Prevalence, Determinants, and Prognostic Significance of Hospital Acquired Pneumonia in Patients with Acute Heart Failure
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Blood Sampling Measurement
2.3. Definition of HAP, Worsening Heart Failure and Worsening Renal Failure
2.4. Clinical Outcomes
2.5. Statistical Analyzes
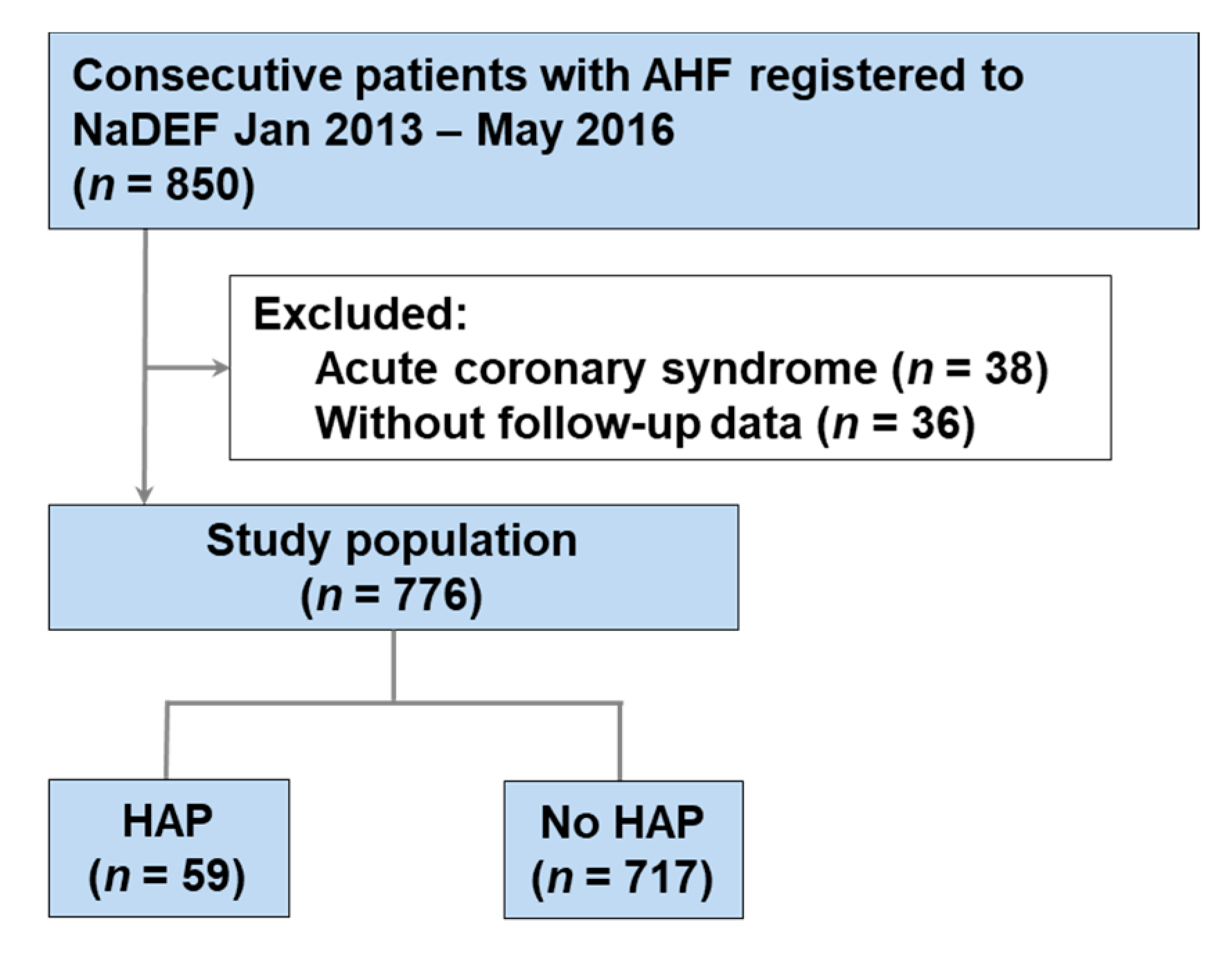
3. Results
3.1. Study Population and Baseline Characteristics
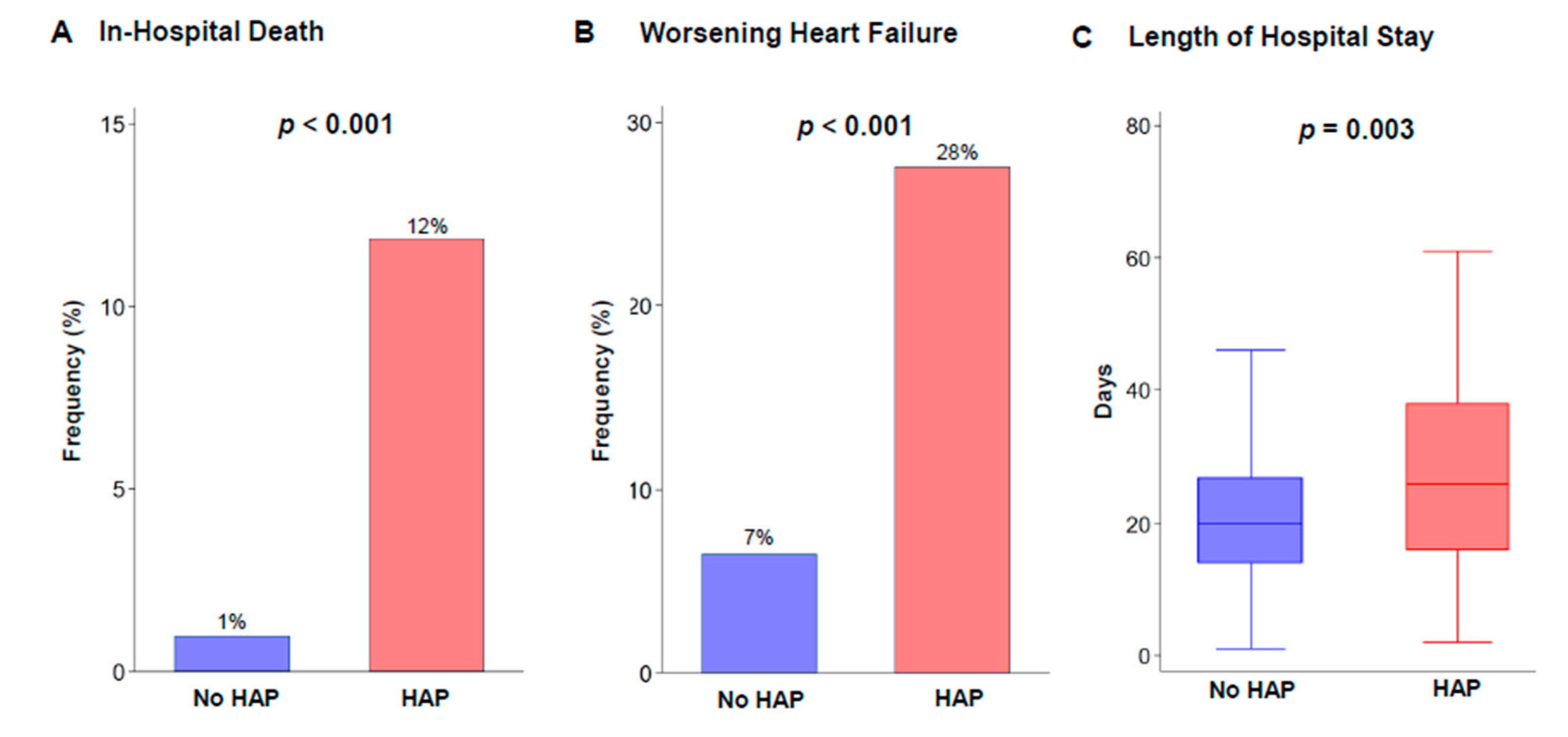
3.2. Clinical Outcomes During Hospitalization
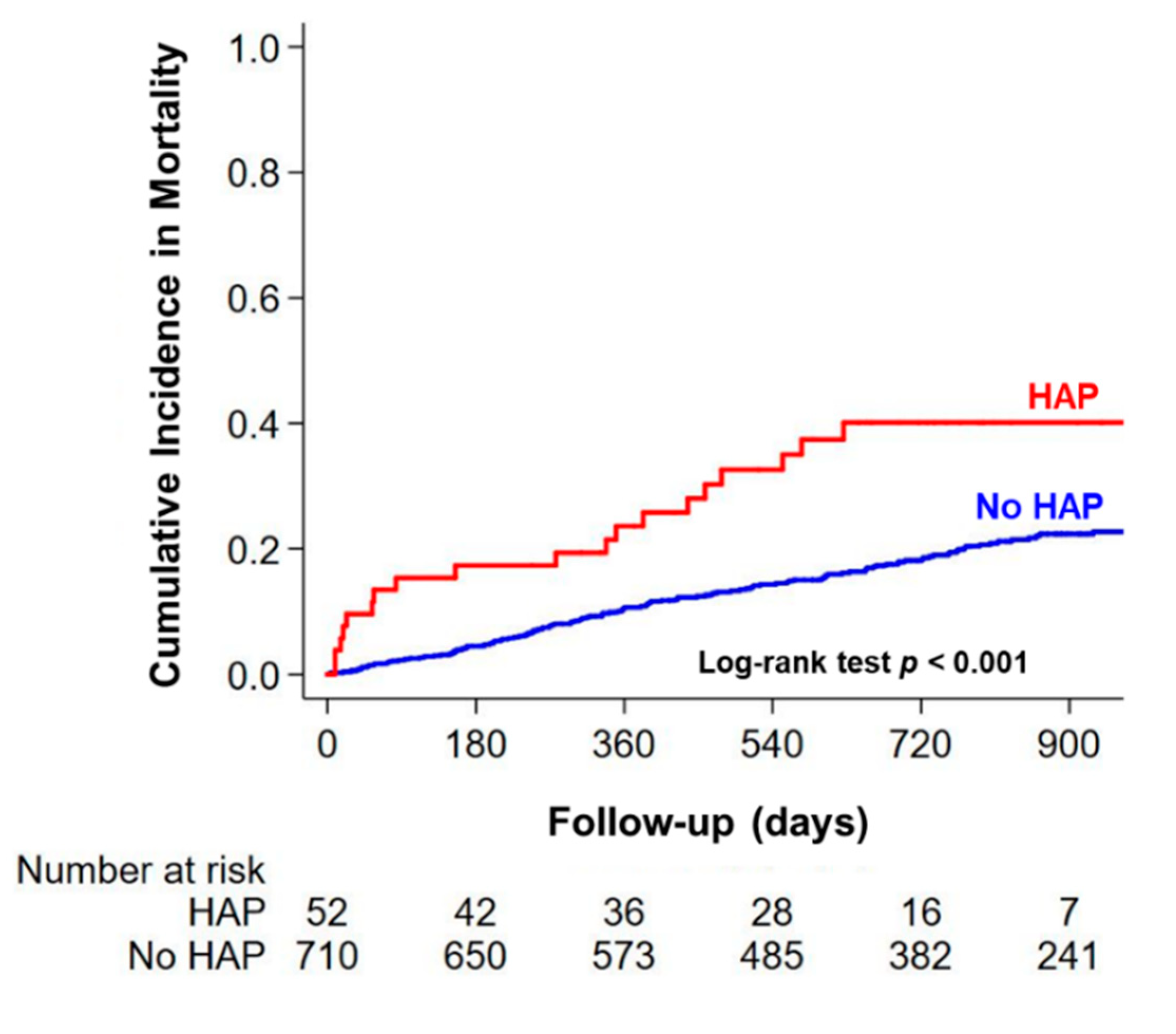
3.3. Long-Term Clinical Outcomes after Discharge and Determinants of HAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murad, K.; Kitzman, D.W. Frailty and multiple comorbidities in the elderly patient with heart failure: Implications for management. Heart Fail. Rev. 2012, 17, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, H.; Miura, M.; Nochioka, K.; Sakata, Y. Heart failure as a general pandemic in Asia. Eur. J. Heart Fail. 2015, 17, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American heart association. Circulation 2015, 131. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.K.; Baker, D.; Quinn, B. The epidemiology of nonventilator hospital-acquired pneumonia in the United States. Am. J. Infect Control 2018, 46, 322–327. [Google Scholar] [CrossRef]
- Rotstein, C.; Evans, G.; Born, A.; Grossman, R.; Light, R.B.; Magder, S.; McTaggart, B.; Weiss, K.; Zhanel, G.G. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 19–53. [Google Scholar] [CrossRef]
- Sopena, N.; Sabrià, M. Multicenter study of hospital-acquired pneumonia in non-icu patients. Chest 2005, 127, 213–219. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care–associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015, 313, 264. [Google Scholar] [CrossRef]
