Receptor-Associated Prorenin System in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma and Neovascular Glaucoma
Abstract
1. Introduction
2. Methods
2.1. Clinical Characteristics of Patients
2.2. Patient Surgical Samples
2.3. Cell Culture and Chemicals
2.4. ELISA
2.5. Immunofluorescence Microscopy
2.6. Immunohistochemistry
2.7. Reverse Transcription-PCR (RT-PCR) and Real-Time Quantitative PCR (qPCR) Analyses
2.8. Statistical Analyses
3. Results
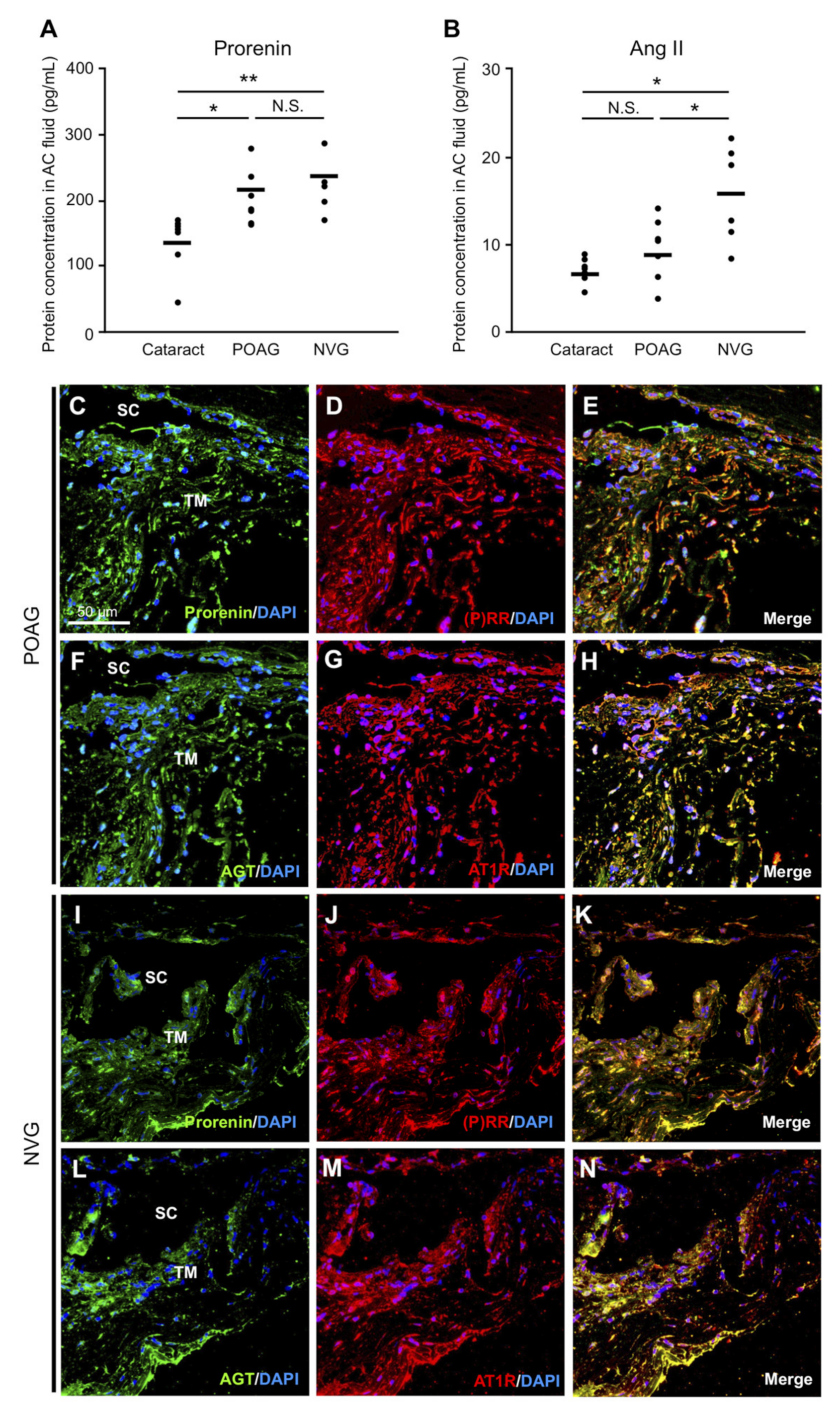
3.1. Elevation of Prorenin and Ang II Levels in AC Fluids from POAG and NVG Patients
3.2. RAPS Ligand–Receptor Co-localization in TM Tissues from POAG and NVG Patients
3.3. Selection of Target Genes Related to the Pathogenesis of POAG and NVG
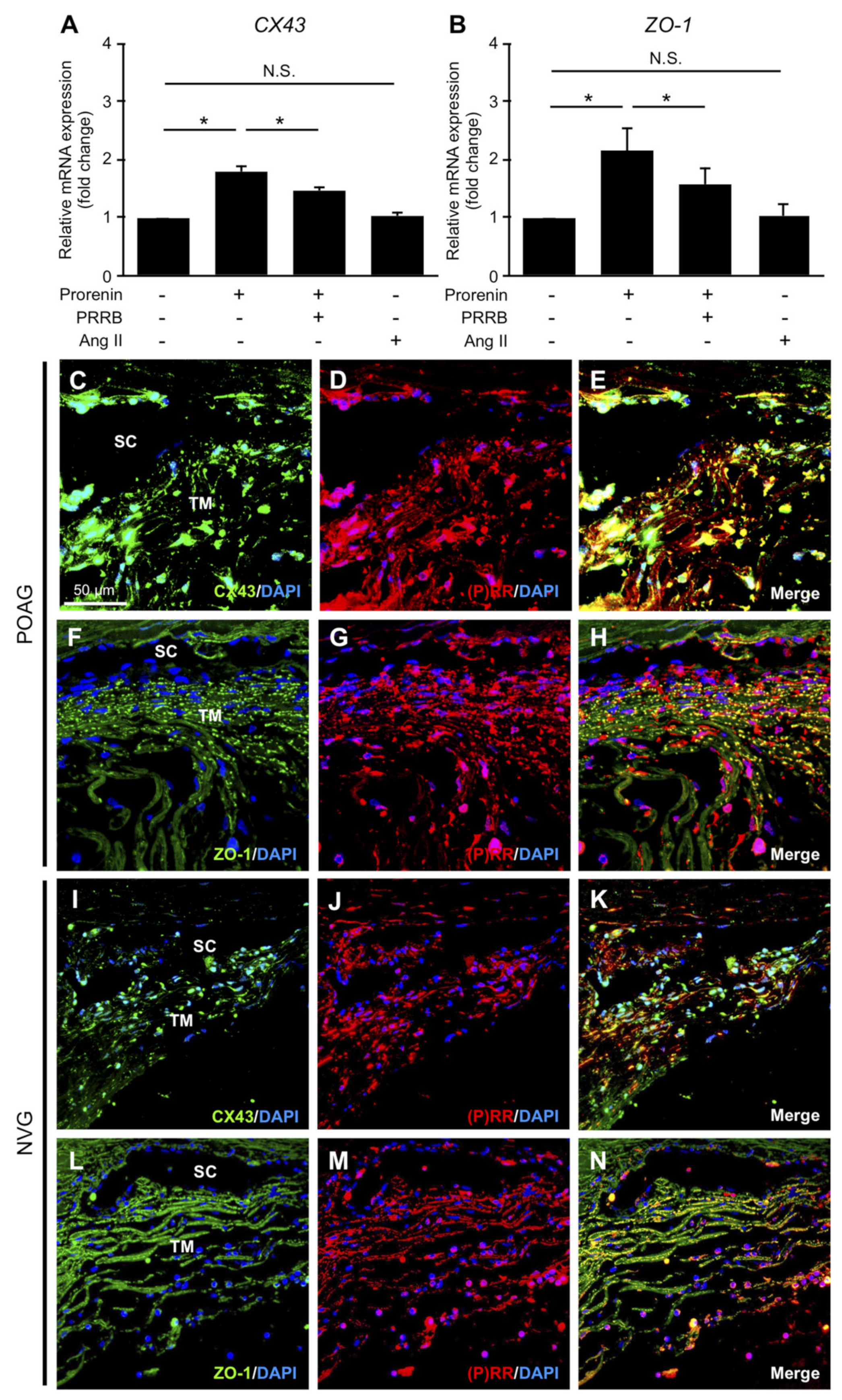
3.4. Upregulation of CX43 and ZO-1 via Prorenin–(P)RR Interaction in TM Cells and Their Production in TM Tissues from POAG and NVG Patients
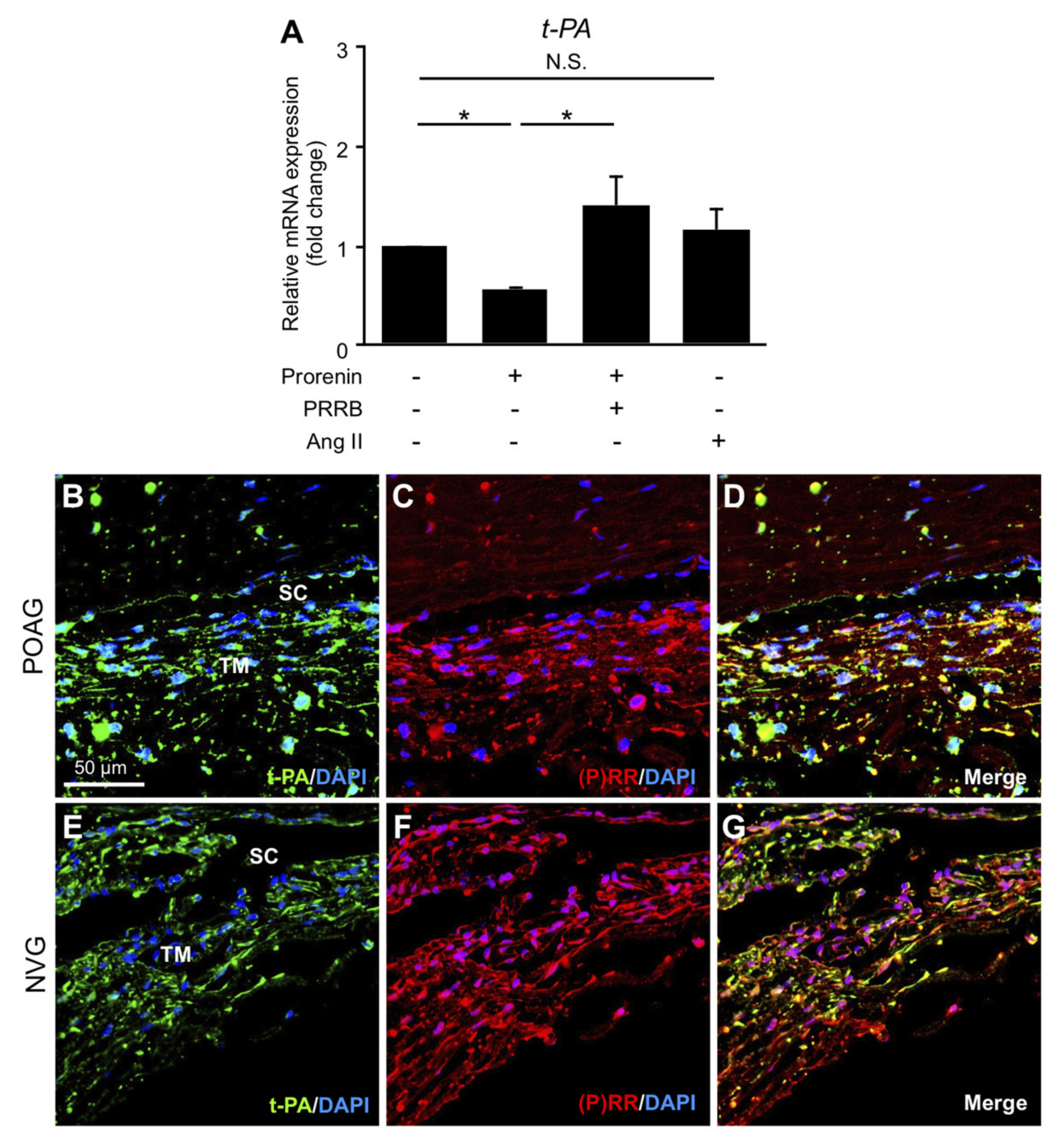
3.5. Downregulation of t-PA via Prorenin–(P)RR Interaction in TM Cells and Its Production in TM Tissues from POAG and NVG Patients
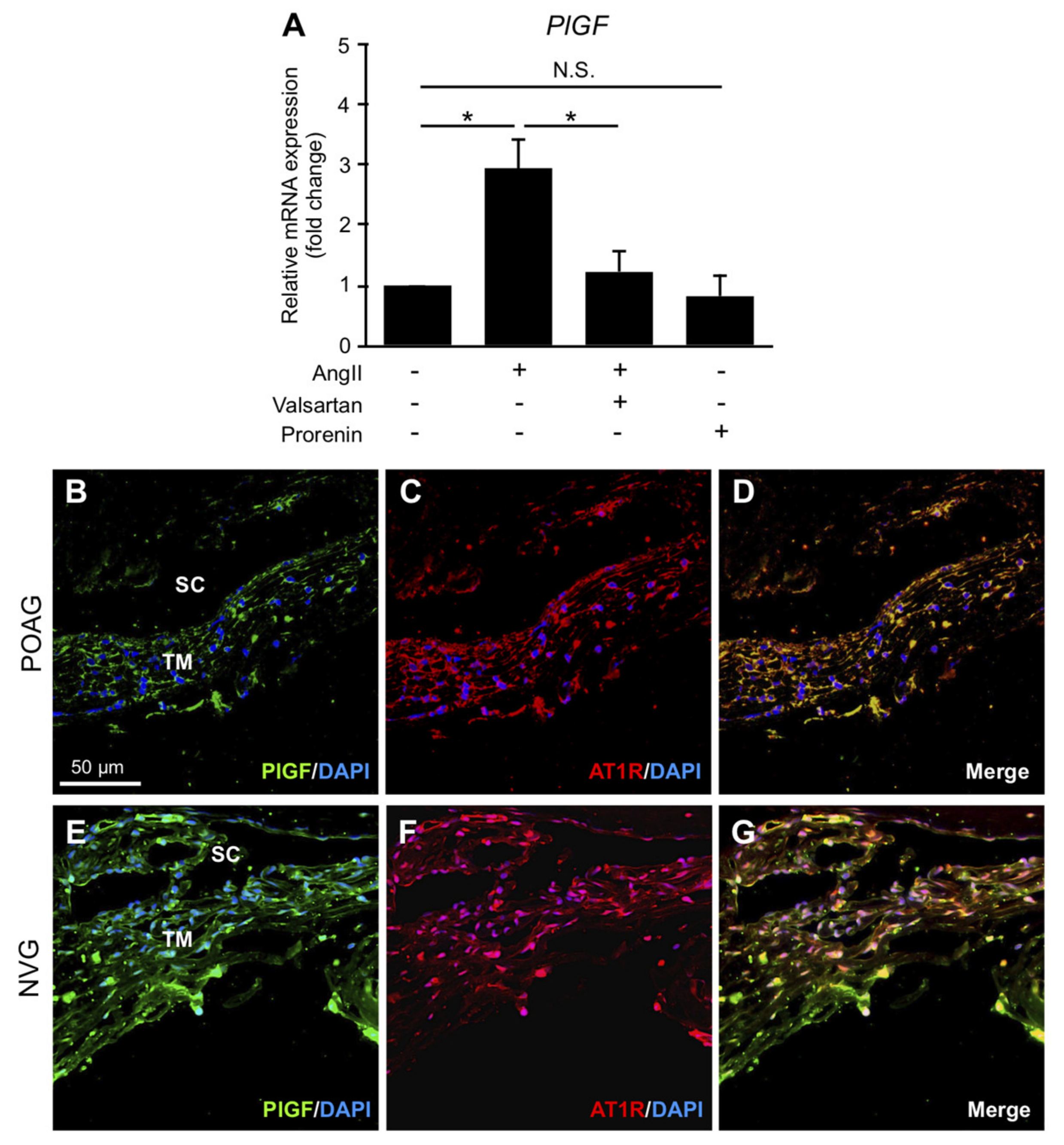
3.6. Upregulation of PlGF via Ang II–AT1R Interaction in TM Cells and Its Production in TM Tissues from POAG and NVG Patients
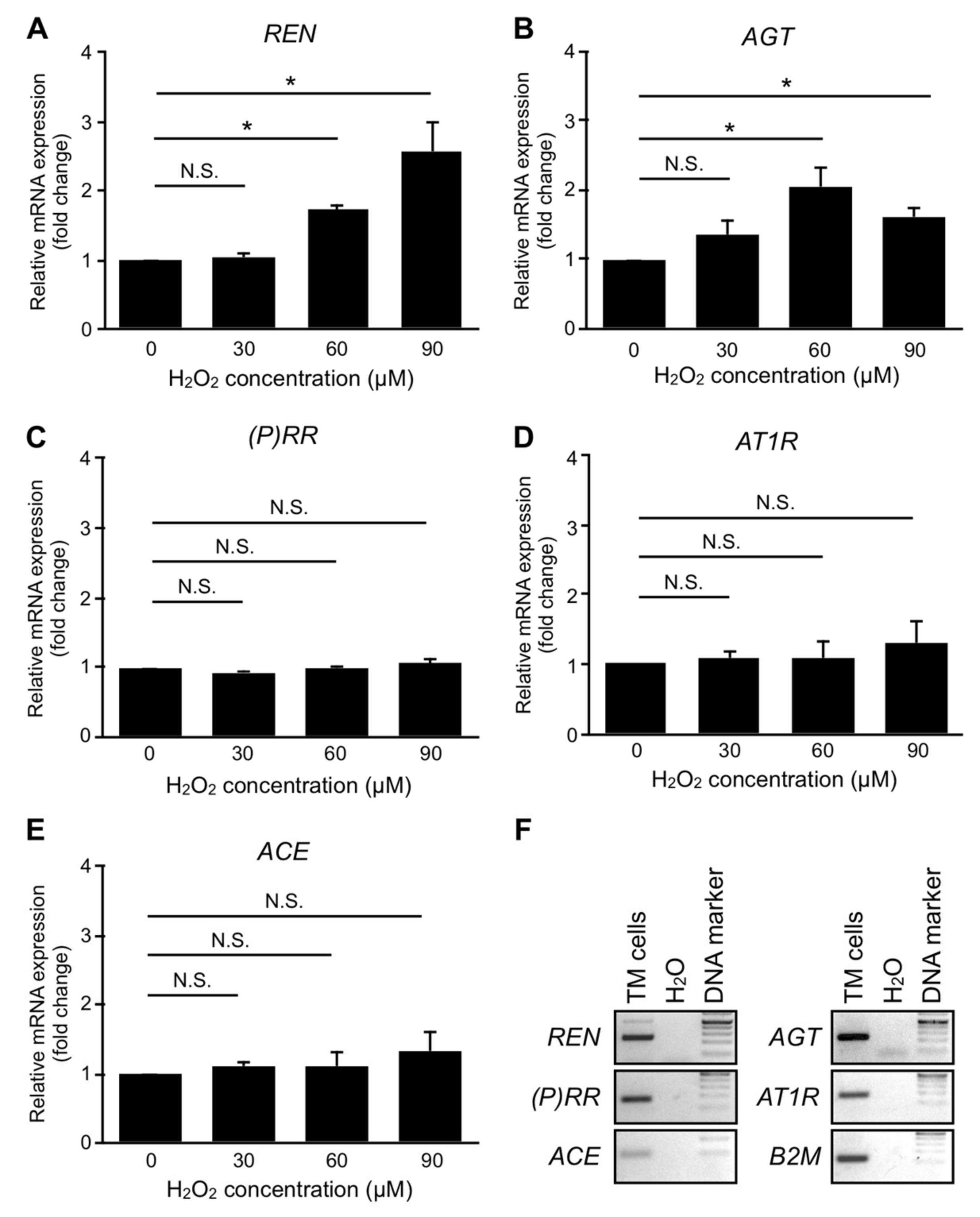
3.7. Upregulation of RAPS Ligands but Not Receptors in TM Cells Exposed to Oxidative Stress
