The Multidimensional Daily Diary of Fatigue-Fibromyalgia-17 Items (MDF-Fibro-17): Evidence from Validity, Reliability and Transcultural Invariance between Portugal and Brazil
Abstract
1. Introduction
1.1. Fibromyalgia and Fatigue
1.2. Past Literature and Current Research
2. Experimental Section
2.1. Participants
2.2. Procedure: Data Collection
2.3. Instrument
2.4. Procedures: Translation of the Questionnaire
- Preliminary Translation: The first stage was carried out by researchers with the help of three Portuguese native bilingual Portuguese-English teachers. The English version of the questionnaire was translated into Portuguese, which resulted in the first draft;
- First Evaluation Panel: An analysis of the initial version of the MDF-fibro-17 Portuguese version was performed individually by Portuguese specialists from different areas, such as two medical doctors specialist in FM and four research specialists in psychometric instrument testing. The items received slight syntax and semantic modifications as proposed by the revisions and feedback;
- Second Evaluation Panel: A revised version of the questionnaire was sent again for evaluation to another panel formed by Brazilian specialists in the same categories as the previous ones (i.e., two medical doctors and four research specialists in psychometric validations). This panel examined all the items in the questionnaire and pointed out some small changes, which were accepted and carried out, so that a new version could be used for preliminary testing;
- Pilot study: The revised questionnaire was answered by a group of 50 patients with FM (22 from Portugal and 28 from Brazil), to determine if all items were clear and understandable. Data from these participants were not considered for psychometric testing of the MDF-fibro-17 nor for test-retest examination;
- Final revision: two Portuguese and two Brazilian teachers revised the final translated version of the MDF-fibro-17 to identify possible syntax, spelling and grammar issues. No differences were found in the semantic, spelling and syntax of the Portuguese version either by Portuguese teachers or by the Brazilian teachers. For this reason, the same measure in Portuguese from Portugal was applied to both samples.
2.5. Statistical Analysis