- Nagai, T.; Nishimura, K.; Honma, T.; Higashiyama, A.; Sugano, Y.; Nakai, M.; Honda, S.; Iwakami, N.; Okada, A.; Kawakami, S.; et al. Prognostic significance of endogenous erythropoietin in long-term outcome of patients with acute decompensated heart failure. Eur. J. Heart Fail. 2016, 18, 803–813. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The natural history of congestive heart failure: The framingham study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2013, 61, e78–e140. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, N.; Nagai, T.; Furukawa, T.A.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Prognostic value of malnutrition assessed by controlling nutritional status score for long-term mortality in patients with acute heart failure. Int. J. Cardiol. 2017, 230, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulibarri, J.; Gonzalez-Madrono, A.; de Villar, N.G.; Gonzalez, P.; Gonzalez, B.; Mancha, A.; Rodriguez, F.; Fernandez, G. Conut: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Niederman, M.S.; Craven, D.E.; Bonten, M.J. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Massie, B.M.; O’onnor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Weatherley, B.D.; Cleland, J.G.F.; Givertz, M.M.; Voors, A.; et al. Rolofylline, an adenosine a1−receptor antagonist, in acute heart failure. N. Engl. J. Med. 2010, 363, 1419–1428. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F., Jr.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Forman, D.E.; Butler, J.; Wang, Y.; Abraham, W.T.; O’Connor, C.M.; Gottlieb, S.S.; Loh, E.; Massie, B.M.; Rich, M.W.; Stevenson, L.W.; et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004, 43, 61–67. [Google Scholar] [CrossRef]
- Cowie, M.R.; Komajda, M.; Murray-Thomas, T.; Underwood, J.; Ticho, B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of the prospective outcomes study in heart failure (POSH). Eur. Heart J. 2006, 27, 1216–1222. [Google Scholar] [CrossRef]
- Harrell, F.E., Jr.; Califf, R.M.; Pryor, D.B.; Lee, K.L.; Rosati, R.A. Evaluating the yield of medical tests. JAMA 1982, 247, 2543–2546. [Google Scholar] [CrossRef]
- Walaszek, M.; Kosiarska, A.; Gniadek, A.; Kolpa, M.; Wolak, Z.; Dobros, W.; Siadek, J. The risk factors for hospital-acquired pneumonia in the intensive care unit. Przegl. Epidemiol. 2016, 70, 15–20. [Google Scholar]
