Organ Stiffness in the Work-Up of Myelofibrosis and Philadelphia-Negative Chronic Myeloproliferative Neoplasms
Abstract
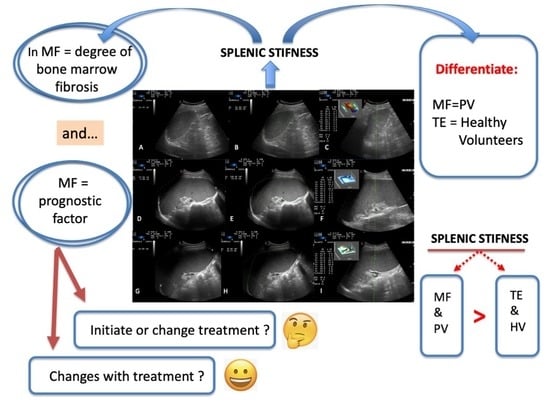
1. Introduction
2. Experimental Section: Patients and Methods
2.1. Patients
2.2. Methods
2.2.1. Ultrasonographic Examination
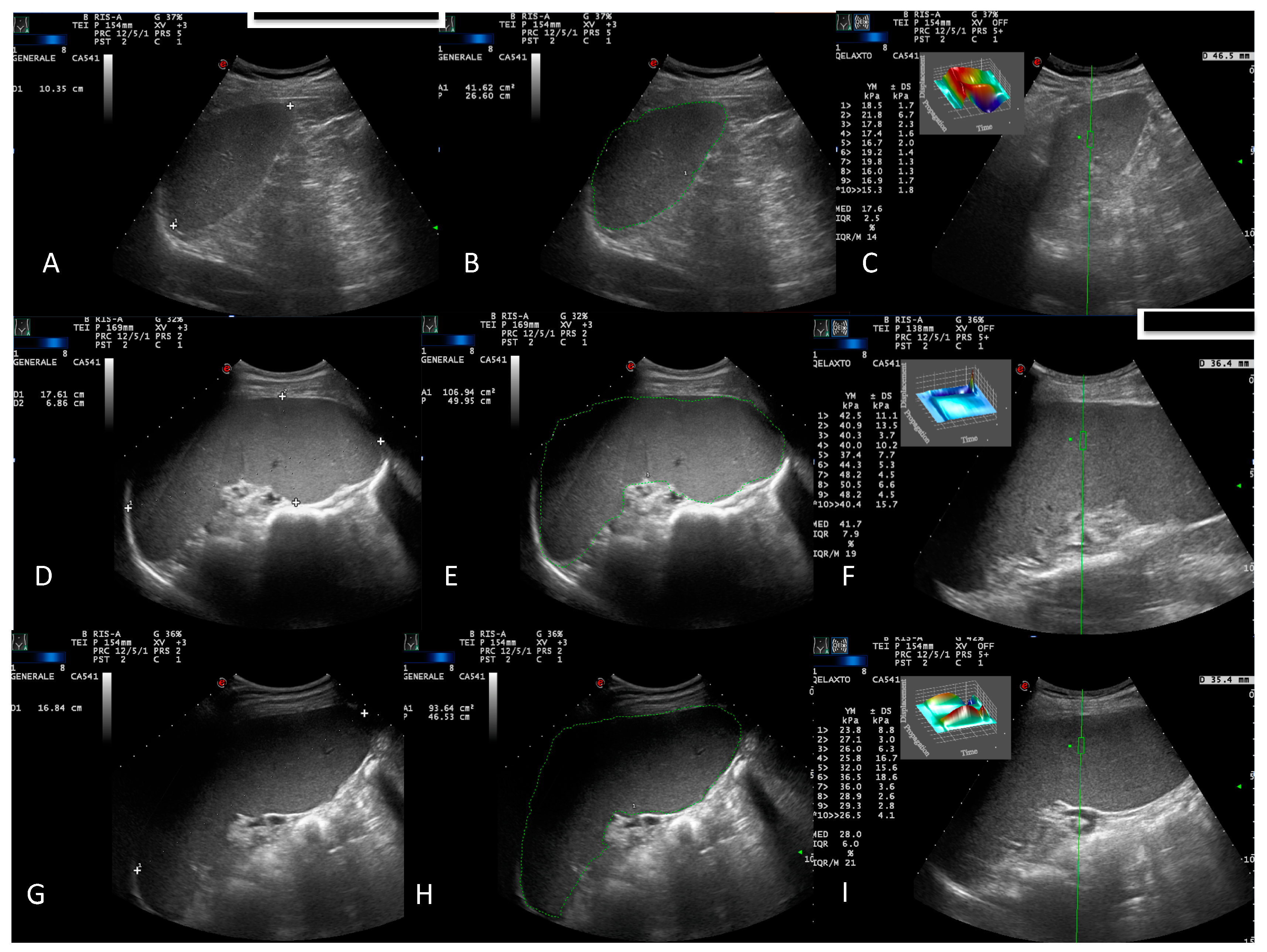
- B-mode US evaluation of the liver, spleen, kidneys, gallbladder, pancreas, bladder, and retroperitoneal and splanchnic abdominal vessels (splenic and portal veins) [15]. US measurements of the liver and spleen were performed and expressed in cm, as previously described [16]. A patient’s spleen was evaluated in supine decubitus through the intercostal window. The US window, which included the splenic hilum, was considered optimal for biometric measurement of the organ. Measurements of the splenic longitudinal diameter (SLD) and cross-sectional area (CSA) were expressed in cm and cm2, respectively (Figure 1A,B,D,E,G,H). An SLD up to 11–12 cm in the cranio-caudal length and a CSA < 45 cm2 were considered normal [17], while moderate and marked splenomegaly were defined as a CSA in the range of 45–65 cm2 and >65 cm2, respectively [18];
- Portal vein diameter measured at the crossing point with the hepatic artery and expressed in mm [19];
- Portal vein flow velocity (PVV), expressed in cm/sec, with an intercostal window and sampled at the hepatic hilum. The maximum velocity (Vmax) and mean velocity (Vm) were taken in all patients and controls [19].