2.6. Multigroup Analysis
3. Results
3.1. Preliminary Analysis
3.2. Test-Retest Analysis
3.3. Descriptive Statistics, Internal Consistency and Convergent and Discriminant Validity
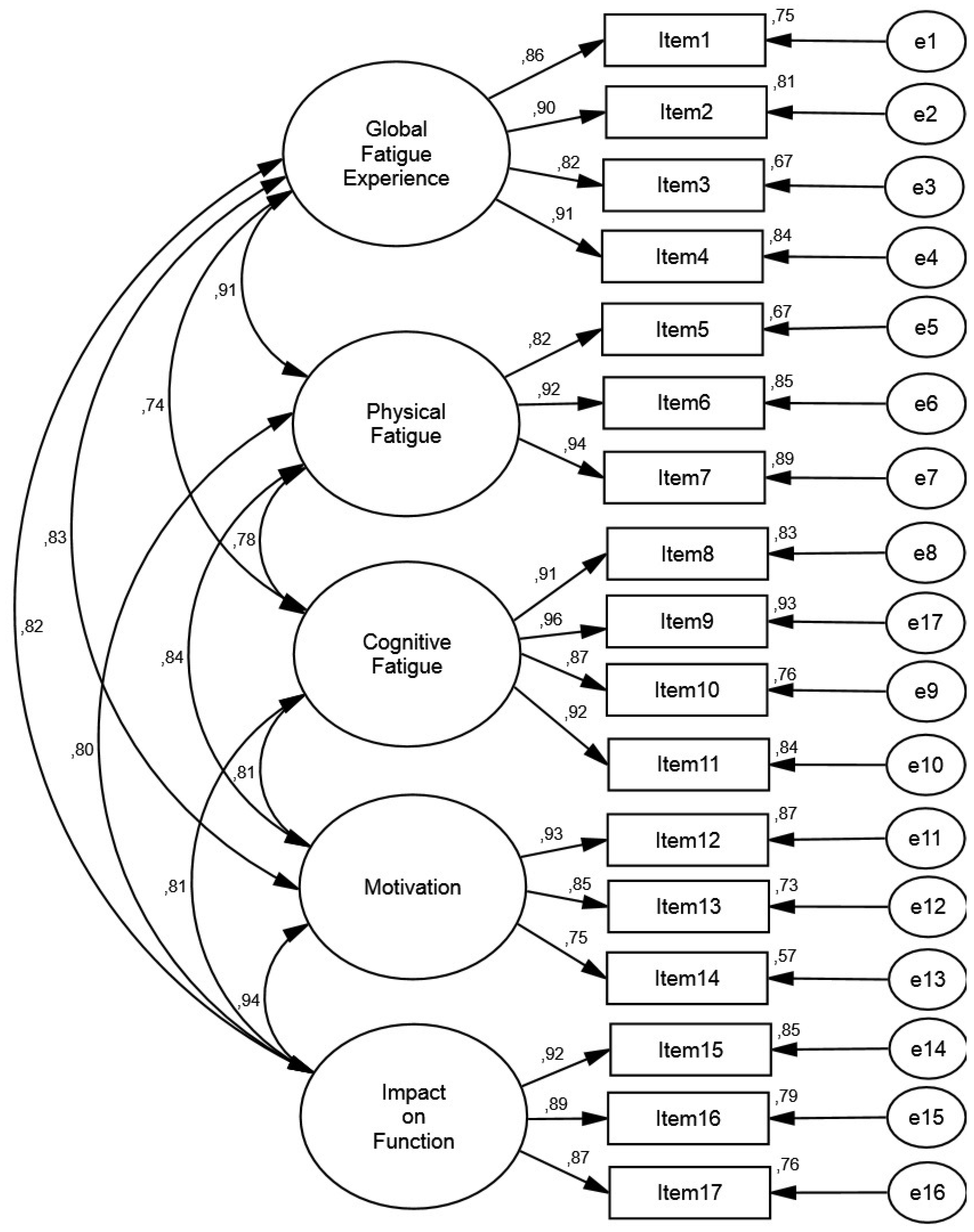
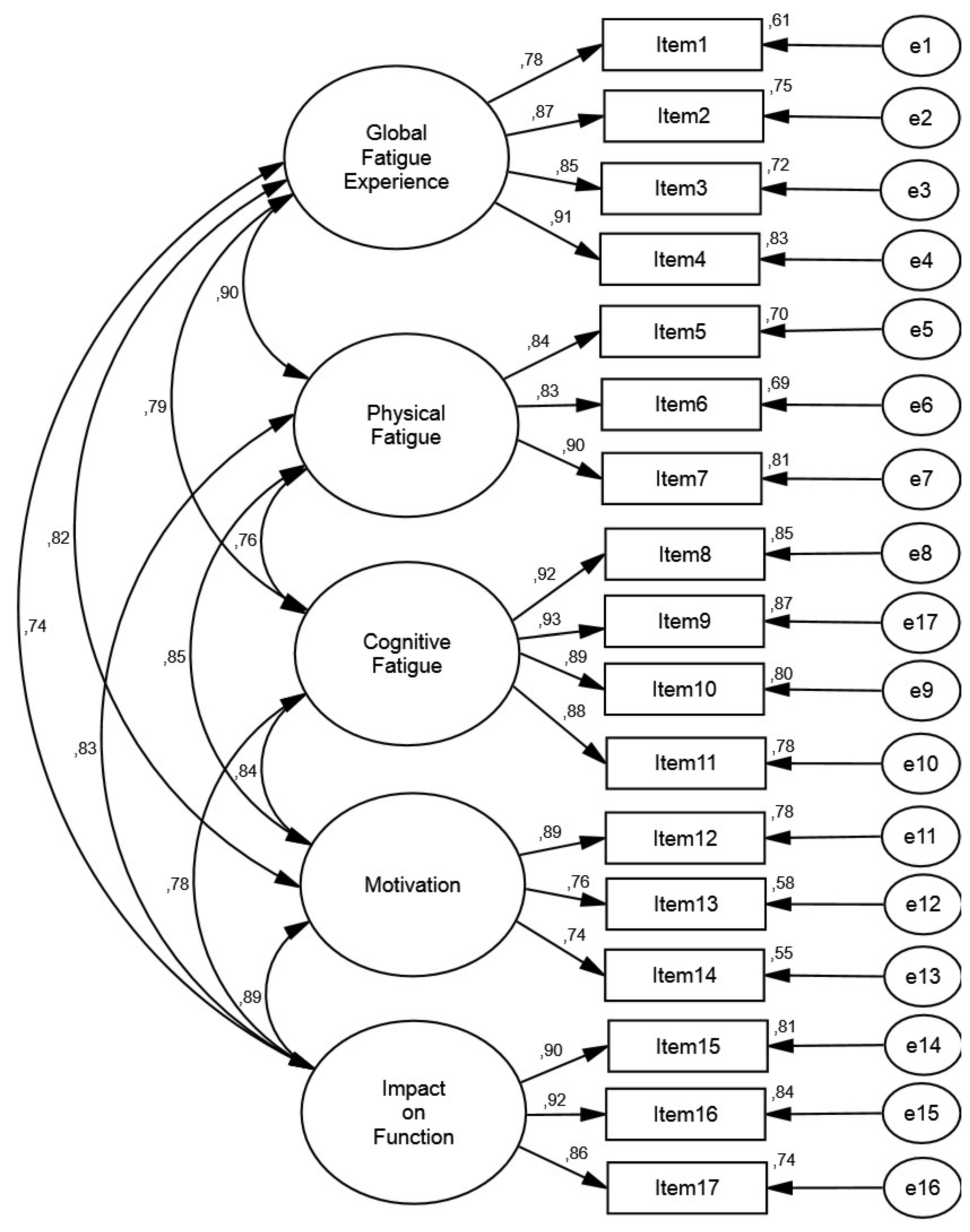
3.4. Confirmatory Factor Analysis
3.5. Measurement Invariance
4. Discussion
4.1. Factorial Validity of the MDF-Fibro-17
4.2. Measurement Invariance
5. Conclusions
5.1. Limitations
5.2. Practical Implications
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Albrecht, P.J.; Rice, F.L. Fibromyalgia syndrome pathology and environmental influences on afflictions with medically unexplained symptoms. Rev. Environ. Health 2016, 31, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.J.; Webber, S.C.; Brachaniec, M.; Bidonde, J.; Bello-Haas, V.D.; Danyliw, A.D.; Overend, T.J.; Richards, R.S.; Sawant, A.; Schachter, C.L. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011, 15, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hung, C.H.; Sun, W.Z.; Wu, W.T.; Lai, C.L.; Han, D.S.; Chen, C.C. Evaluating soreness symptoms of fibromyalgia: Establishment and validation of the Revised Fibromyalgia Impact Questionnaire with Integration of Soreness Assessment. J. Formos. Med. Assoc. 2020, 119, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Saral, I.; Sindel, D.; Esmaeilzadeh, S.; Sertel-Berk, H.O.; Oral, A. The effects of long- and short-term interdisciplinary treatment approaches in women with fibromyalgia: A randomized controlled trial. Rheumatol. Int. 2016, 36, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Assumpcao, A.; Matsutani, L.A.; Yuan, S.L.; Santo, A.S.; Sauer, J.; Mango, P.; Marques, A.P. Muscle stretching exercises and resistance training in fibromyalgia: Which is better? A three-arm randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 663–670. [Google Scholar] [CrossRef]
- Arnold, L.M.; Wang, F.; Ahl, J.; Gaynor, P.J.; Wohlreich, M.M. Improvement in multiple dimensions of fatigue in patients with fibromyalgia treated with duloxetine: Secondary analysis of a randomized, placebo-controlled trial. Arthritis Res. Ther. 2011, 13, R86. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575. [Google Scholar] [CrossRef]
