; Reiter, A.; Roufosse, F.; Weller, P.F.; Hellmann, A.; Metzgeroth, G.; Leiferman, K.M.; Arock, M.; Sotlar, K.; et al. Pathogenesis and classification of eosinophil disorders: A review of recent developments in the field. Expert Rev. Hematol. 2012, 5, 157–176. [Google Scholar] [CrossRef]
- Fries, J.F.; Hunder, G.G.; Bloch, D.A.; Michel, B.A.; Arend, W.P.; Calabrese, L.H.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summ. Arthritis Rheum 1990, 33, 1135–1136. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.Y.; Hernandez, M.L.; Jennette, J.C.; Falk, R.J. Eosinophilic Granulomatosis with Polyangiitis: Clinical Pathology Conference and Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Tsurikisawa, N.; Tsuburai, T.; Oshikata, C.; Akiyama, K. Cytokine production profile of CD4+ T cells from patients with active Churg-Strauss syndrome tends toward Th17. Int. Arch. Allergy Immunol. 2009, 149 (Suppl. 1), 61–65. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
- Furuta, G.T.; Liacouras, C.A.; Collins, M.H.; Gupta, S.K.; Justinich, C.; Putnam, P.E.; Bonis, P.; Hassall, E.; Straumann, A.; Rothenberg, M.E.; et al. Eosinophilic esophagitis in children and adults: A systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007, 133, 1342–1363. [Google Scholar] [CrossRef] [PubMed]
- Liacouras, C.A.; Furuta, G.T.; Hirano, I.; Atkins, D.; Attwood, S.E.; Bonis, P.A.; Burks, A.W.; Chehade, M.; Collins, M.H.; Dellon, E.S.; et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J. Allergy Clin. Immunol. 2011, 128, 3–20.e26. [Google Scholar] [CrossRef] [PubMed]
- Hruz, P.; Straumann, A.; Bussmann, C.; Heer, P.; Simon, H.U.; Zwahlen, M.; Beglinger, C.; Schoepfer, A.M.; Swiss Eo Esg. Escalating incidence of eosinophilic esophagitis: A 20-year prospective, population-based study in Olten County, Switzerland. J. Allergy Clin. Immunol. 2011, 128, 1349–1350.e1345. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Hogan, S.P.; Brandt, E.B.; Rothenberg, M.E. IL-5 promotes eosinophil trafficking to the esophagus. J. Immunol. 2002, 168, 2464–2469. [Google Scholar] [CrossRef]
- Straumann, A.; Conus, S.; Grzonka, P.; Kita, H.; Kephart, G.; Bussmann, C.; Beglinger, C.; Smith, D.A.; Patel, J.; Byrne, M.; et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut 2010, 59, 21–30. [Google Scholar] [CrossRef]
- Spergel, J.M.; Rothenberg, M.E.; Collins, M.H.; Furuta, G.T.; Markowitz, J.E.; Fuchs, G., 3rd; O’Gorman, M.A.; Abonia, J.P.; Young, J.; Henkel, T.; et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2012, 129, 463 e451–453. [Google Scholar] [CrossRef]
- Clayton, F.; Fang, J.C.; Gleich, G.J.; Lucendo, A.J.; Olalla, J.M.; Vinson, L.A.; Lowichik, A.; Chen, X.; Emerson, L.; Cox, K.; et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014, 147, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Hirano, I. Biological therapies for eosinophilic gastrointestinal diseases. J. Allergy Clin. Immunol. 2018, 142, 24–31.e22. [Google Scholar] [CrossRef] [PubMed]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019, 156, 592–603.e510. [Google Scholar] [CrossRef] [PubMed]
- Hekking, P.P.; Wener, R.R.; Amelink, M.; Zwinderman, A.H.; Bouvy, M.L.; Bel, E.H. The prevalence of severe refractory asthma. J. Allergy Clin. Immunol. 2015, 135, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Kerstjens, H.A.; Engel, M.; Dahl, R.; Paggiaro, P.; Beck, E.; Vandewalker, M.; Sigmund, R.; Seibold, W.; Moroni-Zentgraf, P.; Bateman, E.D. Tiotropium in asthma poorly controlled with standard combination therapy. N. Engl. J. Med. 2012, 367, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Fabbri, L.M.; Singh, D.; Vestbo, J.; Celli, B.; Franssen, F.M.E.; Rabe, K.F.; Papi, A. Inhaled corticosteroids in COPD: Friend or foe? Eur. Respir. J. 2018, 52, 1801219. [Google Scholar] [CrossRef]
