Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility and Inclusion/Exclusion Criteria
2.3. Study Selection and Data Collection
2.4. Quality Assessment
2.5. Statistical Analysis
- Mean values of metabolites concentration in patients with or without toxicity
- 2.
- Odds Ratio (OR)
- 3.
- Correlations
3. Results
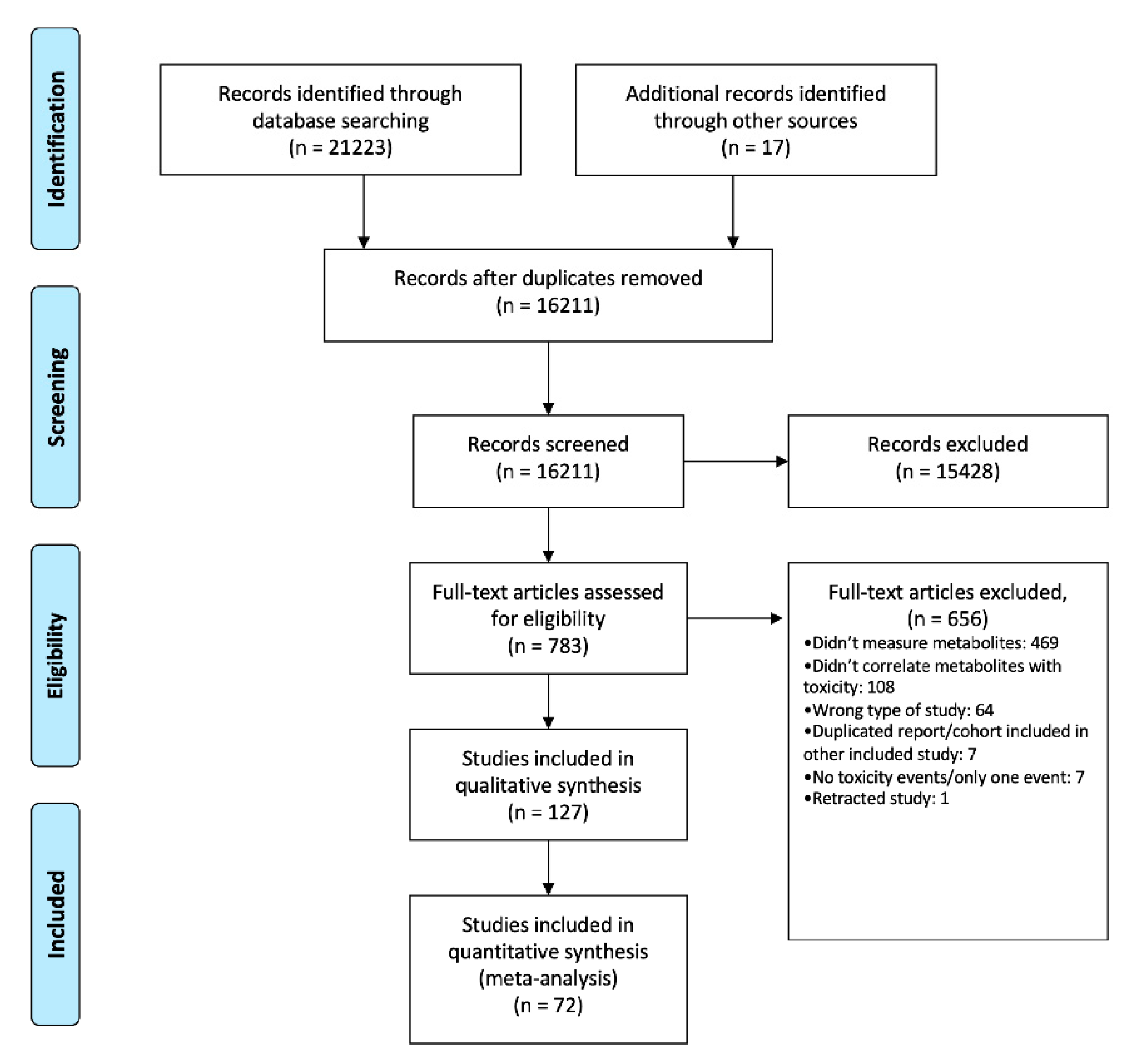
3.1. Bibliographic Search and Study Selection
3.2. Description of the Studies
3.3. Toxicity and Thiopurines’ Metabolites
3.3.1. Overall Adverse Events
6-TGN
6-MMPR
3.3.2. Myelotoxicity
“General” Myelotoxicity
6-TGN
6-MMPR
6-MMPR/6-TGN Ratio
Anemia
Leukopenia
Neutropenia
Lymphopenia
Thrombocytopenia
3.3.3. Liver Toxicity
Altered Liver Enzymes
Veno-occlusive Disease
3.3.4. Gastrointestinal Intolerance
6-TGN
3.3.5. Pancreatitis, Infections and NRH
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Asseldonk, D.P.; de Boer, N.K.; Peters, G.J.; Veldkamp, A.I.; Mulder, C.J.; Van Bodegraven, A.A. On therapeutic drug monitoring of thiopurines in inflammatory bowel disease; pharmacology, pharmacogenomics, drug intolerance and clinical relevance. Curr. Drug Metab. 2009, 10, 981–997. [Google Scholar] [CrossRef]
- Lennard, L.; Singleton, H.J. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: Quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J. Chromatogr. 1992, 583, 83–90. [Google Scholar] [CrossRef]
- Dervieux, T.; Boulieu, R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin. Chem. 1998, 44, 551–555. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gomollon, F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: A review. Am. J. Gastroenterol. 2008, 103, 1783–1800. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, M.; Ordas, I.; Cabre, E.; Garcia-Sanchez, V.; Bastida, G.; Penalva, M.; Gomollon, F.; Garcia-Planella, E.; Merino, O.; Gutierrez, A.; et al. Safety of thiopurine therapy in inflammatory bowel disease: Long-term follow-up study of 3931 patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Van Asseldonk, D.P.; Sanderson, J.; de Boer, N.K.; Sparrow, M.P.; Lemann, M.; Ansari, A.; Almer, S.H.; Florin, T.H.; Gearry, R.B.; Mulder, C.J.; et al. Difficulties and possibilities with thiopurine therapy in inflammatory bowel disease—Proceedings of the first Thiopurine Task Force meeting. Dig. Liver Dis. 2011, 43, 270–276. [Google Scholar] [CrossRef]
- Beswick, L.; Friedman, A.B.; Sparrow, M.P. The role of thiopurine metabolite monitoring in inflammatory bowel disease. Expert Rev. Gastroenterol. 2014, 8, 383–392. [Google Scholar] [CrossRef]
- Lilleyman, J.S.; Lennard, L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet 1994, 343, 1188–1190. [Google Scholar] [CrossRef]
- Lennard, L.; Lilleyman, J.S. Are children with lymphoblastic leukaemia given enough 6-mercaptopurine? Lancet 1987, 330, 785–787. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Afonso, J.; Rosa, I.; Lago, P.; Trindade, E.; Correia, L.; Dias, C.C.; Magro, F. A Systematic Review and Meta-Analysis of 6-Thioguanine Nucleotide Levels and Clinical Remission in Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1381–1392. [Google Scholar] [CrossRef]
- Bergan, S.; Rugstad, H.E.; Bentdal, O.; Sodal, G.; Hartmann, A.; Leivestad, T.; Stokke, O. Monitored high-dose azathioprine treatment reduces acute rejection episodes after renal transplantation. Transplantation 1998, 66, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Osterman, M.T.; Kundu, R.; Lichtenstein, G.R.; Lewis, J.D. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: A meta-analysis. Gastroenterology 2006, 130, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Haglund, S. Interindividual Differences in Thiopurine Metabolism: Studies with Focus on Inflammatory Bowel Disease. Ph.D. Thesis, Linköping University, Linköping, Sweden, 2011. [Google Scholar]
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Theoret, Y.; Seidman, E.G. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118, 705–713. [Google Scholar] [CrossRef]
- Gearry, R.B.; Barclay, M.L. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J. Gastroen. Hepatol. 2005, 20, 1149–1157. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Herfarth, H.; Katz, J.; Falck-Ytter, Y.; Singh, S. American Gastroenterological Association Institute Technical Review on the Role of Therapeutic Drug Monitoring in the Management of Inflammatory Bowel Diseases. Gastroenterology 2017, 153, 835–857. [Google Scholar] [CrossRef]
- Azathioprine- Azathioprine Tablet [Package Insert]. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ceab8e8b-d022-4d0c-a552-cc5782446248 (accessed on 5 February 2020).