- Humphrey, L.; Arbuckle, R.; Mease, P.; Williams, D.A.; Samsoe, B.D.; Gilbert, C. Fatigue in fibromyalgia: A conceptual model informed by patient interviews. BMC Musculoskelet. Disord. 2010, 11, 216. [Google Scholar] [CrossRef]
- Hudson, J.I.; Arnold, L.M.; Bradley, L.A.; Choy, E.H.; Mease, P.J.; Wang, F. What makes patients with fibromyalgia feel better? correlations between patient global impression of improvement and changes in clinical symptoms and function: A pooled analysis of 4 randomized placebo-controlled trials of duloxetine. J. Rheumatol. 2009, 36, 2517. [Google Scholar] [CrossRef]
- Morris, S.; Li, Y.; Smith, J.A.M.; Dube, S.; Burbridge, C.; Symonds, T. Multidimensional daily diary of fatigue-fibromyalgia-17 items (MDF-fibro-17). part 1: Development and content validity. BMC Musculoskelet. Disord. 2017, 18, 195. [Google Scholar] [CrossRef]
- Smets, E.M.A.; Garssen, B.; Bonke, B.; De Haes, J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef]
- Li, Y.; Morris, S.; Cole, J.; Dube’, S.; Smith, J.A.M.; Burbridge, C.; Symonds, T.; Hudgens, S.; Wang, W. Multidimensional daily diary of fatigue-fibromyalgia-17 items (MDF-fibro-17): Part 2 psychometric evaluation in fibromyalgia patients. BMC Musculoskelet. Disord. 2017, 18, 198. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Crofford, L.J.; Mease, P.J.; Burgess, S.M.; Palmer, S.C.; Abetz, L.; Martin, S.A. Patient perspectives on the impact of fibromyalgia. Patient Educ. Couns. 2008, 73, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dailey, D.L.; Keffala, V.J.; Sluka, K.A. Do cognitive and physical fatigue tasks enhance pain, cognitive fatigue, and physical fatigue in people with fibromyalgia? Arthritis Care Res. (Hoboken) 2015, 67, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; Arnold, L.M.; Bennett, R.; Boonen, A.; Buskila, D.; Carville, S. Fibromyalgia syndrome. J. Rheumatol. 2007, 34, 1415–1425. [Google Scholar]
- Mease, P.J.; Clauw, D.J.; Gendreau, R.M.; Rao, S.G.; Kranzler, J.; Chen, W.; Palmer, R.H. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J. Rheumatol. 2009, 36, 398–409. [Google Scholar] [CrossRef]
- Sass, D.A. Testing Measurement Invariance and Comparing Latent Factor Means Within a Confirmatory Factor Analysis Framework. J. Psychoeduc. Assess. 2011, 29, 347–363. [Google Scholar] [CrossRef]