- Bafadhel, M.; McKenna, S.; Terry, S.; Mistry, V.; Reid, C.; Haldar, P.; McCormick, M.; Haldar, K.; Kebadze, T.; Duvoix, A.; et al. Acute exacerbations of chronic obstructive pulmonary disease: Identification of biologic clusters and their biomarkers. Am. J. Respir. Crit. Care Med. 2011, 184, 662–671. [Google Scholar] [CrossRef]
- Criner, G.J.; Celli, B.R.; Singh, D.; Agusti, A.; Papi, A.; Jison, M.; Makulova, N.; Shih, V.H.; Brooks, L.; Barker, P.; et al. Predicting response to benralizumab in chronic obstructive pulmonary disease: Analyses of GALATHEA and TERRANOVA studies. Lancet Respir. Med. 2019, 8, 158–170. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A., Jr.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2017, 5, 881–890. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hawrylowicz, C.M. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest 2018, 153, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, H.M.; Harshfield, B.J.; Laranjo, N.; Carey, V.J.; O’Connor, G.; Sandel, M.; Strunk, R.C.; Bacharier, L.B.; Zeiger, R.S.; Schatz, M.; et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: Secondary analyses from the Vitamin D Antenatal Asthma Reduction Trial. J. Allergy Clin. Immunol. 2017, 140, 1423–1429.e1425. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E., Jr.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Paggiaro, P.; Corradi, M.; Latorre, M.; Raptis, H.; Muraro, A.; Gessner, C.; Siergiejko, Z.; Scuri, M.; Petruzzelli, S. High strength extrafine pMDI beclometasone/formoterol (200/6 mug) is effective in asthma patients not adequately controlled on medium-high dose of inhaled corticosteroids. BMC Pulm. Med. 2016, 16, 180. [Google Scholar] [CrossRef][Green Version]
- Woodcock, A.; Vestbo, J.; Bakerly, N.D.; New, J.; Gibson, J.M.; McCorkindale, S.; Jones, R.; Collier, S.; Lay-Flurrie, J.; Frith, L.; et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: An open-label, parallel group, randomised controlled trial. Lancet 2017, 390, 2247–2255. [Google Scholar] [CrossRef]
- Bousquet, J.; Boulet, L.P.; Peters, M.J.; Magnussen, H.; Quiralte, J.; Martinez-Aguilar, N.E.; Carlsheimer, A. Budesonide/formoterol for maintenance and relief in uncontrolled asthma vs. high-dose salmeterol/fluticasone. Respir. Med. 2007, 101, 2437–2446. [Google Scholar] [CrossRef]
- Virchow, J.C.; Mehta, A.; Ljungblad, L.; Mitfessel, H.; MONICA study group. Add-on montelukast in inadequately controlled asthma patients in a 6-month open-label study: The MONtelukast in Chronic Asthma (MONICA) study. Respir. Med. 2010, 104, 644–651. [Google Scholar] [CrossRef]
- Humbert, M.; Taille, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M.; STELLAIR investigators. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef]
- Sposato, B.; Scalese, M.; Milanese, M.; Masieri, S.; Cavaliere, C.; Latorre, M.; Scichilone, N.; Ricci, A.; Cresti, A.; Santus, P.; et al. Higher blood eosinophil levels after omalizumab treatment may be associated with poorer asthma outcomes. J. Allergy Clin. Immunol. Pract. 2019, 7, 1643–1646. [Google Scholar] [CrossRef]