- Chawla, R. Epidemiology, etiology, and diagnosis of hospital-acquired pneumonia and ventilator-associated pneumonia in Asian countries. Am. J. Infect. Control 2008, 36, S93–S100. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Hospital-acquired pneumonia. Chest 2001, 119, 373S–384S. [Google Scholar] [CrossRef]
- Braunwald, E. Heart failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, R.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Tsukita, Y.; Nihei, M.; Sugiura, H.; Niu, K.; Ebihara, T.; Ichinose, M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachexia Sarcopenia Muscle 2018, 9, 643–653. [Google Scholar] [CrossRef]
- Merx, M.W.; Weber, C. Sepsis and the heart. Circulation 2007, 116, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Van Der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Wells, G.A.; Chirinos, J.A.; Chen, L.; Fine, M.J. Cardiac complications in patients with community-acquired pneumonia. Circulation 2012, 125, 773–781. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Parrillo, J.E.; Parker, M.M.; Natanson, C.; Suffredini, A.F.; Danner, R.L.; Cunnion, R.E.; Ognibene, F.P. Septic shock in humans: Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 1990, 113, 227–242. [Google Scholar] [CrossRef]
- Mankowski, R.T.; Yende, S.; Angus, D.C. Long-term impact of sepsis on cardiovascular health. Intensive Care Med. 2019, 45, 78–81. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Mayr, F.; Kellum, J.A.; Weissfeld, L.; Kaynar, A.M.; Young, T.; Irani, K.; Angus, D.C.; Gen, I.M.S.I. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS ONE 2011, 6, e22847. [Google Scholar] [CrossRef]
- Hamatani, Y.; Nagai, T.; Nakai, M.; Nishimura, K.; Honda, Y.; Nakano, H.; Honda, S.; Iwakami, N.; Sugano, Y.; Asaumi, Y.; et al. Elevated plasma d-dimer level is associated with short-term risk of ischemic stroke in patients with acute heart failure. Stroke 2018, 49, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Lacerna, C.C.; Patey, D.; Block, L.; Naik, S.; Kevorkova, Y.; Galin, J.; Parker, M.; Betts, R.; Parodi, S.; Witt, D. A successful program preventing nonventilator hospital-acquired pneumonia in a large hospital system. Infect. Control Hosp. Epidemiol. 