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Klein, T.E.; et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin. Pharmacol. Ther. 2011, 89, 387–391. [Google Scholar] [CrossRef]
- Relling, M.V.; Gardner, E.E.; Sandborn, W.J.; Schmiegelow, K.; Pui, C.H.; Yee, S.W.; Stein, C.M.; Carrillo, M.; Evans, W.E.; Hicks, J.K.; et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin. Pharmacol. Ther. 2013, 93, 324–325. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Adriani, A.; Caviglia, G.P.; Nicolò, A.; Agnesod, D.; Simiele, M.; Riganò, D.; Pellicano, R.; Canaparo, R.; Perri, G.D.; et al. Correlation between Thiopurine S-Methyltransferase Genotype and Adverse Events in Inflammatory Bowel Disease Patients. Medicina 2019, 55, 441. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Jonathan, J.; Deeks, J.P.H.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2019. [Google Scholar]
- PubMed®. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 3 November 2018).
- Web of Science. Available online: http://www.isiwebofknowledge.com (accessed on 3 November 2018).
- Scopus. Available online: https://www.elsevier.com/solutions/scopus (accessed on 3 November 2018).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing studies with diverse designs: The development and evaluation of a new tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Shipkova, M.; Armstrong, V.W.; Wieland, E.; Oellerich, M. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin. Chem. 2003, 49, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lennard, L. Assay of 6-thioinosinic acid and 6-thioguanine nucleotides, active metabolites of 6-mercaptopurine, in human red blood cells. J. Chromatogr. 1987, 423, 169–178. [Google Scholar] [CrossRef]
- Armstrong, V.W.; Shipkova, M.; von Ahsen, N.; Oellerich, M. Analytic aspects of monitoring therapy with thiopurine medications. Ther. Drug Monit. 2004, 26, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, G.R.; France, L.A.; Bostrom, B.C.; Canafax, D.M. A reversed phase high performance liquid chromatography approach in determining total red blood cell concentrations of 6-thioguanine, 6-mercaptopurine, methylthioguanine, and methylmercaptopurine in a patient receiving thiopurine therapy. Biomed. Chromatogr. 1990, 4, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lowry, P.W.; Franklin, C.L.; Weaver, A.L.; Pike, M.G.; Mays, D.C.; Tremaine, W.J.; Lipsky, J.J.; Sandborn, W.J. Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut 2001, 49, 665–670. [Google Scholar] [CrossRef]
- Thomas, C.W., Jr.; Lowry, P.W.; Franklin, C.L.; Weaver, A.L.; Myhre, G.M.; Mays, D.C.; Tremaine, W.J.; Lipsky, J.J.; Sandborn, W.J. Erythrocyte mean corpuscular volume as a surrogate marker for 6-thioguanine nucleotide concentration monitoring in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Inflamm. Bowel Dis. 2003, 9, 237–245. [Google Scholar] [CrossRef]
- Robijns, K.; van Luin, M.; Jansen, R.T.P.; Neef, C.; Touw, D.J. A design for external quality assessment for the analysis of thiopurine drugs: Pitfalls and opportunities. Clin. Chem. Lab. Med. 2018, 56, 1715–1721. [Google Scholar] [CrossRef]
- Meijer, B.; Wilhelm, A.J.; Mulder, C.J.J.; Bouma, G.; Van Bodegraven, A.A.; de Boer, N.K.H. Pharmacology of Thiopurine Therapy in Inflammatory Bowel Disease and Complete Blood Cell Count Outcomes: A 5-Year Database Study. Ther. Drug Monit. 2017, 39, 399–405. [Google Scholar] [CrossRef]
- Meijer, B.; Kreijne, J.E.; van Moorsel, S.A.W.; Derijks, L.J.J.; Bouma, G.; Mulder, C.J.J.; Wong, D.R.; van der Woude, C.J.; van Bodegraven, A.A.; de Boer, N.K.H. 6-methylmercaptopurine-induced leukocytopenia during thiopurine therapy in inflammatory bowel disease patients. J. Gastroenterol. Hepatol. 2017, 32, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Field, A.P. Meta-analysis of correlation coefficients: A Monte Carlo comparison of fixed- and random-effects methods. Psychol. Methods 2001, 6, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Steinhauser, S.; Schumacher, M.; Rucker, G. Modelling multiple thresholds in meta-analysis of diagnostic test accuracy studies. BMC Med. Res. Methodol. 2016, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Fangbin, Z.; Xiang, G.; Liang, D.; Hui, L.; Xueding, W.; Baili, C.; Huichang, B.; Yinglian, X.; Peng, C.; Lizi, Z.; et al. Prospective Evaluation of Pharmacogenomics and Metabolite Measurements upon Azathioprine Therapy in Inflammatory Bowel Disease: An Observational Study. Medicine 2016, 95, e3326. [Google Scholar] [CrossRef] [PubMed]
- Vikingsson, S.; Carlsson, B.; Almer, S.H.; Peterson, C. Monitoring of thiopurine metabolites in patients with inflammatory bowel disease-what is actually measured? Ther. Drug Monit. 2009, 31, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, S.; Lennard, L.; Coen, P.; Lilleyman, J.; Saha, V. Veno-occlusive disease in patients receiving thiopurines during maintenance therapy for childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2003, 123, 100–102. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Tremaine, W.J.; Wolf, D.C.; Targan, S.R.; Sninsky, C.A.; Sutherland, L.R.; Hanauer, S.B.; McDonald, J.W.D.; Feagan, B.G.; Fedorak, R.N.; et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn’s disease. Gastroenterology 1999, 117, 527–535. [Google Scholar] [CrossRef]
- Adam de Beaumais, T.; Fakhoury, M.; Medard, Y.; Azougagh, S.; Zhang, D.; Yakouben, K.; Jacqz-Aigrain, E. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br. J. Clin. Pharmacol. 2011, 71, 575–584. [Google Scholar] [CrossRef]
- Almer, S.H.; Hjortswang, H.; Hindorf, U. 6-Thioguanine therapy in Crohn’s disease—observational data in Swedish patients. Dig. Liver Dis. 2009, 41, 194–200. [Google Scholar] [CrossRef]
- Alvarez Beltran, M.; Infante Pina, D.; Tormo Carnice, R.; Segarra Canton, O.; Redecillas Ferreiro, S. Optimising azathioprine treatment: Determination of thiopurine methyltransferase activity and thiopurine metabolites. An. Pediatr. 2009, 70, 126–131. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Ban, H.; Hashimoto, T.; Bamba, S.; Ogawa, A.; Sasaki, M.; Saito, Y.; Fujiyama, Y. Monitoring 6-thioguanine nucleotide concentrations in Japanese patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2008, 23, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.; Sharif, J.A.; Galloway, P.; McGrogan, P.; Bishop, J.; Russell, R.K. Evaluating the use of metabolite measurement in children receiving treatment with a thiopurine. Aliment. Pharmacol. Ther. 2011, 34, 1106–1114. [Google Scholar] [CrossRef]