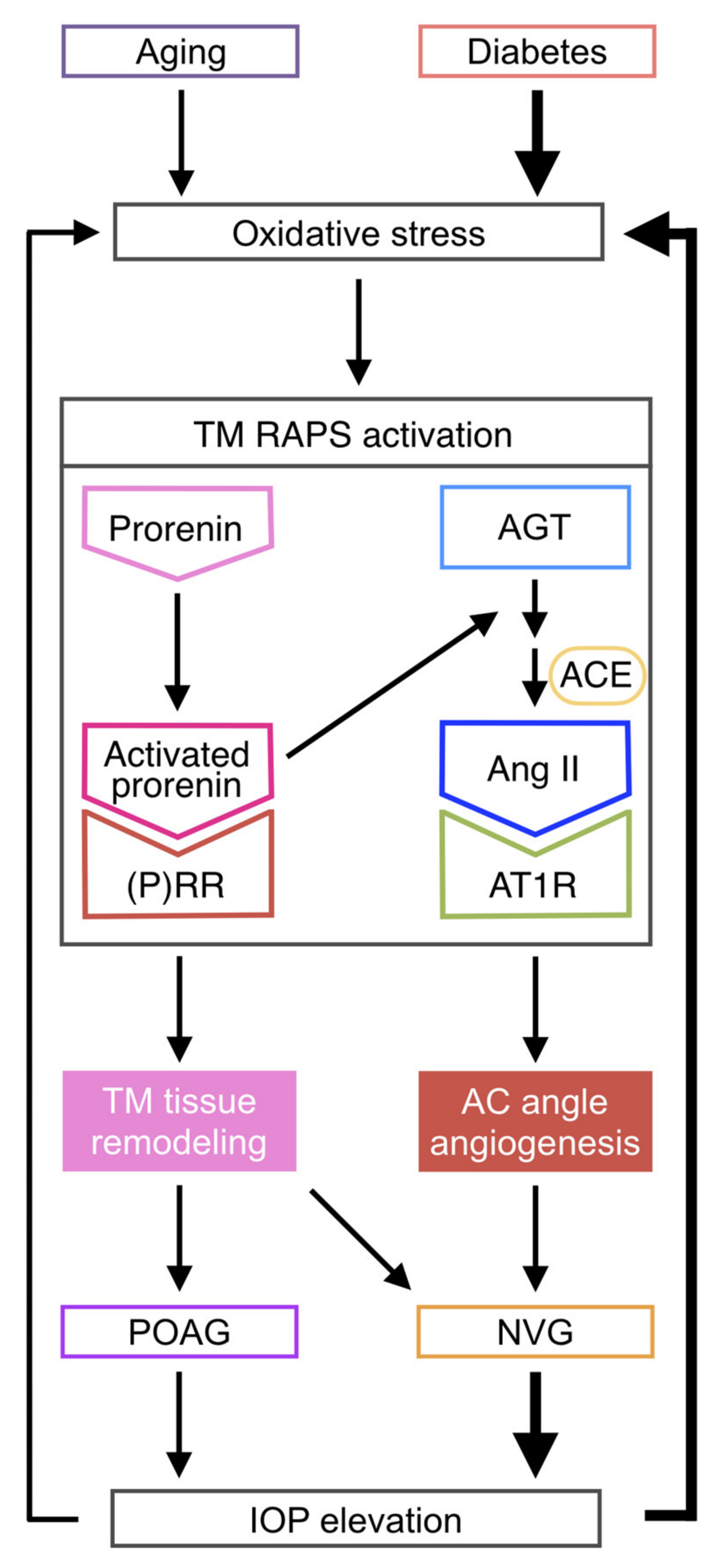
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Leung, C.K.; Crowston, J.G.; Medeiros, F.A.; Friedman, D.S.; Wiggs, J.L.; Martin, K.R. Primary open-angle glaucoma. Nat. Rev. Dis. Primers 2016, 2, 16067. [Google Scholar] [CrossRef] [PubMed]
- Carreon, T.; Van der Merwe, E.; Fellman, R.L.; Johnstone, M.; Bhattacharya, S.K. Aqueous outflow-A continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res. 2017, 57, 108–133. [Google Scholar] [CrossRef]
- Underwood, J.L.; Murphy, C.G.; Chen, J.; Franse-Carman, L.; Wood, I.; Epstein, D.L.; Alvarado, J.A. Glucocorticoids regulate transendothelial fluid flow resistance and formation of intercellular junctions. Am. J. Physiol. 1999, 277, C330–C342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Zhou, C.J.; Li, X.Y.; Sun, P.R.; Li, S.P.; Ren, B.C. Alteration of UCP2 and ZO-1 expression in trabecular meshwork of neovascular glaucoma patients. J. Glaucoma 2015, 24, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.H.; He, Y.; Leung, K.W.; Hou, F.; Li, Y.Q.; Chai, F.; Ge, J. Dexamethasone disrupts intercellular junction formation and cytoskeleton organization in human trabecular meshwork cells. Mol. Vis. 2010, 16, 61–71. [Google Scholar] [PubMed]