2020, 41, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Passaro, L.; Harbarth, S.; Landelle, C. Prevention of hospital-acquired pneumonia in non-ventilated adult patients: A narrative review. Antimicrob. Resist. Infect. Control 2016, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Palli, C.; Fandler, S.; Doppelhofer, K.; Niederkorn, K.; Enzinger, C.; Vetta, C.; Trampusch, E.; Schmidt, R.; Fazekas, F.; Gattringer, T. Early dysphagia screening by trained nurses reduces pneumonia rate in stroke patients. Stroke 2017, 48, 2583–2585. [Google Scholar] [CrossRef]
- Kommuri, N.V.; Koelling, T.M.; Hummel, S.L. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am. J. Med. 2012, 125, 209.e9–209.e15. [Google Scholar] [CrossRef]
| Variable | Overall | HAP | No HAP | p-Value |
|---|---|---|---|---|
| Number | 776 | 59 | 717 | - |
| Age, year | 75 ± 12 | 79 ± 9 | 75 ± 12 | 0.011 |
| Male, n (%) | 467 (60) | 44 (75) | 423 (59) | 0.019 |
| Body mass index, kg/m2 | 22.7 (20.3−25.4) | 21.9 (19.45−24.25) | 22.8 (20.4−25.5) | 0.109 |
| NYHA class III or IV, n (%) | 592 (89) | 45 (96) | 574 (89) | 0.138 |
| Ischemic etiology, n (%) | 185 (24) | 20 (34) | 165 (23) | 0.059 |
| LVEF, % | 38 ± 17 | 41 ± 18 | 38 ± 17 | 0.24 |
| Past History, n (%) | ||||
| Hypertension | 545 (74) | 50 (85) | 495 (73) | 0.051 |
| Diabetes | 266 (36) | 18 (31) | 248 (37) | 0.34 |
| HF admission | 333 (45) | 28 (47) | 305 (45) | 0.72 |
| Atrial arrhythmia | 386 (52) | 36 (61) | 350 (52) | 0.169 |
| Cerebrovascular disease | 196 (27) | 19 (32) | 177 (26) | 0.32 |
| Malignancy | 111 (15) | 11 (19) | 100 (15) | 0.42 |
| Chronic kidney disease | 378 (52) | 32 (54) | 346 (51) | 0.67 |
| COPD | 33 (4) | 5 (8) | 28 (4) | 0.123 |
| Etiology, n (%) | ||||
| ICM | 185 (24) | 20 (34) | 165 (23) | 0.059 |
| DCM | 81 (10) | 3 (5) | 78 (11) | 0.162 |
| HHD | 182 (24) | 10 (17) | 172 (24) | 0.22 |
| Other | 328 (42) | 26 (44) | 302 (42) | 0.77 |
| Current smoking, n (%) | 77 (19) | 4 (11) | 73 (20) | 0.21 |
| Habitual drinking, n (%) | 164 (47) | 12 (44) | 152 (48) | 0.76 |