- Ban, H.; Andoh, A.; Imaeda, H.; Kobori, A.; Bamba, S.; Tsujikawa, T.; Sasaki, M.; Saito, Y.; Fujiyama, Y. The multidrug-resistance protein 4 polymorphism is a new factor accounting for thiopurine sensitivity in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 2010, 45, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bishop, W.P. Evolution of thiopurine use in pediatric inflammatory bowel disease in an academic center. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Belaiche, J.; Desager, J.P.; Horsmans, Y.; Louis, E. Therapeutic drug monitoring of azathioprine and 6-mercaptopurine metabolites in Crohn disease. Scand. J. Gastroenterol. 2001, 36, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Bergan, S.; Rugstad, H.E.; Bentdal, O.; Stokke, O. Monitoring of azathioprine treatment by determination of 6-thioguanine nucleotide concentrations in erythrocytes. Transplantation 1994, 58, 803–808. [Google Scholar] [CrossRef]
- Berkovitch, M.; Matsui, D.; Zipursky, A.; Blanchette, V.S.; Verjee, Z.; Giesbrecht, E.; Saunders, E.F.; Evans, W.E.; Koren, G. Hepatotoxicity of 6-mercaptopurine in childhood acute lymphocytic leukemia: Pharmacokinetic characteristics. Med. Pediatr. Oncol. 1996, 26, 85–89. [Google Scholar] [CrossRef]
- Boulieu, R.; Lenoir, A.; Bertocchi, M.; Mornex, J.F. Intracellular thiopurine nucleotides and azathioprine myelotoxicity in organ transplant patients. Br. J. Clin. Pharmacol. 1997, 43, 116–118. [Google Scholar] [CrossRef]
- Boulieu, R.; Dervieux, T.; Gallant, I.; Sauviat, M.; Bertocchi, M.; Mornex, J.F. Methylated and non methylated thiopurine nucleotide ratio (Me6-MPN/6-TGN): Usefulness in the monitoring of azathioprine therapy? Adv. Exp. Med. Biol. 2000, 486, 361–367. [Google Scholar] [CrossRef]
- Broekman, M.; Coenen, M.J.H.; Wanten, G.J.; van Marrewijk, C.J.; Klungel, O.H.; Verbeek, A.L.M.; Hooymans, P.M.; Guchelaar, H.J.; Scheffer, H.; Derijks, L.J.J.; et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment. Pharmacol. Ther. 2017, 46, 953–963. [Google Scholar] [CrossRef]
- Chapdelaine, A.; Mansour, A.M.; Troyanov, Y.; Williamson, D.R.; Dore, M. Metabolite monitoring to guide thiopurine therapy in systemic autoimmune diseases. Clin. Rheumatol. 2017, 36, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowska, M.; Kolecki, P.; Duczmal-Cichocka, B.; Fiet, J. Metabolites of mercaptopurine in red blood cells: A relationship between 6-thioguanine nucleotides and 6-methylmercaptopurine metabolite concentrations in children with lymphoblastic leukemia. Eur. J. Pharm. Sci. 1999, 8, 329–334. [Google Scholar] [CrossRef]
- Chrzanowska, M.; Krzymanski, M. Determination of 6-thioguanine and 6-methylmercaptopurine metabolites in renal transplantation recipients and patients with glomerulonephritis treated with azathioprine. Ther. Drug Monit. 1999, 21, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Cuffari, C.; Théorêt, Y.; Latour, S.; Seidman, G. 6-Mercaptopurine metabolism in Crohn’s disease: Correlation with efficacy and toxicity. Gut 1996, 39, 401–406. [Google Scholar] [CrossRef]
- Cuffari, C.; Li, D.Y.; Mahoney, J.; Barnes, Y.; Bayless, T.M. Peripheral blood mononuclear cell DNA 6-thioguanine metabolite levels correlate with decreased interferon-gamma production in patients with Crohn’s disease on AZA therapy. Dig. Dis. Sci. 2004, 49, 133–137. [Google Scholar] [CrossRef]
- Dassopoulos, T.; Dubinsky, M.C.; Bentsen, J.L.; Martin, C.F.; Galanko, J.A.; Seidman, E.G.; Sandler, R.S.; Hanauer, S.B. Randomised clinical trial: Individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 39, 163–175. [Google Scholar] [CrossRef]
- De Boer, N.K.; Derijks, L.J.; Gilissen, L.P.; Hommes, D.W.; Engels, L.G.; de-Boer, S.Y.; den Hartog, G.; Hooymans, P.M.; Makelburg, A.B.; Westerveld, B.D.; et al. On tolerability and safety of a maintenance treatment with 6-thioguanine in azathioprine or 6-mercaptopurine intolerant IBD patients. World J. Gastroenterol. 2005, 11, 5540–5544. [Google Scholar] [CrossRef]
- Derijks, L.J.; de Jong, D.J.; Gilissen, L.P.; Engels, L.G.; Hooymans, P.M.; Jansen, J.B.; Mulder, C.J. 6-Thioguanine seems promising in azathioprine- or 6-mercaptopurine-intolerant inflammatory bowel disease patients: A short-term safety assessment. Eur. J. Gastroenterol. Hepatol. 2003, 15, 63–67. [Google Scholar] [CrossRef]
- Derijks, L.J.; Gilissen, L.P.; Engels, L.G.; Bos, L.P.; Bus, P.J.; Lohman, J.J.; Curvers, W.L.; Van Deventer, S.J.; Hommes, D.W.; Hooymans, P.M. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: Implications for therapy. Ther. Drug Monit. 2004, 26, 311–318. [Google Scholar] [CrossRef]
- Dervieux, T.; Medard, Y.; Verpillat, P.; Guigonis, V.; Duval, M.; Lescoeur, B.; Suciu, S.; Vilmer, E.; Jacqz-Aigrain, E. Possible implication of thiopurine S-methyltransferase in occurrence of infectious episodes during maintenance therapy for childhood lymphoblastic leukemia with mercaptopurine. Leukemia 2001, 15, 1706–1712. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, F.B.; Liu, H.; Gao, X.; Bi, H.C.; Wang, X.D.; Chen, B.L.; Zhang, Y.; Zhao, L.Z.; Zhong, G.P.; et al. Hypoxanthine guanine phosphoribosyltransferase activity is related to 6-thioguanine nucleotide concentrations and thiopurine-induced leukopenia in the treatment of inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 63–73. [Google Scholar] [CrossRef]
- Dubinsky, M.C.; Yang, H.; Hassard, P.V.; Seidman, E.G.; Kam, L.Y.; Abreu, M.T.; Targan, S.R.; Vasiliauskas, E.A. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology 2002, 122, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, M.C.; Vasiliauskas, E.A.; Singh, H.; Abreu, M.T.; Papadakis, K.A.; Tran, T.; Martin, P.; Vierling, J.M.; Geller, S.A.; Targan, S.R.; et al. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology 2003, 125, 298–303. [Google Scholar] [CrossRef]
- Fei, X.; Shu, Q.; Zhu, H.; Hua, B.; Wang, S.; Guo, L.; Fang, Y.; Ge, W. NUDT15 R139C Variants Increase the Risk of Azathioprine-Induced Leukopenia in Chinese Autoimmune Patients. Front. Pharmacol. 2018, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Guo, J.; Zhang, S.H.; Qiu, Y.; Chen, B.L.; He, Y.; Zeng, Z.R.; Ben-Horin, S.; Chen, M.H.; Mao, R. Low 6-thioguanine nucleotide level: Effective in maintaining remission in Chinese patients with Crohn’s disease. J. Gastroenterol. Hepatol. 2019, 34, 679–685. [Google Scholar] [CrossRef]