- De Groef, L.; Van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the trabecular meshwork: Promising targets for future glaucoma therapies? Investig. Ophthalmol. Vis. Sci. 2013, 54, 7756–7763. [Google Scholar] [CrossRef]
- Kumar, S.; Shah, S.; Tang, H.M.; Smith, M.; Borras, T.; Danias, J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS ONE 2013, 8, e72447. [Google Scholar] [CrossRef]
- Seftor, R.E.; Stamer, W.D.; Seftor, E.A.; Snyder, R.W. Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J. Glaucoma 1994, 3, 323–328. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Abe, R.Y.; Zangalli, C.; Sodre, S.L.; Donini, F.A.; Costa, D.C.; Leite, A.; Felix, J.P.; Torigoe, M.; Diniz-Filho, A.; et al. Neovascular glaucoma: A review. Int. J. Retina Vitreous 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, T.; Tsuda, S.; Kunikata, H.; Yasuda, M.; Himori, N.; Kunimatsu-Sanuki, S.; Maruyama, K.; Nakazawa, T. Characteristic Profiles of Inflammatory Cytokines in the Aqueous Humor of Glaucomatous Eyes. Ocul. Immunol. Inflamm. 2018, 26, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Inoue, T.; Shobayashi, K.; Iwao, K.; Fukushima, M.; Tanihara, H. Simultaneous increase in multiple proinflammatory cytokines in the aqueous humor in neovascular glaucoma with and without intravitreal bevacizumab injection. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Sun, X.H.; Dahan, E.; Guo, W.Y.; Qian, S.H.; Meng, F.R.; Song, Y.L.; Simon, G.J. Increased levels of transforming growth factor-betal and -beta2 in the aqueous humor of patients with neovascular glaucoma. Ophthalmic Surg. Lasers Imaging 2007, 38, 6–14. [Google Scholar] [CrossRef]