- Cid, L.; Lettnin, C.; Stobaus, C.; Monteiro, D.; Davoglio, T.; Moutao, J. Cross-Cultural Validation of the Basic Psychological Needs in Physical Education Scale between Portugal and Brazil Samples. Span. J. Psychol. 2016, 19, E5. [Google Scholar] [CrossRef]
- Vlachopoulos, S.P.; Asci, F.H.; Cid, L.; Ersoz, G.; González-Cutre, D.; Moreno-Murcia, J.A.; Moutão, J. Cross-cultural Invariance of the Basic Psychological Needs in Exercise Scale and Latent Mean Differences Among Greek, Spanish, Portuguese, and Turkish Samples. Psychol. Exerc. Sport 2013, 14, 622–631. [Google Scholar] [CrossRef]
- He, J.; van de Vijver, F. Bias and equivalence in cross-cultural research. Online Read. Psychol. Cult. 2012, 2. [Google Scholar] [CrossRef]
- Karl, J.A.; Prado, S.M.M.; Gračanin, A.; Verhaeghen, P.; Ramos, A.; Mandal, S.P.; Michalak, J.; Zhang, C.Q.; Schmidt, C.; Tran, U.S.; et al. The Cross-cultural Validity of the Five-Facet Mindfulness Questionnaire Across 16 Countries. Mindfulness 2020. [Google Scholar] [CrossRef]
- EpiReuma. Estudo Epidemiológico das Doenças Reumáticas em Portugal. Soc. Port. Reumatol. 2011, 36, 203–204. [Google Scholar]
- Gomes, P.; Campos, C. Fibromialgia: Abordagem terapêutica. Rev. Port. Med. Geral E Fam. 2010, 26. [Google Scholar] [CrossRef]
- Branco, J.C.; Bannwarth, B.; Failde, I.; Abello Carbonell, J.; Blotman, F.; Spaeth, M.; Saraiva, F.; Nacci, F.; Thomas, E.; Caubere, J.P.; et al. Prevalence of fibromyalgia: A survey in five European countries. Semin Arthritis Rheum 2010, 39, 448–453. [Google Scholar] [CrossRef]
- Souza, J.B.; Perissinotti, D.M.N. The prevalence of fibromyalgia in Brazil—A population-based study with secondary data of the study on chronic pain prevalence in Brazil. Braz. J. Pain 2018, 1. [Google Scholar] [CrossRef]
- Byrne, B. Structural Equation Modeling with Mplus. Basic Concepts, Applications, and Programming (Multivariate Application Series), 1st ed.; Routledge/Taylor & Francis Group: New York, NY, USA, 2012. [Google Scholar]
- Cheung, G.W.; Rensvold, R.B. Evaluating Goodness-of-Fit Indexes for Testing Measurement Invariance. Struct. Equ. Model. Multidiscip. J. 2002, 9, 233–255. [Google Scholar] [CrossRef]