| Systolic BP on admission, mm Hg | 140 ± 32 | 139 ± 32 | 140 ± 32 | 0.90 |
| Heart rate on admission, beat/min | 92 ± 28 | 96 ± 28 | 91 ± 28 | 0.25 |
| Laboratory Data on Admission | ||||
| White blood cell count, 103/μL | 6.4 (5.1−8.4) | 8.3 (6.0−12.4) | 6.4 (5.1−8.1) | <0.001 |
| CRP, mg/dL | 0.42 (0.14−1.41) | 2.18 (0.65−6.17) | 0.38 (0.13−1.14) | <0.001 |
| Serum albumin, g/dL | 3.8 ± 0.4 | 3.6 ± 0.5 | 3.8 ± 0.4 | 0.004 |
| Total bilirubin, mg/dL | 0.7 (0.5−1.1) | 0.7 (0.4−1.0) | 0.7 (0.5−1.1) | 0.159 |
| Sodium, mEq/L | 139.5 ± 4.2 | 139.3 ± 4.5 | 139.6 ± 4.2 | 0.65 |
| BNP, pg/mL | 600 (323−1116) | 736 (230−1395) | 595 (326−1092) | 0.63 |
| Troponin T, ng/mL | 0.04 (0.02−0.07) | 0.04 (0.03−0.09) | 0.04 (0.02−0.07) | 0.051 |
| Creatinine, mg/dL | 1.1 (0.9−1.6) | 1.2 (0.9−1.9) | 1.1 (0.9−1.5) | 0.042 |
| BUN, mg/dL | 23 (17−33) | 25 (18−41) | 23 (17−32) | 0.093 |
| eGFR, mL/min/1.73m2 | 60.0 (39.2−78.2) | 50.0 (34.7−72.1) | 60.8 (40.3−78.4) | 0.100 |
| Hemoglobin, g/dL | 12.0 ± 2.1 | 11.4 ± 2.3 | 12.1 ± 2.1 | 0.033 |
| Blood glucose, mg/dL | 126 (106−166) | 130 (103−195) | 126 (106−164) | 0.50 |
| HbA1c, % | 6.2 ± 1.0 | 6.2 ± 1.0 | 6.2 ± 1.0 | 0.59 |
| D-dimer, mg/dL | 2.0 (1.1−4.0) | 3.0 (1.4−7.1) | 2.0 (1.1−3.9) | 0.009 |
| CONUT score | 2.0 (2.0−4.0) | 4.0 (2.0−6.0) | 2.0 (2.0−4.0) | <0.001 |
| Medications before Admission, n (%) | ||||
| ACE inhibitors/ARBs | 384 (49) | 28 (47) | 356 (50) | 0.75 |
| Beta-blockers | 383 (49) | 26 (44) | 357 (50) | 0.40 |
| Diuretics | 414 (53) | 33 (56) | 381 (53) | 0.68 |
| Spironolactone | 149 (19) | 15 (25) | 134 (19) | 0.21 |
| Medications at Discharge, n (%) | ||||
| ACE inhibitors/ARBs | 512 (71) | 33 (63) | 479 (71) | 0.22 |
| Beta-blockers | 520 (72) | 32 (62) | 488 (73) | 0.077 |
| Diuretics | 598 (83) | 41 (79) | 557 (83) | 0.43 |
| Spironolactone | 285 (40) | 17 (33) | 268 (40) | 0.34 |
| Intravenous Treatments, n (%) | ||||
| Loop diuretics | 554 (71) | 49 (83) | 505 (70) | 0.039 |
| Vasodilators | 627 (81) | 52 (88) | 575 (80) | 0.137 |
| Inotropes | 103 (13) | 7 (12) | 96 (13) | 0.74 |
| Dose of intravenous loop diuretics, mg/day | 20 (20−40) | 20 (20−40) | 20 (20−40) | 0.186 |