- Ando, R.; Noda, K.; Namba, S.; Saito, W.; Kanda, A.; Ishida, S. Aqueous humour levels of placental growth factor in diabetic retinopathy. Acta Ophthalmol. 2014, 92, e245–e246. [Google Scholar] [CrossRef]
- Nagai, N.; Oike, Y.; Izumi-Nagai, K.; Urano, T.; Kubota, Y.; Noda, K.; Ozawa, Y.; Inoue, M.; Tsubota, K.; Suda, T.; et al. Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2252–2259. [Google Scholar] [CrossRef]
- Nagai, N.; Izumi-Nagai, K.; Oike, Y.; Koto, T.; Satofuka, S.; Ozawa, Y.; Yamashiro, K.; Inoue, M.; Tsubota, K.; Umezawa, K.; et al. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4342–4350. [Google Scholar] [CrossRef]
- Nagai, N.; Noda, K.; Urano, T.; Kubota, Y.; Shinoda, H.; Koto, T.; Shinoda, K.; Inoue, M.; Shiomi, T.; Ikeda, E.; et al. Selective suppression of pathologic, but not physiologic, retinal neovascularization by blocking the angiotensin II type 1 receptor. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1078–1084. [Google Scholar] [CrossRef]
- Nagai, N.; Oike, Y.; Noda, K.; Urano, T.; Kubota, Y.; Ozawa, Y.; Shinoda, H.; Koto, T.; Shinoda, K.; Inoue, M.; et al. Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2925–2931. [Google Scholar] [CrossRef]