- Brislin, R.W. Translation and content analysis for oral and written material. In Handbook of Cross-Cultural Psychology; Triandis, H., Berry, J., Eds.; Allyn and Bacon: Needham Heights, MA, USA, 1980; Volume 2, pp. 389–444. [Google Scholar]
- Banville, D.; Desorosiers, P.; Genet-Volet, Y. Translating Questionnaires and Inventories Using a Cross-Cultural Translation Technique. J. Teach. Phys. Educ. 2000, 19, 374–387. [Google Scholar] [CrossRef]
- Hair, J.; Babin, B.; Anderson, R.; Black, W. Multivariate Data Analysis, 8th ed.; Pearson Educational, Inc.: Hoboken, NJ, USA, 2019; ISBN 978-1473756540. [Google Scholar]
- Arbuckle, J.L. IBM® SPSS® Amos(TM) 23 User Guide. 2014. Available online: ftp://public.dhe.ibm.com/software/analytics/spss/documentation/amos/23.0/en/Manuals/IBM_SPSS_Amos_User_Guide.pdf (accessed on 2 May 2020).
- Byrne, B. Structural Equation Modeling with AMOS. Basic Concepts, Applications, and Programming, 3rd ed.; Taylor & Francis Group, LLC.: New York, NY, USA, 2016; ISBN 978-1138797024. [Google Scholar]
- Marsh, H.W.; Hau, K.-T.; Wen, Z. In Search of Golden Rules: Comment on Hypothesis-Testing Approaches to Setting Cutoff Values for Fit Indexes and Dangers in Overgeneralizing Hu and Bentler‘s (1999) Findings. Struct. Equ. Model. Multidiscip. J. 2004, 11, 320–341. [Google Scholar] [CrossRef]
- Hair, J.; Black, W.; Anderson, R. Multivariate Data Analysis, 7th ed.; Pearson Educational, Inc.: Hoboken, NJ, USA, 2014; ISBN 978-0138132637. [Google Scholar]
- Chen, F.F. Sensitivity of Goodness of Fit Indexes to Lack of Measurement Invariance. Struct. Equ. Model. Multidiscip. J. 2007, 14, 464–504. [Google Scholar] [CrossRef]
- Nevitt, J.; Hancock, G. Performance of Bootstrapping Approaches to Model Test Statistics and Parameter Standard Error Estimation in Structural Equation Modeling. Struct. Equ. Model. Multidiscip. J. 2001, 8, 353–377. [Google Scholar] [CrossRef]
- Seo, S.R.; Park, D.J.; Kang, J.H.; Lee, J.W.; Lee, K.E.; Wen, L.; Kim, T.J.; Park, Y.W.; Lee, S.S. Cross-cultural adaptation of the Revised Korean version of the Fibromyalgia Impact Questionnaire: Its association with physical function and quality of life. Int. J. Rheum. Dis. 2015, 19, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Calvente, M.; Medina-Porqueres, I.; Fontalba-Navas, A.; Pena-Andreu, J.M.; de Vos-Martin, C. Validation and cross-cultural adaptation of the ‘Fibromyalgia Participation Questionnaire’ to the Spanish population: Study protocol. Rheumatol. Int. 2015, 35, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