| CICU admission, n (%) | 177 (23) | 24 (41) | 153 (21) | 0.001 |
| WRF during hospitalization, n (%) | 325 (42) | 33 (50) | 292 (42) | 0.190 |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| HAP | 5.69 (2.97−10.9) | <0.001 | 4.91 (2.44−9.89) | <0.001 |
| eGFR, mL/min/1.73 m2 | 0.96 (0.96−0.98) | <0.001 | 0.98 (0.96−0.99) | 0.004 |
| Systolic BP, 10 mm Hg | 0.85 (0.77−0.93) | 0.001 | 0.88 (0.79−0.97) | 0.011 |
| Log BNP, pg/mL | 1.55 (1.15−2.10) | 0.004 | 1.28 (0.94−1.74) | 0.122 |
| Serum sodium, mEq/L | 0.94 (0.88−0.99) | 0.018 | 0.96 (0.91−1.02) | 0.39 |
| LVEF, % | 0.99 (0.98−1.01) | 0.28 | Not selected | - |
| History of cerebrovascular disease | 0.59 (0.30−1.17) | 0.130 | Not selected | - |
| Serum albumin, 0.2 g/dL | 0.91 (0.81−1.02) | 0.108 | Not selected | - |
| NYHA class III or IV | 1.31 (0.84−2.06) | 0.23 | Not selected | - |
| Age, year | 0.99 (0.97−1.01) | 0.51 | Not selected | - |
| Sex, male | 0.92 (0.54−1.57) | 0.76 | Not selected | - |
| Body mass index, kg/m2 | 0.98 (0.92−1.04) | 0.49 | Not selected | - |
| Variables | Univariable Analysis | Multivariable Analysis: Model 1 | Multivariable Analysis: Model 2 | Multivariable Analysis: Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| HAP | 2.45 (1.51−3.97) | <0.001 | 1.86 (1.08−3.19) | 0.025 | 1.47 (0.77−2.79) | 0.024 | 2.14 (1.31−3.49) | 0.002 |
| Serum albumin, 0.2 g/dL | 0.78 (0.73−0.84) | <0.001 | 0.82 (0.76−0.89) | <0.001 | 0.80 (0.74−0.88) | <0.001 | 0.79 (0.74−0.85) | <0.001 |
| Prior HF admission | 2.90 (2.05−4.10) | <0.001 | 2.04 (1.40−2.97) | <0.001 | 1.76 (1.17−2.65) | 0.007 | 2.41 (1.69−3.43) | <0.001 |
| eGFR, mL/min/1.73 m2 | 0.98 (0.98−0.99) | <0.001 | 0.99 (0.98−0.997) | 0.006 | 0.99 (0.98−0.99) | <0.001 | 0.99 (0.98−0.994) | <0.001 |
| Serum sodium, mEq/L | 0.93 (0.90−0.97) | <0.001 | 0.96 (0.92−0.997) | 0.033 | 0.96 (0.92−0.998) | 0.0042 | 0.96 (0.93−0.99) | 0.024 |
| Use of Loop diuretics | 0.78 (0.56−1.09) | 0.142 | Not selected | - | Not selected | - | 0.73 (0.51−1.03) | 0.077 |
| Heart rate, 10 beat/min | 0.92 (0.86−0.97) | 0.006 | 0.99 (0.92−1.06) | 0.70 | 0.97 (0.89−1.05) | 0.48 | Not selected | - |