- Okunuki, Y.; Usui, Y.; Nagai, N.; Kezuka, T.; Ishida, S.; Takeuchi, M.; Goto, H. Suppression of experimental autoimmune uveitis by angiotensin II type 1 receptor blocker telmisartan. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2255–2261. [Google Scholar] [CrossRef]
- Kurihara, T.; Ozawa, Y.; Nagai, N.; Shinoda, K.; Noda, K.; Imamura, Y.; Tsubota, K.; Okano, H.; Oike, Y.; Ishida, S. Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes 2008, 57, 2191–2198. [Google Scholar] [CrossRef]
- Usui, T.; Sugisaki, K.; Iriyama, A.; Yokoo, S.; Yamagami, S.; Nagai, N.; Ishida, S.; Amano, S. Inhibition of corneal neovascularization by blocking the angiotensin II type 1 receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4370–4376. [Google Scholar] [CrossRef] [PubMed]
- Yaguchi, S.; Ogawa, Y.; Shimmura, S.; Kawakita, T.; Hatou, S.; Satofuka, S.; Nakamura, S.; Imada, T.; Miyashita, H.; Yoshida, S.; et al. Angiotensin II type 1 receptor antagonist attenuates lacrimal gland, lung, and liver fibrosis in a murine model of chronic graft-versus-host disease. PLoS ONE 2013, 8, e64724. [Google Scholar] [CrossRef]
- Costagliola, C.; Verolino, M.; De Rosa, M.L.; Iaccarino, G.; Ciancaglini, M.; Mastropasqua, L. Effect of oral losartan potassium administration on intraocular pressure in normotensive and glaucomatous human subjects. Exp. Eye Res. 2000, 71, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Podos, S.M.; Mittag, T.W.; Yokoyoma, T. Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp. Eye Res. 2005, 80, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Semba, K.; Namekata, K.; Guo, X.; Harada, C.; Harada, T.; Mitamura, Y. Renin-angiotensin system regulates neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014, 5, e1333. [Google Scholar] [CrossRef] [PubMed]
- White, A.J.; Heller, J.P.; Leung, J.; Tassoni, A.; Martin, K.R. Retinal ganglion cell neuroprotection by an angiotensin II blocker in an ex vivo retinal explant model. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Agarwal, R. Trabecular meshwork ECM remodeling in glaucoma: Could RAS be a target? Expert Opin. Ther. Targets 2018, 22, 629–638. [Google Scholar] [CrossRef]
- Vaajanen, A.; Vapaatalo, H. Local ocular renin-angiotensin system-A target for glaucoma therapy? Basic Clin. Pharmacol. Toxicol. 2011, 109, 217–224. [Google Scholar] [CrossRef]
- Holappa, M.; Vapaatalo, H.; Vaajanen, A. Local ocular renin-angiotensin-aldosterone system: Any connection with intraocular pressure? A comprehensive review. Ann. Med. 2020, 52, 1–16. [Google Scholar] [CrossRef]
- Olmesartan Ophthalmic-AdisInsight. Available online: https://adisinsight.springer.com/drugs/800015154 (accessed on 27 May 2020).