| Items | M ± SD | r | p | Alpha |
|---|---|---|---|---|
| Item 1 Pre-Post | 7.24 ± 1.62–7.38 ± 1.77 | 0.83 | <0.001 | |
| Item 2 Pre-Post | 7.24 ± 1.68–7.72 ± 1.91 | 0.82 | <0.001 | |
| Item 3 Pre-Post | 7.88 ± 1.34–7.82 ± 1.56 | 0.86 | <0.001 | |
| Item 4 Pre-Post | 7.39 ± 1.71–7.74 ± 1.82 | 0.76 | <0.001 | |
| Item 5 Pre-Post | 7.48 ± 1.64–7.46 ± 2.05 | 0.81 | <0.001 | |
| Item 6 Pre-Post | 7.82 ± 1.79–8.03 ± 1.86 | 0.80 | <0.001 | |
| Item 7 Pre-Post | 7.70 ± 1.55–8.00 ± 1.72 | 0.81 | <0.001 | |
| Item 8 Pre-Post | 7.06 ± 2.16–7.33 ± 2.02 | 0.78 | <0.001 | |
| Item 9 Pre-Post | 7.06 ± 2.20–6.23 ± 2.04 | 0.76 | <0.001 | |
| Item 10 Pre-Post | 7.48 ± 2.03–7.69 ± 1.72 | 0.71 | <0.001 | |
| Item 11 Pre-Post | 7.27 ± 1.86–7.36 ± 1.97 | 0.81 | <0.001 | |
| Item 12 Pre-Post | 7.79 ± 1.45–7.90 ± 1.68 | 0.84 | <0.001 | |
| Item 13 Pre-Post | 7.89 ± 2.05–7.67 ± 2.19 | 0.76 | <0.001 | |
| Item 14 Pre-Post | 7.45 ± 2.18–7.56 ± 2.43 | 0.72 | <0.001 | |
| Item 15 Pre-Post | 7.64 ± 1.64–7.77 ± 1.95 | 0.89 | <0.001 | |
| Item 16 Pre-Post | 7.64 ± 1.95–7.51 ± 2.45 | 0.74 | <0.001 | |
| Item 17 Pre-Post | 7.85 ± 1.73–7.82 ± 2.32 | 0.86 | <0.001 | |
| Global Fatigue Experience Pre-Post | 7.44 ± 1.47–7.67 ± 1.58 | 0.87 | <0.001 | 0.77–0.78 |
| Physical Fatigue Pre-Post | 7.67 ± 1.47–7.83 ± 1.75 | 0.76 | <0.001 | 0.80–0.77 |
| Cognitive Fatigue Pre-Post | 7.22 ± 1.94–7.40 ± 1.83 | 0.84 | <0.001 | 0.81–0.83 |
| Motivation Pre-Post | 7.70 ± 1.61–7.71 ± 1.74 | 0.81 | <0.001 | 0.82–0.81 |
| Impact on Function Pre-Post | 7.71 ± 1.67–7.71 ± 2.18 | 0.74 | <0.001 | 0.78–0.77 |
| Items | M ± SD | r | p | Alpha |
|---|---|---|---|---|
| Item 1 Pre-Post | 6.39 ± 2.43–7.03 ± 2.34 | 0.80 | <0.001 | |
| Item 2 Pre-Post | 6.70 ± 2.16–7.32 ± 2.17 | 0.86 | <0.001 | |
| Item 3 Pre-Post | 7.04 ± 2.08–7.46 ± 2.18 | 0.80 | <0.001 | |
| Item 4 Pre-Post | 7.00 ± 2.52–7.59 ± 2.41 | 0.77 | <0.001 | |
| Item 5 Pre-Post | 7.43 ± 2.31–7.78 ± 2.12 | 0.84 | <0.001 | |
| Item 6 Pre-Post | 7.78 ± 2.28–8.05 ± 2.05 | 0.83 | <0.001 | |