- Ferucci, E.D.; Hurlburt, K.J.; Mayo, M.J.; Livingston, S.; Deubner, H.; Gove, J.; Plotnik, J.; McMahon, B.J. Azathioprine metabolite measurements are not useful in following treatment of autoimmune hepatitis in Alaska Native and other non-Caucasian people. Can. J. Gastroenterol. 2011, 25, 21–27. [Google Scholar] [CrossRef]
- Ganping, Z.; Zuofu, C.; Shedian, Z.; Zhichun, F.; Feng, X. A Simplistic Individualization Method for 6-Mercaptopurine in Acute Lymphoblastic Leukemia Children. Int. J. Pharmacol. 2008, 4, 64–66. [Google Scholar] [CrossRef]
- Gardiner, S.J.; Gearry, R.B.; Begg, E.J.; Zhang, M.; Barclay, M.L. Thiopurine dose in intermediate and normal metabolizers of thiopurine methyltransferase may differ three-fold. Clin. Gastroenterol. Hepatol. 2008, 6, 654–660. [Google Scholar] [CrossRef]
- Gupta, P.; Gokhale, R.; Kirschner, B.S. 6-mercaptopurine metabolite levels in children with inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 450–454. [Google Scholar] [CrossRef]
- Halonen, P.; Mattila, J.; Makipernaa, A.; Ruuska, T.; Schmiegelow, K. Erythrocyte concentrations of metabolites or cumulative doses of 6-mercaptopurine and methotrexate do not predict liver changes in children treated for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2006, 46, 762–766. [Google Scholar] [CrossRef]
- Hande, S.; Wilson-Rich, N.; Bousvaros, A.; Zholudev, A.; Maurer, R.; Banks, P.; Makrauer, F.; Reddy, S.; Burakoff, R.; Friedman, S. 5-aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm. Bowel Dis. 2006, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Heerasing, N.M.; Ng, J.F.; Dowling, D. Does lymphopenia or macrocytosis reflect 6-thioguanine levels in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine? Intern. Med. J. 2016, 46, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Hindorf, U.; Lindqvist, M.; Hildebrand, H.; Fagerberg, U.; Almer, S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 24, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Hindorf, U.; Lindqvist, M.; Peterson, C.; Soderkvist, P.; Strom, M.; Hjortswang, H.; Pousette, A.; Almer, S. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut 2006, 55, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, F.; Danesi, R.; Favre, C.; Nardi, M.; Menconi, M.C.; Di Paolo, A.; Bocci, G.; Fogli, S.; Barbara, C.; Barachini, S.; et al. Variable correlation between 6-mercaptopurine metabolites in erythrocytes and hematologic toxicity: Implications for drug monitoring in children with acute lymphoblastic leukemia. Ther. Drug Monit. 2000, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, U.; Amre, D.; Theoret, Y.; Deslandres, C.; Seidman, E.G. Thiopurine metabolite ratios for monitoring therapy in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 511–515. [Google Scholar] [CrossRef]
- Lancaster, D.L.; Lennard, L.; Rowland, K.; Vora, A.J.; Lilleyman, J.S. Thioguanine versus mercaptopurine for therapy of childhood lymphoblastic leukaemia: A comparison of haematological toxicity and drug metabolite concentrations. Br. J. Haematol. 1998, 102, 439–443. [Google Scholar] [CrossRef]
- Lee, M.N.; Kang, B.; Choi, S.Y.; Kim, M.J.; Woo, S.Y.; Kim, J.W.; Choe, Y.H.; Lee, S.Y. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm. Bowel Dis. 2015, 21, 1054–1062. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Choi, L.H.; Woo, H.I.; Lee, S.Y.; Kim, Y.H. Measurements of 6-thioguanine nucleotide levels with TPMT and NUDT15 genotyping in patients with Crohn’s disease. PLoS ONE 2017, 12, e0188925. [Google Scholar] [CrossRef]
- Lennard, L.; Rees, C.A.; Lilleyman, J.S.; Maddocks, J.L. Childhood leukaemia: A relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br. J. Clin. Pharmacol. 1983, 16, 359–363. [Google Scholar] [CrossRef]
- Lennard, L.; Brown, C.B.; Fox, M.; Maddocks, J.L. Azathioprine metabolism in kidney transplant recipients. Br. J. Clin. Pharmacol. 1984, 18, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lennard, L.; Lilleyman, J.S.; Van Loon, J.; Weinshilboum, R.M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet 1990, 336, 225–229. [Google Scholar] [CrossRef]
- Lennard, L.; Richards, S.; Cartwright, C.S.; Mitchell, C.; Lilleyman, J.S.; Vora, A. The thiopurine methyltransferase genetic polymorphism is associated with thioguanine-related veno-occlusive disease of the liver in children with acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 2006, 80, 375–383. [Google Scholar] [CrossRef]
- Lilleyman, J.S.; Lennard, L.; Rees, C.A.; Morgan, G.; Maddocks, J.L. Childhood lymphoblastic leukaemia: Sex difference in 6-mercaptopurine utilization. Br. J. Cancer 1984, 49, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Mei, Q.; Han, W.; Hu, J.; Hu, N. Measurement of red blood cell 6-thioguanine nucleotide is beneficial in azathioprine maintenance therapy of Chinese Crohn’s disease patients. Scand. J. Gastroenterol. 2016, 51, 1093–1099. [Google Scholar] [CrossRef]
- Melaouhia, S.; Fekih, M.; Ferchichi, H.; Bouissorra, H.; Ben Mustapha, N.; Boubaker, J.; Filali, A.; Lakhal, M.; Klouz, A. Is there any interest to dose the azathioprine’s metabolites during inflammatory bowel diseases? Therapie 2013, 68, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Le Gall, C.; Lachaux, A.; Boulieu, R. High thiopurine metabolite concentrations associated with lymphopenia in inflammatory bowel disease (IBD) pediatric patients receiving aminosalicylates combined with azathioprine. Int. J. Clin. Pharmacol. Ther. 2010, 48, 275–281. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Daubard, M.; Le Gall, C.; Larger, M.; Lachaux, A.; Boulieu, R. Monitoring of azathioprine metabolites in pediatric patients with autoimmune hepatitis. Ther. Drug Monit. 2010, 32, 433–437. [Google Scholar] [CrossRef]
- Nygaard, U.; Toft, N.; Schmiegelow, K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin. Pharmacol. Ther. 2004, 75, 274–281. [Google Scholar] [CrossRef]