- Satofuka, S.; Ichihara, A.; Nagai, N.; Noda, K.; Ozawa, Y.; Fukamizu, A.; Tsubota, K.; Itoh, H.; Oike, Y.; Ishida, S. (Pro)renin receptor-mediated signal transduction and tissue renin-angiotensin system contribute to diabetes-induced retinal inflammation. Diabetes 2009, 58, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, A.; Sakoda, M.; Kurauchi-Mito, A.; Narita, T.; Kinouchi, K.; Murohashi-Bokuda, K.; Itoh, H. Possible roles of human (pro)renin receptor suggested by recent clinical and experimental findings. Hypertens Res. 2010, 33, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Satofuka, S.; Ichihara, A.; Nagai, N.; Noda, K.; Ozawa, Y.; Fukamizu, A.; Tsubota, K.; Itoh, H.; Oike, Y.; Ishida, S. (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin-angiotensin system. Am. J. Pathol. 2008, 173, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Ishida, S. (Pro)renin receptor: Involvement in diabetic retinopathy and development of molecular targeted therapy. J. Diabetes Investig. 2019, 10, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ichihara, A.; Kaneshiro, Y.; Inomata, K.; Sakoda, M.; Takemitsu, T.; Nishiyama, A.; Itoh, H. Regression of nephropathy developed in diabetes by (Pro)renin receptor blockade. J. Am. Soc. Nephrol. 2007, 18, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Noda, K.; Saito, W.; Ishida, S. (Pro)renin receptor is associated with angiogenic activity in proliferative diabetic retinopathy. Diabetologia 2012, 55, 3104–3113. [Google Scholar] [CrossRef]
- Kanda, A.; Noda, K.; Saito, W.; Ishida, S. Vitreous renin activity correlates with vascular endothelial growth factor in proliferative diabetic retinopathy. Br. J. Ophthalmol. 2013, 97, 666–668. [Google Scholar] [CrossRef]
- Hase, K.; Kanda, A.; Hirose, I.; Noda, K.; Ishida, S. Systemic factors related to soluble (pro)renin receptor in plasma of patients with proliferative diabetic retinopathy. PLoS ONE 2017, 12, e0189696. [Google Scholar] [CrossRef]
- Liu, Y.; Kanda, A.; Wu, D.; Ishizuka, E.T.; Kase, S.; Noda, K.; Ichihara, A.; Ishida, S. Suppression of Choroidal Neovascularization and Fibrosis by a Novel RNAi Therapeutic Agent against (Pro)renin Receptor. Mol. Ther. Nucleic Acids 2019, 17, 113–125. [Google Scholar] [CrossRef]
- Dong, Y.; Kanda, A.; Noda, K.; Saito, W.; Ishida, S. Pathologic Roles of Receptor-Associated Prorenin System in Idiopathic Epiretinal Membrane. Sci. Rep. 2017, 7, 44266. [Google Scholar] [CrossRef]
- Kanda, A.; Ishizuka, E.T.; Shibata, A.; Matsumoto, T.; Toyofuku, H.; Noda, K.; Namba, K.; Ishida, S. A Novel Single-Strand RNAi Therapeutic Agent Targeting the (Pro)renin Receptor Suppresses Ocular Inflammation. Mol. Ther. Nucleic Acids 2017, 7, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, E.T.; Kanda, A.; Kase, S.; Noda, K.; Ishida, S. Involvement of the receptor-associated prorenin system in the pathogenesis of human conjunctival lymphoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 74–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashizume, K.; Mashima, Y.; Fumayama, T.; Ohtake, Y.; Kimura, I.; Yoshida, K.; Ishikawa, K.; Yasuda, N.; Fujimaki, T.; Asaoka, R.; et al. Genetic polymorphisms in the angiotensin II receptor gene and their association with open-angle glaucoma in a Japanese population. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Pang, I.H.; Shade, D.L.; Clark, A.F.; Steely, H.T.; DeSantis, L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr. Eye Res. 1994, 13, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Sacca, S.C.; Cartiglia, C.; De Flora, S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am. J. Med. 2003, 114, 638–646. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Lerner, S.F.; Brunzini, R.; Reides, C.G.; Evelson, P.A.; Llesuy, S.F. Time course changes of oxidative stress markers in a rat experimental glaucoma model. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4635–4640. [Google Scholar] [CrossRef]