| Item 7 Pre-Post | 7.74 ± 2.09–8.11 ± 1.98 | 0.84 | <0.001 | |
| Item 8 Pre-Post | 6.70 ± 2.34–7.51 ± 2.24 | 0.80 | <0.001 | |
| Item 9 Pre-Post | 6.65 ± 2.34–7.38 ± 2.67 | 0.79 | <0.001 | |
| Item 10 Pre-Post | 6.17 ± 2.64–7.14 ± 2.58 | 0.72 | <0.001 | |
| Item 11 Pre-Post | 6.43 ± 2.57–7.19 ± 2.45 | 0.83 | <0.001 | |
| Item 12 Pre-Post | 7.39 ± 2.25–7.89 ± 2.12 | 0.87 | <0.001 | |
| Item 13 Pre-Post | 7.43v1.95–7.89 ± 2.05 | 0.75 | <0.001 | |
| Item 14 Pre-Post | 7.22 ± 2.76–7.54 ± 2.56 | 0.73 | <0.001 | |
| Item 15 Pre-Post | 7.35 ± 1.99–7.95 ± 1.94 | 0.88 | <0.001 | |
| Item 16 Pre-Post | 7.00 ± 2.45–7.76 ± 2.35 | 0.71 | <0.001 | |
| Item 17 Pre-Post | 7.26 ±2.40–7.92 ± 2.29 | 0.84 | <0.001 | |
| Global Fatigue Experience Pre-Post | 6.78 ±2.06–7.35 ± 2.05 | 0.88 | <0.001 | 0.76–0.74 |
| Physical Fatigue Pre-Post | 7.65 ± 2.10–7.98 ± 1.44 | 0.79 | <0.001 | 0.81–0.79 |
| Cognitive Fatigue Pre-Post | 6.49 ± 2.37–7.30 ± 2.30 | 0.86 | <0.001 | 0.79–0.78 |
| Motivation Pre-Post | 7.35 ± 2.10–7.77 ± 2.03 | 0.85 | <0.001 | 0.80–0.81 |
| Impact on Function Pre-Post | 7.20 ± 2.18–7.87 ± 2.12 | 0.77 | <0.001 | 0.78–0.76 |
| Samples | M | SD | CR | AVE | r2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Portuguese | 1 | 2 | 3 | 4 | 5 | ||||
| (1) Global Fatigue Experience | 7.25 | 1.58 | 0.93 | 0.77 | 1 | - | - | - | - |
| (2) Physical Fatigue | 7.49 | 1.64 | 0.92 | 0.80 | 0.83 | 1 | - | - | - |
| (3) Cognitive Fatigue | 7.20 | 1.85 | 0.95 | 0.84 | 0.55 | 0.59 | 1 | - | - |
| (4) Motivation | 7.50 | 1.70 | 0.88 | 0.72 | 0.69 | 0.71 | 0.65 | 1 | - |
| (5) Impact on Function | 7.45 | 1.83 | 0.92 | 0.80 | 0.67 | 0.65 | 0.65 | 0.89 | 1 |
| Brazilian | |||||||||
| (1) Global Fatigue Experience | 7.72 | 1.73 | 0.92 | 0.73 | 1 | - | - | - | - |
| (2) Physical Fatigue | 8.22 | 1.58 | 0.89 | 0.73 | 0.80 | 1 | - | - | - |
| (3) Cognitive Fatigue | 7.88 | 1.82 | 0.95 | 0.83 | 0.63 | 0.58 | 1 | - | - |
| (4) Motivation | 8.19 | 1.71 | 0.84 | 0.64 | 0.67 | 0.72 | 0.70 | 1 | - |
| (5) Impact on Function | 8.26 | 1.80 | 0.92 | 0.80 | 0.55 | 0.70 | 0.61 | 0.80 | 1 |
| Models | χ2 | df | B-S p | CFI | TLI | SRMR | RMSEA | RMSEA CI90% |
|---|---|---|---|---|---|---|---|---|
| MDF-fibro-17- PT version | 369.21 | 109 | <0.001 | 0.954 | 0.943 | 0.030 | 0.080 | 0.076–0.082 |