| Body mass index, kg/m2 | 0.88 (0.84−0.92) | <0.001 | 0.92 (0.88−0.97) | 0.002 | 0.92 (0.87−0.97) | 0.004 | Not selected | - |
| Systolic BP, 10 mmHg | 0.90 (0.85−0.96) | <0.001 | 0.97 (0.91−1.03) | 0.31 | 0.99 (0.93−1.06) | 0.80 | Not selected | - |
| Age, year | 1.03 (1.02−1.05) | <0.001 | 1.02 (0.997−1.04) | 0.096 | 1.01 (0.99−1.03) | 0.45 | Not selected | - |
| Log BNP, pg/mL | 1.38 (1.16−1.65) | <0.001 | 1.07 (0.88−1.29) | 0.49 | 1.03 (0.83−1.29) | 0.76 | Not selected | - |
| History of COPD | 2.31 (1.31−4.09) | 0.004 | 1.69 (0.92−3.10) | 0.090 | 1.82 (0.90−3.66) | 0.093 | Not selected | - |
| Hemoglobin, g/dL | 0.83 (0.76−0.89) | <0.001 | 0.996 (0.91−1.09) | 0.93 | 0.98 (0.89−1.09) | 0.72 | Not selected | - |
| CRP, mg/dL | 1.06 (1.02−1.10) | 0.004 | 1.00 (0.96−1.05) | 0.89 | 1.00 (0.94−1.05) | 0.88 | Not selected | - |
| LVEF ≥ 50% | 0.85 (0.58−1.24) | 0.40 | Not selected | - | 0.85 (0.54−1.35) | 0.49 | Not selected | - |
| LVEF, % | 1.00 (0.99−1.01) | 0.80 | Not selected | - | Not selected | - | Not selected | - |
| WBC, 103/μL | 0.96 (0.90−1.02) | 0.157 | Not selected | - | Not selected | - | Not selected | - |
| ICM | 0.87 (0.59−1.27) | 0.47 | Not selected | - | Not selected | - | Not selected | - |
| DCM | 0.90 (0.69−1.17) | 0.44 | Not selected | - | Not selected | - | Not selected | - |
| HHD | 1.09 (0.97−1.26) | 0.153 | Not selected | - | Not selected | - | Not selected | - |
| NYHA class III or IV | 1.26 (0.71−2.24) | 0.43 | Not selected | - | Not selected | - | Not selected | - |
| Sex, male | 1.20 (0.86−1.67) | 0.29 | Not selected | - | Not selected | - | Not selected | - |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Age, year | 1.04 (1.01−1.06) | 0.011 | 1.04 (1.01−1.08) | 0.006 |
| Sex, male | 2.04 (1.11−3.73) | 0.021 | 2.21 (1.14−4.28) | 0.019 |
| WBC, 103/μL | 1.20 (1.121.29) | <0.001 | 1.18 (1.09−1.29) | <0.001 |
| CRP, mg/dL | 1.15 (1.09−1.21) | <0.001 | 1.08 (1.01−1.16) | 0.017 |
| Serum albumin, 0.2 g/dL | 0.84 (0.75−0.95) | 0.004 | 0.95 (0.83−1.09) | 0.45 |
| Creatinine, mg/dL | 1.16 (0.995−1.36) | 0.051 | 1.08 (0.88−1.33) | 0.46 |