- Odahara, S.; Uchiyama, K.; Kubota, T.; Ito, Z.; Takami, S.; Kobayashi, H.; Saito, K.; Koido, S.; Ohkusa, T. A Prospective Study Evaluating Metabolic Capacity of Thiopurine and Associated Adverse Reactions in Japanese Patients with Inflammatory Bowel Disease (IBD). PLoS ONE 2015, 10, e0137798. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Arai, K.; Aoyagi, Y.; Fujii, T.; Yamakawa, Y.; Ohtani, K.; Ikuse, T.; Baba, Y.; Inage, E.; Kudo, T.; et al. Monitoring 6-thioguanine nucleotide concentrations in Japanese children and adolescents with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2010, 25, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Bohane, T.D.; Lee, D.; Naidoo, D.; Day, A.S. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007, 25, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Pranzatelli, M.R.; Tate, E.D.; Allison, T.J. 6-Mercaptopurine modifies cerebrospinal fluid T cell abnormalities in paediatric opsoclonus-myoclonus as steroid sparer. Clin. Exp. Immunol. 2017, 190, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Rae, W.; Burke, G.; Pinto, A. A study of the utility of azathioprine metabolite testing in myasthenia gravis. J. Neuroimmunol. 2016, 293, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, K.; Bruunshuus, I. 6-Thioguanine nucleotide accumulation in red blood cells during maintenance chemotherapy for childhood acute lymphoblastic leukemia, and its relation to leukopenia. Cancer Chemother. Pharmacol. 1990, 26, 288–292. [Google Scholar] [CrossRef]
- Shaye, O.A.; Yadegari, M.; Abreu, M.T.; Poordad, F.; Simon, K.; Martin, P.; Papadakis, K.A.; Ippoliti, A.; Vasiliauskas, E.; Tran, T.T. Hepatotoxicity of 6-mercaptopurine (6-MP) and Azathioprine (AZA) in adult IBD patients. Am. J. Gastroenterol. 2007, 102, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Wojtuszkiewicz, A.; Barcelos, A.; Dubbelman, B.; De Abreu, R.; Brouwer, C.; Bokkerink, J.P.; de Haas, V.; de Groot-Kruseman, H.; Jansen, G.; Kaspers, G.L.; et al. Assessment of mercaptopurine (6MP) metabolites and 6MP metabolic key-enzymes in childhood acute lymphoblastic leukemia. Nucleosides Nucleotides Nucleic Acids 2014, 33, 422–433. [Google Scholar] [CrossRef]
- Wong, D.R.; Coenen, M.J.; Derijks, L.J.; Vermeulen, S.H.; van Marrewijk, C.J.; Klungel, O.H.; Scheffer, H.; Franke, B.; Guchelaar, H.J.; de Jong, D.J.; et al. Early prediction of thiopurine-induced hepatotoxicity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 391–402. [Google Scholar] [CrossRef]
- Wong, D.R.; Coenen, M.J.; Vermeulen, S.H.; Derijks, L.J.; van Marrewijk, C.J.; Klungel, O.H.; Scheffer, H.; Franke, B.; Guchelaar, H.J.; de Jong, D.J.; et al. Early Assessment of Thiopurine Metabolites Identifies Patients at Risk of Thiopurine-induced Leukopenia in Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 175–184. [Google Scholar] [CrossRef]
- Wright, S.; Sanders, D.S.; Lobo, A.J.; Lennard, L. Clinical significance of azathioprine active metabolite concentrations in inflammatory bowel disease. Gut 2004, 53, 1123–1128. [Google Scholar] [CrossRef]
- Yarur, A.J.; Gondal, B.; Hirsch, A.; Christensen, B.; Cohen, R.D.; Rubin, D.T. Higher Thioguanine Nucleotide Metabolite Levels Are Associated with Better Long-Term Outcomes in Patients with Inflammatory Bowel Diseases. J. Clin. Gastroenterol. 2018, 52, 537–544. [Google Scholar] [CrossRef]
- Zochowska, D.; Zegarska, J.; Hryniewiecka, E.; Samborowska, E.; Jazwiec, R.; Tszyrsznic, W.; Borowiec, A.; Dadlez, M.; Paczek, L. Determination of Concentrations of Azathioprine Metabolites 6-Thioguanine and 6-Methylmercaptopurine in Whole Blood With the Use of Liquid Chromatography Combined With Mass Spectrometry. Transplant. Proc. 2016, 48, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Van Asseldonk, D.P.; Jharap, B.; Verheij, J.; den Hartog, G.; Westerveld, D.B.; Becx, M.C.; Russel, M.G.; Engels, L.G.; de Jong, D.J.; Witte, B.I.; et al. The Prevalence of Nodular Regenerative Hyperplasia in Inflammatory Bowel Disease Patients Treated with Thioguanine Is Not Associated with Clinically Significant Liver Disease. Inflamm. Bowel Dis. 2016, 22, 2112–2120. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Heap, G.A.; Green, H.D.; Hamilton, B.; Bewshea, C.; Walker, G.J.; Thomas, A.; Nice, R.; Perry, M.H.; Bouri, S.; et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: A prospective, multicentre, cohort study. Lancet Gastroenterol. 2019, 4, 341–353. [Google Scholar] [CrossRef]
- Roblin, X.; Williet, N.; Boschetti, G.; Phelip, J.M.; Del Tedesco, E.; Berger, A.E.; Vedrines, P.; Duru, G.; Peyrin-Biroulet, L.; Nancey, S.; et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: A prospective randomised trial. Gut 2020, 69, 1206–1212. [Google Scholar] [CrossRef]
- Yarur, A.J.; Kubiliun, M.J.; Czul, F.; Sussman, D.A.; Quintero, M.A.; Jain, A.; Drake, K.A.; Hauenstein, S.I.; Lockton, S.; Deshpande, A.R.; et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin. Gastroenterol. Hepatol. 2015, 13, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Boschetti, G.; Williet, N.; Nancey, S.; Marotte, H.; Berger, A.; Phelip, J.M.; Peyrin-Biroulet, L.; Colombel, J.F.; Del Tedesco, E.; et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: An open-label, prospective and randomised clinical trial. Aliment. Pharmacol. Ther. 2017, 46, 142–149. [Google Scholar] [CrossRef] [PubMed]
- De Boer, N.K.; de Graaf, P.; Wilhelm, A.J.; Mulder, C.J.; van Bodegraven, A.A. On the limitation of 6-tioguaninenucleotide monitoring during tioguanine treatment. Aliment. Pharmacol. Ther. 2005, 22, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Duley, J.A.; Florin, T.H. Thiopurine therapies: Problems, complexities, and progress with monitoring thioguanine nucleotides. Ther. Drug Monit. 2005, 27, 647–654. [Google Scholar] [CrossRef]
- Dervieux, T.; Blanco, J.G.; Krynetski, E.Y.; Vanin, E.F.; Roussel, M.F.; Relling, M.V. Differing contribution of thiopurine methyltransferase to mercaptopurine versus thioguanine effects in human leukemic cells. Cancer Res. 2001, 61, 5810–5816. [Google Scholar]
- Lim, S.Z.; Chua, E.W. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front. Pharmacol. 2018, 9, 1107. [Google Scholar] [CrossRef]
- Fraser, A.G.; Jewell, D.P. Side effects of azathioprine treatment given for inflammatory bowel disease-a 30 year audit. Gastroenterology 2000, 118, A787. [Google Scholar] [CrossRef]