| MDF-fibro-17- BR version | 381.48 | 109 | 0.002 | 0.962 | 0.953 | 0.026 | 0.076 | 0.068–0.085 |
| MDF-fibro-17- Original version | 213.43 | 109 | - | 0.950 | 0.930 | 0.025 | 0.071 | 0.109–0.186 |
| Model | χ2 | df | Δχ2 | Δdf | p | CFI | ΔCFI | RMSEA | ΔRMSEA | SRMR | ΔSRMR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Configural Invariance | 750.94 | 218 | - | - | - | 0.959 | - | 0.058 | - | 0.030 | |
| Metric Invariance | 769.69 | 230 | 18,755 | 12 | 0.095 | 0.958 | 0.001 | 0.057 | 0.001 | 0.029 | 0.009 |
| Scale Invariance | 817.25 | 245 | 66,313 | 27 | <0.001 | 0.956 | 0.003 | 0.057 | 0.001 | 0.034 | 0.004 |
| Residual Invariance | 968.21 | 262 | 217.27 | 44 | <0.001 | 0.945 | 0.014 | 0.061 | 0.003 | 0.045 | 0.015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.C.; Albuquerque, M.L.L.; Neiva, H.P.; Cid, L.; Rodrigues, F.; Teixeira, D.S.; Monteiro, D. The Multidimensional Daily Diary of Fatigue-Fibromyalgia-17 Items (MDF-Fibro-17): Evidence from Validity, Reliability and Transcultural Invariance between Portugal and Brazil. J. Clin. Med. 2020, 9, 2330. https://doi.org/10.3390/jcm9082330
Álvarez MC, Albuquerque MLL, Neiva HP, Cid L, Rodrigues F, Teixeira DS, Monteiro D. The Multidimensional Daily Diary of Fatigue-Fibromyalgia-17 Items (MDF-Fibro-17): Evidence from Validity, Reliability and Transcultural Invariance between Portugal and Brazil. Journal of Clinical Medicine. 2020; 9(8):2330. https://doi.org/10.3390/jcm9082330
Chicago/Turabian StyleÁlvarez, Marcos C., Maria Luiza L. Albuquerque, Henrique P. Neiva, Luís Cid, Filipe Rodrigues, Diogo S. Teixeira, and Diogo Monteiro. 2020. "The Multidimensional Daily Diary of Fatigue-Fibromyalgia-17 Items (MDF-Fibro-17): Evidence from Validity, Reliability and Transcultural Invariance between Portugal and Brazil" Journal of Clinical Medicine 9, no. 8: 2330. https://doi.org/10.3390/jcm9082330
APA StyleÁlvarez, M. C., Albuquerque, M. L. L., Neiva, H. P., Cid, L., Rodrigues, F., Teixeira, D. S., & Monteiro, D. (2020). The Multidimensional Daily Diary of Fatigue-Fibromyalgia-17 Items (MDF-Fibro-17): Evidence from Validity, Reliability and Transcultural Invariance between Portugal and Brazil. Journal of Clinical Medicine, 9(8), 2330. https://doi.org/10.3390/jcm9082330