| Hemoglobin, g/dL | 0.87 (0.77−0.99) | 0.033 | 0.93 (0.80−1.08) | 0.34 |
| D-dimer, mg/dL | 1.01 (0.99−1.02) | 0.33 | Not selected | - |
| Body mass index, kg/m2 | 0.97 (0.90−1.03) | 0.32 | Not selected | - |
| History of COPD | 2.14 (0.80−5.77) | 0.132 | Not selected | - |
| History of diabetes | 0.76 (0.43−1.35) | 0.35 | Not selected | - |
| History of cerebrovascular disease | 1.33 (0.75−2.37) | 0.32 | Not selected | - |
| Systolic BP, 10 mm Hg | 0.99 (0.91−1.08) | 0.90 | Not selected | - |
| Log BNP, pg/mL | 1.03 (0.78−1.37) | 0.81 | Not selected | - |
| LVEF, % | 1.01 (0.99−1.03) | 0.24 | Not selected | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, A.; Omote, K.; Nagai, T.; Honda, Y.; Nakano, H.; Honda, S.; Iwakami, N.; Hamatani, Y.; Nakai, M.; Nishimura, K.; et al. Prevalence, Determinants, and Prognostic Significance of Hospital Acquired Pneumonia in Patients with Acute Heart Failure. J. Clin. Med. 2020, 9, 2219. https://doi.org/10.3390/jcm9072219
Tada A, Omote K, Nagai T, Honda Y, Nakano H, Honda S, Iwakami N, Hamatani Y, Nakai M, Nishimura K, et al. Prevalence, Determinants, and Prognostic Significance of Hospital Acquired Pneumonia in Patients with Acute Heart Failure. Journal of Clinical Medicine. 2020; 9(7):2219. https://doi.org/10.3390/jcm9072219
Chicago/Turabian StyleTada, Atsushi, Kazunori Omote, Toshiyuki Nagai, Yasuyuki Honda, Hiroki Nakano, Satoshi Honda, Naotsugu Iwakami, Yasuhiro Hamatani, Michikazu Nakai, Kunihiro Nishimura, and et al. 2020. "Prevalence, Determinants, and Prognostic Significance of Hospital Acquired Pneumonia in Patients with Acute Heart Failure" Journal of Clinical Medicine 9, no. 7: 2219. https://doi.org/10.3390/jcm9072219
APA StyleTada, A., Omote, K., Nagai, T., Honda, Y., Nakano, H., Honda, S., Iwakami, N., Hamatani, Y., Nakai, M., Nishimura, K., Asaumi, Y., Aiba, T., Noguchi, T., Kusano, K., Yokoyama, H., Yasuda, S., Ogawa, H., & Anzai, T. (2020). Prevalence, Determinants, and Prognostic Significance of Hospital Acquired Pneumonia in Patients with Acute Heart Failure. Journal of Clinical Medicine, 9(7), 2219. https://doi.org/10.3390/jcm9072219