- McGovern, D.P.B.; Travis, S.P.L.; Duley, J.; Shobowale-Bakre, E.M.; Dalton, H.R. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology 2002, 122, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Domenech, E.; Nos, P.; Papo, M.; Lopez-San Roman, A.; Garcia-Planella, E.; Gassull, M.A. 6-mercaptopurine in patients with inflammatory bowel disease and previous digestive intolerance of azathioprine. Scand. J. Gastroenterol. 2005, 40, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Chan, F.K.L.; Leung, W.K.; Li, M.K.K.; Leung, C.M.; Sze, S.F.; Ching, J.Y.L.; Lo, F.H.; Tsang, S.W.C.; Shan, E.H.S.; et al. Low-dose azathioprine is effective in maintaining remission in steroid-dependent ulcerative colitis: Results from a territory-wide Chinese population-based IBD registry. Therap. Adv. Gastroenterol. 2016, 9, 449–456. [Google Scholar] [CrossRef] [PubMed]






| Study | Study Design | Study Population | Population with Evaluation of Metabolites and Toxicity (If Different from Total Number) | Disease | Treatment Regimen and Duration | Concomitant Medication | Measured Metabolites | Measurements Per Patient (If Multiple, Used Value) | Method | QAT Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Adam de Beaumais et al., Br J Clin Pharm 2011 [44] | Prospective | 66 patients, pediatric | Same | Leukemia | 6-MP >4 weeks | Methotrexate | 6-MMPR | Multiple (average of all samples per patient) | Dervieux and Boulieu | 67.8 |
| Almer et al., Dig Liver Dis 2009 [45] | Prospective | 23 patients, adult | Same | IBD | 6-TG From the start (timeline not discriminated) | Mesalazine, steroids and antibiotics. | 6-TGN | Multiple (maximum value of metabolites) | Lennard and Singleton | 40.5 |
| Alvarez Beltran et al., An Pediatr 2009 [46] | Retrospective | 107 patients, pediatric | 18 patients | IBD and AIH | AZA >2 months | NS | 6-TGN and 6-MMPR | NS | NS | 42.9 |
| Andoh et al., J Gastroenterol Hepatol 2008 [47] | Retrospective | 83 patients, adult | Same | IBD | AZA + 6-MP >4 months | Mesalazine | 6-TGN | NS | Erdmann | 41.7 |
| Armstrong et al., Aliment Pharmacol Ther 2011 [48] | Retrospective | 70 patients, pediatric | Same | IBD | AZA + 6-MP >3 months | Mesalazine | 6-TGN | Multiple (NS) | Dervieux and Boulieu | 38.1 |
| Ban et al., J Gastroenterol 2010 [49] | Prospective | 279 patients, pediatric and adult | 130 patients | IBD | AZA + 6-MP NS | Mesalazine | 6-TGN | NS | Erdmann | 54.7 |
| Banerjee et al., J Pediatr Gastroenterol Nutr 2006 [50] | Retrospective | 101 patients, pediatric | 64 patients | IBD | AZA + 6-MP >6 months | Mesalazine, steroids, antibiotics and infliximab | 6-MMP | Multiple (evaluation per-sample) | Prometheus | 65.5 |
| Belaiche et al., Scand J Gastroenterol 2001 [51] | Prospective | 28 patients, adult | Same | IBD | AZA + 6-MP >3 months | Steroids | 6-TGN | Single | Lennard and Singleton | 52.4 |
| Bergan et al., Transplantation 1994 [52] | Prospective | 65 patients, pediatric and adult | 62 patients | Renal transplant | AZA Initial 40 days | Ciclosporin and steroids | 6-TGN | Multiple (division in 2 groups: patients with all 6-TGN below threshold, and patients with at least one 6-TGN measure above threshold) | Lennard | 67.8 |
| Berkovitch et al., Med Pediatr Oncol 1996 [53] | Retrospective | 29 patients, pediatric | 8 patients | Leukemia | 6-MP NS | Chemotherapy | 6-MMPR | Single | Lennard and Singleton | 40.5 |
| Boulieu et al., Br J Clin Pharm 1997 [54] | Prospective | 47 patients, adult | Same | Transplant | AZA >3 months | Cyclosporine and steroids | 6-TGN | Single | Dervieux and Boulieu | 39.3 |
| Boulieu et al., Adv Exp Med Biol 2000 [55] | Prospective | 27 patients, adult | Same | Transplant | AZA >3 months | Steroids and Cyclosporine | 6-TGN and 6-MMPR | Single | Dervieux and Boulieu | 45.2 |
| Broekman et al., Aliment Pharm Ther 2017 [56] | Prospective | 695 patients, adult | 301 patients | IBD | AZA + 6-MP Week 8 | Mesalazine, steroids, biologics | 6-TGN and 6-MMPR | Single | Lennard and Singleton | 76.2 |
| Chapdelaine et al., J Clin Rheumatol 2017 [57] | Retrospective | 71 patients, adult | Same | Rheumatologic disorders | AZA NS | NS | 6-TGN and 6-MMPR | Multiple (NS) | Lennard and Singleton | 65.5 |
| Chrzanowska et al., Eur J Pharm Sci 1999 [58] | Prospective | 19 patients, pediatric | Same | Leukemia | 6-MP >1 month | Methotrexate | 6-TGN and 6-MMPR | Single | Lennard and Singleton | 63.1 |
| Chrzanowska et al., Ther Drug Monit 1999 [59] | Prospective | 37 patients, pediatric and adult | Same | Transplant and glomerulonephritis | AZA >1 month | Cyclosporine and steroids | 6-TGN | Single | Lennard and Singleton | 46.4 |
| Cuffari et al., Gut 1996 [60] | Prospective | 25 patients, pediatric | Same | IBD | 6-MP >4 months | Low-dose steroids | 6-TGN and 6-MMP | Single | Lennard and Singleton | 61.9 |
| Cuffari et al., Dig Dis Sci 2004 [61] | Prospective | 46 patients, adult | Same | IBD | AZA NS | Mesalazine and “other medications” allowed (NS) | 6-TGN | NS | Lennard and Singleton | 60.7 |
| Dassopoulos et al., Aliment Pharmacol Ther 2014 [62] | Prospective | 50 patients, pediatric and adult | Same | IBD | AZA Week 4 to week 44 | Steroids; other immunosuppressants not allowed | 6-TGN | NS | Prometheus | 71.4 |
| De Boer et al., World J Gastroenterol 2005 [63] | Retrospective | 95 patients, adult | 55 patients | IBD | TG >4 weeks | Other immunosuppressants not allowed (cyclosporine, infliximab, methotrexate, thalidomide) | 6-TGN | Single | Lennard and Singleton | 41.7 |
| Derijks et al., Eur J Gastroen Hepat 2003 [64] | Prospective | 32 patients, adult | Same | IBD | 6-TG Week 1 to week 8 | Other immunosuppressants not allowed | 6-TGN | Multiple (correlation per event) | Lennard and Singleton | 51.2 |
| Derijks et al., Ther Drug Monit 2004 [65] | Prospective | 30 patients, adult | 17 patients | IBD | 6-MP Week 1 to week 8 | Mesalazine; other immunosuppressants not allowed | 6-TGN | Multiple (level of metabolites at the time of AE; for the non-AE group, levels at week 8) | Lennard and Singleton | 64.3 |
| Dervieux et al., Leukemia 2001 [66] | Prospective | 78 patients, pediatric | 25 patients | Leukemia | 6-MP At least >4 weeks | Methotrexate | 6-TGN | Multiple (steady-state concentration) | Dervieux and Boulieu | 51.2 |
| Ding et al., Inflamm Bowel Dis 2012 [67] | Prospective | 120 patients, pediatric and adult | 104 patients | IBD | AZA + 6-MP Week 8 | Mesalazine and infliximab; methotrexate and cyclosporine not allowed | 6-TGN | Single | Dervieux and Boulieu | 76.2 |