- Oshida, E.; Matsumoto, Y.; Arai, K. Free radicals in the aqueous humor of patients with glaucoma. Clin. Ophthalmol. 2010, 4, 653–660. [Google Scholar] [CrossRef][Green Version]
- Sorkhabi, R.; Ghorbanihaghjo, A.; Javadzadeh, A.; Rashtchizadeh, N.; Moharrery, M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol. Vis. 2011, 17, 41–46. [Google Scholar]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res. Rev. 2016, 29, 26–41. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Chan, P.S. Oxidative stress and diabetic retinopathy. Exp. Diabetes Res. 2007, 2007, 43603. [Google Scholar] [CrossRef]
- Kusaka, I.; Kusaka, G.; Zhou, C.; Ishikawa, M.; Nanda, A.; Granger, D.N.; Zhang, J.H.; Tang, J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2442–H2451. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Nakanishi, Y.; Hori, S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Br. J. Ophthalmol. 2002, 86, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Maruichi, M.; Oku, H.; Takai, S.; Muramatsu, M.; Sugiyama, T.; Imamura, Y.; Minami, M.; Ueki, M.; Satoh, B.; Sakaguchi, M.; et al. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: A possible involvement of chymase in the pathogenesis of macular hole patients. Curr. Eye Res. 2004, 29, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Fu, H.; Zhang, L.; Huang, H.; Luo, F.; Wu, W.; Guo, Y.; Liu, X. Angiotensin II upregulates the expression of placental growth factor in human vascular endothelial cells and smooth muscle cells. BMC Cell Biol. 2010, 11, 36. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | Age | Sex | Systemic Complications | Pre-Operative IOP (mmHg) | Surgical Samples | Purpose of Use |
|---|---|---|---|---|---|---|
| POAG | 80 | M | Myocardial infarction | 17 | AC fluid | ELISA |
| 75 | F | None | 27 | |||
| 50 | F | Internal carotid aneurysm | 15 | |||
| 50 | M | DL | 19 | |||
| 57 | F | None | 21 | |||
| 64 | M | None | 17 | |||
| 60 | M | None | 16 | |||
| 85 | F | HT | 14 | TM tissue | IF, IHC, RT-PCR | |
| 64 | F | None | 28 | |||
| 80 | F | HT | 10 | |||
| 57 | M | HT | 16 | |||
| NVG secondary to PDR | 76 | M | DM | 40 | AC fluid | ELISA |
| 55 | F | DM, DL, HT | 46 | |||
| 66 | F | DM | 29 | |||
| 68 | M | DM | 34 | |||
| 57 | M | DM, Maxillary sinusitis | 38 | |||
| 65 | M | DM, HT | 35 | |||
| 61 | F | DM, DN, HT | 29 | TM tissue | IF, IHC, RT-PCR | |
| 44 | M | DM, HT | 21 | |||
| 65 | F | DM, Megaloblastic anemia | 40 | |||
| 64 | F | DM, DN, HT | 21 | |||
| 67 | M | DM, DN, HT | 18 | |||
| Cataract | 78 | F | HT | 13 | AC fluid | ELISA |
| 64 | F | DL, HT | 13 | |||
| 73 | M | DL | 14 | |||
| 69 | M | HT | 19 | |||
| 76 | M | DL | 15 | |||
| 70 | M | DL | 15 | |||
| 65 | F | DL | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishizuka, E.T.; Kanda, A.; Shinmei, Y.; Ohguchi, T.; Tagawa, Y.; Hase, K.; Yamamoto, T.; Noda, K.; Chin, S.; Ishida, S. Receptor-Associated Prorenin System in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma and Neovascular Glaucoma. J. Clin. Med. 2020, 9, 2336. https://doi.org/10.3390/jcm9082336
Ishizuka ET, Kanda A, Shinmei Y, Ohguchi T, Tagawa Y, Hase K, Yamamoto T, Noda K, Chin S, Ishida S. Receptor-Associated Prorenin System in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma and Neovascular Glaucoma. Journal of Clinical Medicine. 2020; 9(8):2336. https://doi.org/10.3390/jcm9082336
Chicago/Turabian StyleIshizuka, Erdal Tan, Atsuhiro Kanda, Yasuhiro Shinmei, Takeshi Ohguchi, Yoshiaki Tagawa, Keitaro Hase, Taku Yamamoto, Kousuke Noda, Shinki Chin, and Susumu Ishida. 2020. "Receptor-Associated Prorenin System in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma and Neovascular Glaucoma" Journal of Clinical Medicine 9, no. 8: 2336. https://doi.org/10.3390/jcm9082336
APA StyleIshizuka, E. T., Kanda, A., Shinmei, Y., Ohguchi, T., Tagawa, Y., Hase, K., Yamamoto, T., Noda, K., Chin, S., & Ishida, S. (2020). Receptor-Associated Prorenin System in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma and Neovascular Glaucoma. Journal of Clinical Medicine, 9(8), 2336. https://doi.org/10.3390/jcm9082336