- Benfante, A.; Principe, S.; Battaglia, S.; Scichilone, N. Are biological drugs effective and safe in older severe asthmatics? Expert Opin. Drug Saf. 2019, 6, 369–380. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Menzies-Gow, A.; Zangrilli, J.G.; Hirsch, I.; Metcalfe, P.; Newbold, P.; Goldman, M. Predictors of enhanced response with benralizumab for patients with severe asthma: Pooled analysis of the SIROCCO and CALIMA studies. Lancet Respir. Med. 2018, 6, 51–64. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaci, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.E.; O’Byrne, P.M.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P.; et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 2012, 42, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2014, 43, 1067–1076. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Jevnikar, Z.; Ostling, J.; Ax, E.; Calven, J.; Thorn, K.; Israelsson, E.; Oberg, L.; Singhania, A.; Lau, L.C.K.; Wilson, S.J.; et al. Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes study g. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 577–590. [Google Scholar] [CrossRef]
- Fenwick, P.S.; Macedo, P.; Kilty, I.C.; Barnes, P.J.; Donnelly, L.E. Effect of JAK Inhibitors on Release of CXCL9, CXCL10 and CXCL11 from Human Airway Epithelial Cells. PLoS ONE 2015, 10, e0128757. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.D.; Goehring, U.M.; Richard, F.; Watz, H. Roflumilast combined with montelukast versus montelukast alone as add-on treatment in patients with moderate-to-severe asthma. J. Allergy Clin. Immunol. 2016, 138, 142–149.e148. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Vanderstichele, C.; Jordens, P.; Deman, R.; Slabbynck, H.; Ringoet, V.; Verleden, G.; Demedts, I.K.; Verhamme, K.; Delporte, A.; et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): A multicentre randomised double-blind placebo-controlled trial. Thorax 2013, 68, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef]
- Tan, L.D.; Yoneda, K.Y.; Louie, S.; Hogarth, D.K.; Castro, M. Bronchial Thermoplasty: A Decade of Experience: State of the Art. J. Allergy Clin. Immunol. Pract. 2019, 7, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Thomson, N.C.; Rubin, A.S.; Niven, R.M.; Corris, P.A.; Siersted, H.C.; Olivenstein, R.; Pavord, I.D.; McCormack, D.; Chaudhuri, R.; et al. AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N. Engl. J. Med. 2007, 356, 1327–1337. [Google Scholar] [CrossRef]
- Pavord, I.D.; Cox, G.; Thomson, N.C.; Rubin, A.S.; Corris, P.A.; Niven, R.M.; Chung, K.F.; Laviolette, M. RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 1185–1191. [Google Scholar] [CrossRef]
- Castro, M.; Rubin, A.S.; Laviolette, M.; Fiterman, J.; De Andrade Lima, M.; Shah, P.L.; Fiss, E.; Olivenstein, R.; Thomson, N.C.; Niven, R.M.; et al. AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: A multicenter, randomized, double-blind, sham-controlled clinical trial. Am. J. Respir. Crit. Care Med. 2010, 181, 116–124. [Google Scholar] [CrossRef]
- Arrigo, R.; Failla, G.; Scichilone, N.; La Sala, A.; Galeone, C.; Battaglia, S.; Benfante, A.; Facciolongo, N. How Effective and Safe Is Bronchial Thermoplasty in “Real Life” Asthmatics Compared to Those Enrolled in Randomized Clinical Trials? Biomed. Res. Int. 2016, 2016, 9132198. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.; et al. Rehabilitation AETFoP. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Benfante, A.; Di Marco, F.; Terraneo, S.; Centanni, S.; Scichilone, N. Dynamic hyperinflation during the 6-min walk test in severely asthmatic subjects. ERJ Open Res. 2018, 4, 00143-2017. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Dabrowska, G.; Pawik, M.; Rozek, K. Evaluation of a Home-Based Pulmonary Rehabilitation Program for Older Females Suffering from Bronchial Asthma. Adv Clin. Exp. Med. 2015, 24, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Trevor, J.L.; Bhatt, S.P.; Wells, J.M.; Kirkpatrick, D.; Schumann, C.; Hitchcock, J.; Dransfield, M.T. Benefits of completing pulmonary rehabilitation in patients with asthma. J. Asthma 2015, 52, 969–973. [Google Scholar] [CrossRef]