| Dubinsky et al., Gastroenterology 2000 [14] | Prospective | 92 patients, pediatric | Same | IBD | AZA + 6-MP >4 months | Mesalazine | 6-MMPR | Multiple (NS) | Lennard and Singleton | 66.7 |
| Dubinsky et al., Gastroenterology 2002 [68] | Retrospective | 51 patients, pediatric and adult | Same | IBD | AZA + 6-MP >3 months | Mesalazine and steroids | 6-MMPR | Multiple (median values) | Prometheus | 70.2 |
| Dubinsky et al., Gastroenterology 2003 [69] | Retrospective | 111 patients, pediatric and adult | Same | IBD | 6-TG 1 to 28 months | Steroids, mesalazine and infliximab | 6-TGN | NS | Prometheus | 54.8 |
| Fangbin et al., Medicine 2016 [40] | Prospective | 132 patients, adult | Same | IBD | AZA Week 1 to week 48 | Mesalazine and infliximab | 6-TGN | Multiple (maximum tgn at the time of AE) For optimal cut-off calculus, all 471 samples were used | Dervieux and Boulieu for 6-TGN and Lennard and Singleton for 6-MMP | 64.3 |
| Fei et al., Front Pharmacol 2018 [70] | Retrospective | 87 patients, adult | Same | Multiple (NS) | AZA >2 months | Medications interfering with metabolite levels and/or causing leukopenia were excluded (cycloscporine, tacrolimus, mesalazine, allopurinol, diuretics) | 6-TGN | Single | Dervieux and Boulieu | 70.3 |
| Feng et al., J Gastroenterol Hepatol 2018 [71] | Retrospective | 252 patients, adult | Same | IBD | AZA >3 months | Mesalazine and antibiotics; biologics, thalidomide and steroids not allowed | 6-TGN | Multiple (evaluation per-sample) | Dervieux and Boulieu | 73.8 |
| Ferucci et al., Can J Gastroenterol 2011 [72] | Retrospective | 71 patients, adult | 48 patients | AIH | AZA NS | NS | 6-TGN and 6-MMPR | Multiple (most recent value available) | Prometheus | 70.2 |
| Ganping et al., Int J Pharmacol 2008 [73] | Prospective | 10 patients, pediatric | Same | Leukemia | 6-MP >2 months | Methotrexate | 6-TGN | Multiple (level of metabolites measured 7 days before laboratorial evaluation of AE) | Lennard and Singleton | 39.3 |
| Gardiner et al., Clin Gastroenterol Hepatol 2008 [74] | Prospective | 69 patients, >16 years old | 61 patients | IBD | AZA + 6-MP Month 1 to month 9 | No patient was excluded based on concomitant medication; concomitant drugs NS | 6-TGN and 6-MMPR | Multiple (level of metabolites within 2 days of stopping treatment in the AE group; for the non-AE group, values at month 1) | Dervieux and Boulieu | 66.7 |
| Gupta et al., J Pediatr Gastroenterol Nutr 2001 [75] | Retrospective | 101 patients, pediatric | Same | IBD | AZA + 6-MP >4 months | NS | 6-TGN and 6-MMPR | Multiple (NS) | Prometheus | 52.4 |
| Halonen et al., Pediatr Blood Cancer 2006 [76] | Prospective | 16 patients, pediatric | Same | Leukemia | 6-MP NS | Chemotherapy | 6-TGN | Multiple (average of all samples per patient) | Bruunshuus | 59.5 |
| Hande et al., Inflamm Bowel Dis 2006 [77] | Retrospective | 126 patients, pediatric and adult | 121 patients | IBD | AZA + 6-MP >3 months | Mesalazine; steroids, infliximab and other immunosuppressants not allowed | 6-TGN and 6-MMPR | Multiple (most recent values) | Prometheus | 73.8 |
| Heerasing et al., Intern Med J 2016 [78] | Retrospective | 67 patients, NS | Same | IBD | AZA + 6-MP NS | NS | 6-TGN | NS | NS | 42.9 |
| Hindorf et al., Aliment Pharmacol Ther 2006 [79] | Retrospective | 364 patients, pediatric and adult | 266 patients | IBD | AZA + 6-MP + 6-TG NS | Only mesalazine and steroids | 6-TGN and 6-MMPR | Multiple (at the time of AE; for the non-AE group, last result available) | Lennard and Singleton | 81.0 |
| Hindorf et al., Gut et al. 2006 [80] | Prospective | 60 patients, adult | 54 patients | IBD | AZA + 6-MP Week 1 to week 20 | Mesalazine, steroids, infliximab | 6-TGN and 6-MMPR | Multiple (maximum value of metabolites) | Lennard and Singleton | 59.5 |
| Innocenti et al., Ther Drug Monit 2000 [81] | Prospective | 19 patients, pediatric | Same | Leukemia | 6-MP >3 months | Chemotherapy | 6-TGN | Multiple (evaluation per-sample) | Lennard and Singleton | 65.5 |
| Kopylov et al., J Pediatr Gastroenterol Nutr 2014 [82] | Prospective | 237 patients, pediatric | Same | IBD | AZA + 6-MP >3 months | Mesalazine and steroids; methotrexate and biologics not allowed | 6-MMPR | Multiple (evaluation per-sample) | Lennard and Singleton | 63.1 |
| Lancaster et al., Br J Haematol 1998 [83] | Prospective | 46 patients, pediatric | 37 patients | Leukemia | 6-MP + 6-TG Measurements available from at least week 3 (not mentioned if for all patients) | Chemotherapy | 6-TGN | Multiple (earliest essay) | Lennard and Singleton | 53.6 |
| Lee at al., Inflamm Bowel Dis 2015 [84] | Retrospective | 137 patients, pediatric | Same | IBD | AZA >2 months | Mesalazine, steroids, infliximab | 6-TGN | Multiple (evaluation per-sample) | Dervieux and Boulieu | 63.1 |
| Lee et al., PLoS One 2017 [85] | Retrospective | 165 patients, adult | Same | IBD | AZA + 6-MP >3 months | Steroids and mesalazine; patients using anti-TNF were excluded | 6-TGN and 6-MMPR | NS | Dervieux and Boulieu | 67.8 |
| Lennard et al., Br J Clin Pharm 1983 [86] | Prospective | 22 patients, pediatric | Same | Leukemia | 6-MP >4 weeks | Chemotherapy | 6-TGN | Multiple (level of metabolites measured 14 days before laboratorial evaluation) | Lennard and Singleton | 70.2 |
| Lennard et al., Br J Clin Pharm 1984 [87] | Prospective | 54 patients, NS | 46 patients | Transplant | AZA >6 months | Steroids | 6-TGN | Multiple (evaluation per-sample) | Lennard and Singleton | 51.2 |
| Lennard et al., Lancet 1990 [88] | Retrospective | 225 patients, pediatric | 82 patients | Leukemia | 6-MP >2 months | Chemotherapy | 6-TGN | Single | Lennard and Singleton | 46.4 |
| Lennard et al., Clin Pharm Ther 2006 [89] | Prospective | 1492 patients, pediatric | 134 patients | Leukemia | TG >7 days | Chemotherapy | 6-TGN | Single | Lennard and Singleton | 67.8 |
| Lilleyman et al., Br J Cancer 1984 [90] | Prospective | 22 patients, pediatric | Same | Leukemia | 6-MP >7 months | Chemotherapy | 6-TGN | Multiple (level of metabolites measured 14 days before laboratorial evaluation) | Lennard and Singleton | 63.1 |
| Liu et al., Scand J Gastroenterol 2016 [91] | Prospective | 69 patients, adult | Same | IBD | AZA >3 months | Steroids and Infliximab | 6-TGN | NS | Dervieux and Boulieu | 69.1 |