- Sahin, H.; Naz, I. Comparing the effect of pulmonary rehabilitation in patients with uncontrolled and partially controlled asthma. J. Asthma 2019, 56, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Oto, T.; Griffiths, A.; Levvey, B.; Whitford, H.; Kotsimbos, T.C.; Rabinov, M.; Esmore, D.S.; Williams, T.J.; Snell, G.I. Donor history of asthma is not a contraindication to lung transplantation: 12-year single-center experience. J. Heart Lung Transplant. 2004, 23, 309–316. [Google Scholar] [CrossRef]
- Solomonov, A.; Yigla, M.; Amir, G.; Rudis, E.; Berkman, N. Single lung transplantation in refractory asthma with irreversible airflow obstruction. Isr. Med. Assoc. J. 2004, 6, 246–248. [Google Scholar]
- Wirtz, H.R.; Kroegel, C.; Caffier, P.; Bittner, H. Bilateral lung transplantation for severe persistent and difficult asthma. J. Heart Lung Transplant. 2005, 24, 1700–1703. [Google Scholar] [CrossRef]
- Wiebe, K.; Rowe, B.H. Nebulized racemic epinephrine used in the treatment of severe asthmatic exacerbation: A case report and literature review. CJEM 2007, 9, 304–308. [Google Scholar] [CrossRef][Green Version]
- Travers, A.H.; Milan, S.J.; Jones, A.P.; Camargo, C.A., Jr.; Rowe, B.H. Addition of intravenous beta(2)-agonists to inhaled beta(2)-agonists for acute asthma. Cochrane Database Syst. Rev. 2012, 12, CD010179. [Google Scholar]
- Watts, K.; Chavasse, R.J. Leukotriene receptor antagonists in addition to usual care for acute asthma in adults and children. Cochrane Database Syst. Rev. 2012, 16, CD006100. [Google Scholar] [CrossRef]
- Parameswaran, K.; Belda, J.; Rowe, B.H. Addition of intravenous aminophylline to beta2-agonists in adults with acute asthma. Cochrane Database Syst. Rev. 2000, CD002742. [Google Scholar] [PubMed]
- Rodrigo, G.; Pollack, C.; Rodrigo, C.; Rowe, B.H. Heliox for nonintubated acute asthma patients. Cochrane Database Syst. Rev. 2006, 18, CD002884. [Google Scholar]
- Maselli, D.J.; Peters, J.I. Medication Regimens for Managing Acute Asthma. Respir. Care 2018, 63, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Rochwerg, B.; Brochard, L.; Elliott, M.W.; Hess, D.; Hill, N.S.; Nava, S.; Navalesi PMOTSC; Antonelli, M.; Brozek, J.; Conti, G.; et al. Official ERS/ATS clinical practice guidelines: Noninvasive ventilation for acute respiratory failure. Eur. Respir. J. 2017, 50, 1602426. [Google Scholar] [CrossRef]
- Murphy, V.E.; Namazy, J.A.; Powell, H.; Schatz, M.; Chambers, C.; Attia, J.; Gibson, P.G. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011, 118, 1314–1323. [Google Scholar] [CrossRef]
- Murphy, V.E.; Clifton, V.L.; Gibson, P.G. Asthma exacerbations during pregnancy: Incidence and association with adverse pregnancy outcomes. Thorax 2006, 61, 169–176. [Google Scholar] [CrossRef]
- Ali, Z.; Ulrik, C.S. Incidence and risk factors for exacerbations of asthma during pregnancy. J. Asthma Allergy 2013, 6, 53–60. [Google Scholar]
- Schatz, M.; Dombrowski, M.P.; Wise, R.; Thom, E.A.; Landon, M.; Mabie, W.; Newman, R.B.; Hauth, J.C.; Lindheimer, M.; Caritis, S.N.; et al. Asthma morbidity during pregnancy can be predicted by severity classification. J. Allergy Clin. Immunol. 2003, 112, 283–288. [Google Scholar] [CrossRef]
- Tata, L.J.; Lewis, S.A.; McKeever, T.M.; Smith, C.J.; Doyle, P.; Smeeth, L.; Gibson, J.E.; Hubbard, R.B. Effect of maternal asthma, exacerbations and asthma medication use on congenital malformations in offspring: A UK population-based study. Thorax 2008, 63, 981–987. [Google Scholar] [CrossRef]
- Namazy, J.; Cabana, M.D.; Scheuerle, A.E.; Thorp, J.M., Jr.; Chen, H.; Carrigan, G.; Wang, Y.; Veith, J.; Andrews, E.B. The Xolair Pregnancy Registry (EXPECT): The safety of omalizumab use during pregnancy. J. Allergy Clin. Immunol. 2015, 135, 407–412. [Google Scholar] [CrossRef]
| Unanswered Questions | Specific Topics |
|---|---|
| What are the different faces of severe asthma? | The patient’s perspective |
| The clinician’s perspective | |
| The researcher perspective | |
| The scientific society’s perspective | |
| The academic’s perspective | |
| What makes asthma a severe disease? | Comorbidities |
| Tolerance and resistance to β2-agonists | |
| Aging | |
| What are the most appropriate tools to assess/monitor severe asthma? | Second level functional assessment |
| Imaging | |
| Biomarkers | |
| Expert systems and artificial intelligence | |
| Has severe asthma masked facades? | COPD and ACO |
| Eosinophilic disorders | |
| What are the challenges in treating severe asthma? | Current algorithm |
| Drugs for COPD | |
| Vitamin D | |
| Future directions | |
| Biologic drugs | |
| Non pharmacological treatments | |
| Severe asthma in acute or difficult settings | Acute settings |
| Difficult settings (pregnancy) |
| Year | Definition | Endorsement |
|---|---|---|
| 1999 (Chung K.F., Eur. Respir. J.) [1] | difficult/therapy resistant asthma | ERS |
| 2000 (ATS workshop, Am. J. Respir. Crit. Care Med.) [2] | refractory asthma | ATS |
| 2007 (Chanez P., J. Allergy. Clin. Immunol.) [3] | severe asthma | GINA |
| 2014 (Chung K.F., Eur. Respir. J.) [4] | severe controlled and uncontrolled asthma | ERS/ATS |
| 2018 (GINA pocket guide) [5] | difficult-to-treat asthma severe asthma | GINA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scichilone, N.; Barnes, P.J.; Battaglia, S.; Benfante, A.; Brown, R.; Canonica, G.W.; Caramori, G.; Cazzola, M.; Centanni, S.; Cianferoni, A.; et al. The Hidden Burden of Severe Asthma: From Patient Perspective to New Opportunities for Clinicians. J. Clin. Med. 2020, 9, 2397. https://doi.org/10.3390/jcm9082397
Scichilone N, Barnes PJ, Battaglia S, Benfante A, Brown R, Canonica GW, Caramori G, Cazzola M, Centanni S, Cianferoni A, et al. The Hidden Burden of Severe Asthma: From Patient Perspective to New Opportunities for Clinicians. Journal of Clinical Medicine. 2020; 9(8):2397. https://doi.org/10.3390/jcm9082397
Chicago/Turabian StyleScichilone, Nicola, Peter John Barnes, Salvatore Battaglia, Alida Benfante, Robert Brown, Giorgio Walter Canonica, Gaetano Caramori, Mario Cazzola, Stefano Centanni, Antonella Cianferoni, and et al. 2020. "The Hidden Burden of Severe Asthma: From Patient Perspective to New Opportunities for Clinicians" Journal of Clinical Medicine 9, no. 8: 2397. https://doi.org/10.3390/jcm9082397
APA StyleScichilone, N., Barnes, P. J., Battaglia, S., Benfante, A., Brown, R., Canonica, G. W., Caramori, G., Cazzola, M., Centanni, S., Cianferoni, A., Corsico, A., De Carlo, G., Di Marco, F., Gaga, M., Hawrylowicz, C., Heffler, E., Matera, M. G., Matucci, A., Paggiaro, P., ... Boulet, L.-P. (2020). The Hidden Burden of Severe Asthma: From Patient Perspective to New Opportunities for Clinicians. Journal of Clinical Medicine, 9(8), 2397. https://doi.org/10.3390/jcm9082397