| Meijer et al., J Gastroenterol Hepatol 2017 [37] | Retrospective | 24 patients, adult | Same | IBD | AZA + 6-MP Median 11 weeks (IQR 6-46) | Steroids; no mention to additional medication | 6-MMPR | Multiple (level of metabolites within 3 days of AE) | Lennard and Singleton | 50.0 |
| Meijer et al., Ther Drug Monit 2017 [36] | Retrospective | 424 patients, adult | Same | IBD, AIH and celiac disease | AZA + 6-MP + TG NS | NS | 6-TGN and 6-MMP | Multiple (evaluation per-sample when laboratory data within 3 days are available) | Dervieux and Boulieu (but converted to Lennard by a factor of 2.6) | 63.1 |
| Melaouhia et al., Therapie 2013 [92] | Prospective | 50 patients, adult | Same | IBD | AZA >12 months | Mesalazine and steroids | 6-TGN and 6-MMPR | Multiple (NS) | Dervieux and Boulieu | 44.1 |
| Nguyen et al., Int J Clin Pharm 2010 [93] | Retrospective | 71 patients, pediatric | Same | IBD | AZA >1 year | Mesalazine | 6-TGN and 6-MMPR | Multiple (evaluation per-sample) | Dervieux and Boulieu | 38.1 |
| Nguyen et al., Ther Drug Monitor 2010 [94] | Retrospective | 28 patients, pediatric | Same | AIH | AZA >3 months | Steroids | 6-TGN and 6-MMPR | Multiple (NS) | Dervieux and Boulieu | 48.8 |
| Nygaard et al., Clin Pharm Ther 2004 [95] | Retrospective | 43 patients, pediatric | Same | Leukemia | 6-MP >4 weeks | Methotrexate | 6-TGN and 6-MMPR | Multiple (average of all samples per patient) | Erdmann | 54.7 |
| Odahara et al., PLoS One 2015 [96] | Prospective | 48 patients, adult | Same | IBD | AZA NS | Mesalazine and Infliximab | 6-TGN | Multiple (level of metabolites at the time of AE; for the non-AE group, mean-value between weeks 8 and 52) | Lennard and Singleton | 59.5 |
| Ohtsuka et al., J Gastroenterol Hepatol 2010 [97] | Retrospective | 51 patients, pediatric | Same | IBD | AZA + 6-MP >3 weeks | Mesalazine and steroids | 6-TGN | Multiple (evaluation per-sample) | Erdmann | 40.5 |
| Ooi et al., Aliment Pharm Ther 2007 [98] | Retrospective | 56 patients, pediatric | Same | IBD | AZA + 6-MP >1 month | Steroids > 10 mg/day, infliximab, tacrolimus, methotrexate and cyclosporine not allowed | 6-TGN | Multiple (evaluation per-sample) | Lennard and Singleton | 53.6 |
| Pranzatelli et al., J Clin Exp Immunol 2017 [99] | Retrospective | 10 patients, pediatric | Same | Opsoclonus-myoclonus | 6-MP >7 months | Adrenocorticotrophic hormone, intravenous immunoglobulin and steroids | 6-TGN | Multiple (NS) | Prometheus | 53.6 |
| Rae et al., J Neuroimmunol 2016 [100] | Prospective | 19 patients, adult | Same | Myasthenia gravis | AZA ≥52 weeks | Steroids | 6-TGN and 6-MMP | NS | Dervieux and Boulieu | 57.1 |
| Sandborn et al., Gastroenterology 1999 [43] | Prospective | 96 patients, adult | Same | IBD | AZA From week 0.2 to week 16 | Steroids | 6-TGN | Multiple (evaluation per sample) | Erdmann | 88.1 |
| Schmiegelow et al., Cancer Chemother Pharmacol 1990 [101] | Prospective | 31 patients, pediatric | Same | Leukemia | 6-MP >5 weeks | Chemotherapy | 6-TGN | Multiple (mean of measurements) | Bruunshuus | 52.4 |
| Shaye et al., Am J Gastroenterol 2007 [102] | Retrospective | 173 patients, adult | Same | IBD | AZA + 6-MP >1 month | Mesalazine | 6-MMPR | NS | Prometheus | 59.5 |
| Stoneham et al., Br J Haematol 2003 [42] | Retrospective | 99 patients, pediatric | Same | Leukemia | 6-MP + TG Week 4 | NS | 6-TGN | Single | Lennard and Singleton | 34.5 |
| Thomas et al., Inflamm Bowel Dis 2003 [34] | Prospective | 166 patients, adult | 158 patients | IBD | AZA + 6-MP >3 months | Sulfassalazine | 6-TGN | Single | Erdmann | 63.1 |
| Wojtuskiewicz et al., Nucleos Nucleot Nucl 2014 [103] | Prospective | 236 patients, pediatric and adult | 41 patients | Leukemia | 6-MP Measurements from week 25 to 109 | Chemotherapy | 6-TGN | Multiple (metabolite levels at week 25) | Keuzenkamp | 63.1 |
| Wong et al., Aliment Pharmacol Ther 2016 [104] | Prospective | 270 patients, adult | Same | IBD | AZA + 6-MP Week 1 | Mesalazine, steroids and anti-TNF | 6-MMPR | Single | Lennard and Singleton | 82.1 |
| Wong et al., J Crohn Colitis 2017 [105] | Prospective | 194 patients, adult | Data for 194 patients available; data from 181 patients were used in the means comparison and pooled OR analyses (exclusion of patients using anti-TNF) | IBD | AZA + 6-MP Week 1 | Mesalazine and steroids; (patients using anti-TNF were excluded from means comparison and pooled OR analysis; for calculation of an optimal cutoff, data from all patients were used) | 6-TGN and 6-MMPR | Single | Lennard and Singleton | 82.1 |
| Wright et al., Gut 2004 [106] | Prospective | 159 patients, NS | 123 patients | IBD | AZA >4 months | Mesalazine and steroids | 6-MMPR | Multiple (average of all samples per patient) | Lennard and Singleton | 78.6 |
| Yarur et al., J Clin Gastroenterol 2018 [107] | Retrospective | 87 patients, adult | Same | IBD | AZA + 6-MP >8 weeks | Mesalazine; biologics, cyclosporine and tacrolimus not allowed | 6-TGN | Multiple (nadir values, median and peak available; analysis made with median) | NS | 63.1 |
| Zochowska et al., Transplant Proc 2016 [108] | NS | 33 patients, adult | Same | Transplant | AZA NS | Calcineurin inhibitors, steroids | 6-TGN and 6-MMPR | NS | Other (description provided) | 51.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, P.; Estevinho, M.M.; Dias, C.C.; Ministro, P.; Kopylov, U.; Danese, S.; Peyrin-Biroulet, L.; Magro, F. Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis. J. Clin. Med. 2020, 9, 2216. https://doi.org/10.3390/jcm9072216
Sousa P, Estevinho MM, Dias CC, Ministro P, Kopylov U, Danese S, Peyrin-Biroulet L, Magro F. Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis. Journal of Clinical Medicine. 2020; 9(7):2216. https://doi.org/10.3390/jcm9072216
Chicago/Turabian StyleSousa, Paula, Maria Manuela Estevinho, Cláudia Camila Dias, Paula Ministro, Uri Kopylov, Silvio Danese, Laurent Peyrin-Biroulet, and Fernando Magro. 2020. "Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis" Journal of Clinical Medicine 9, no. 7: 2216. https://doi.org/10.3390/jcm9072216
APA StyleSousa, P., Estevinho, M. M., Dias, C. C., Ministro, P., Kopylov, U., Danese, S., Peyrin-Biroulet, L., & Magro, F. (2020). Thiopurines’ Metabolites and Drug Toxicity: A Meta-Analysis. Journal of Clinical Medicine, 9(7), 2216. https://doi.org/10.3390/jcm9072216