2.2.2. Elastosonographic Examination
2.2.3. Statistical Analysis
3. Results
3.1. Study Population
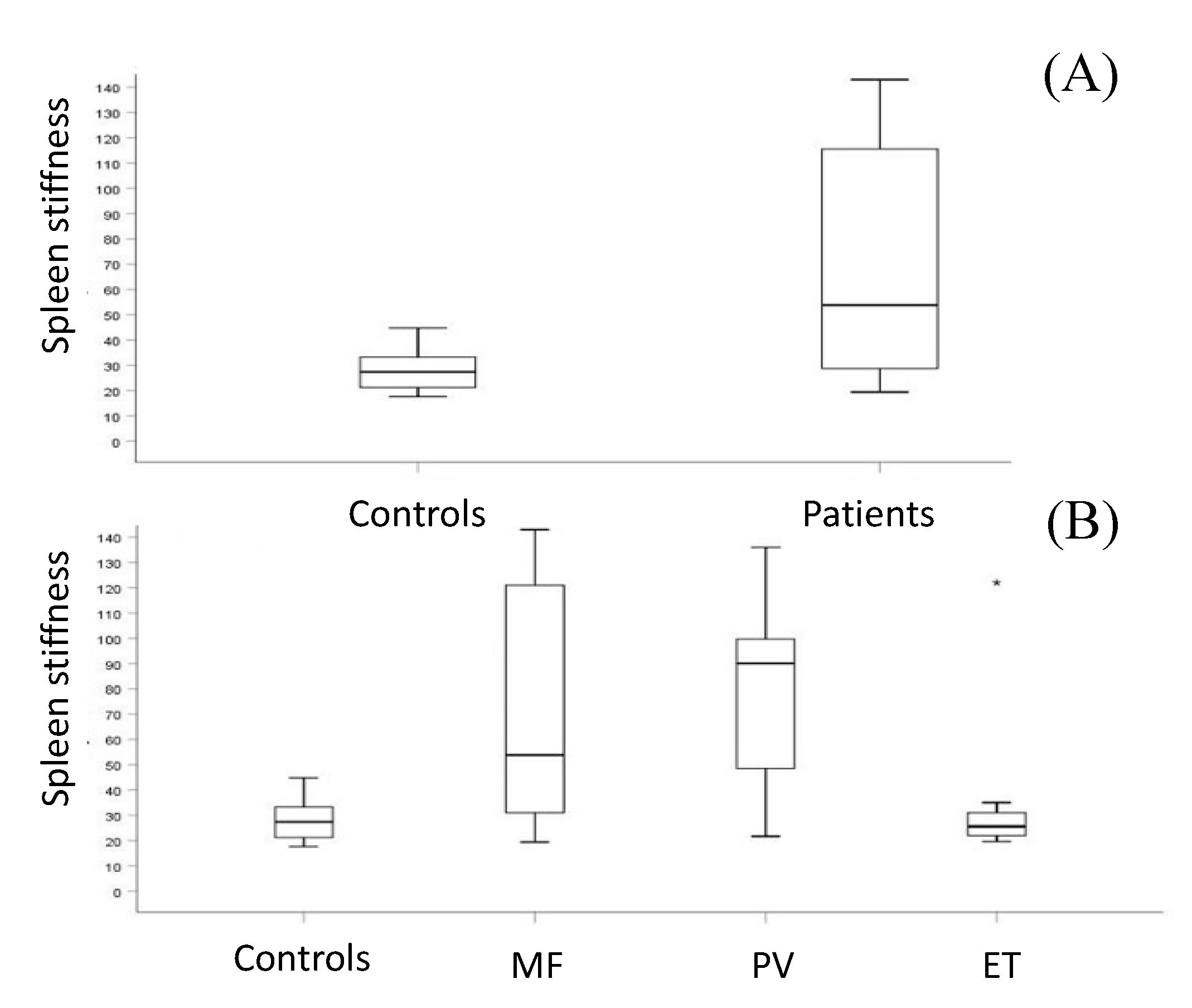
3.2. Splenic and Liver Assessment
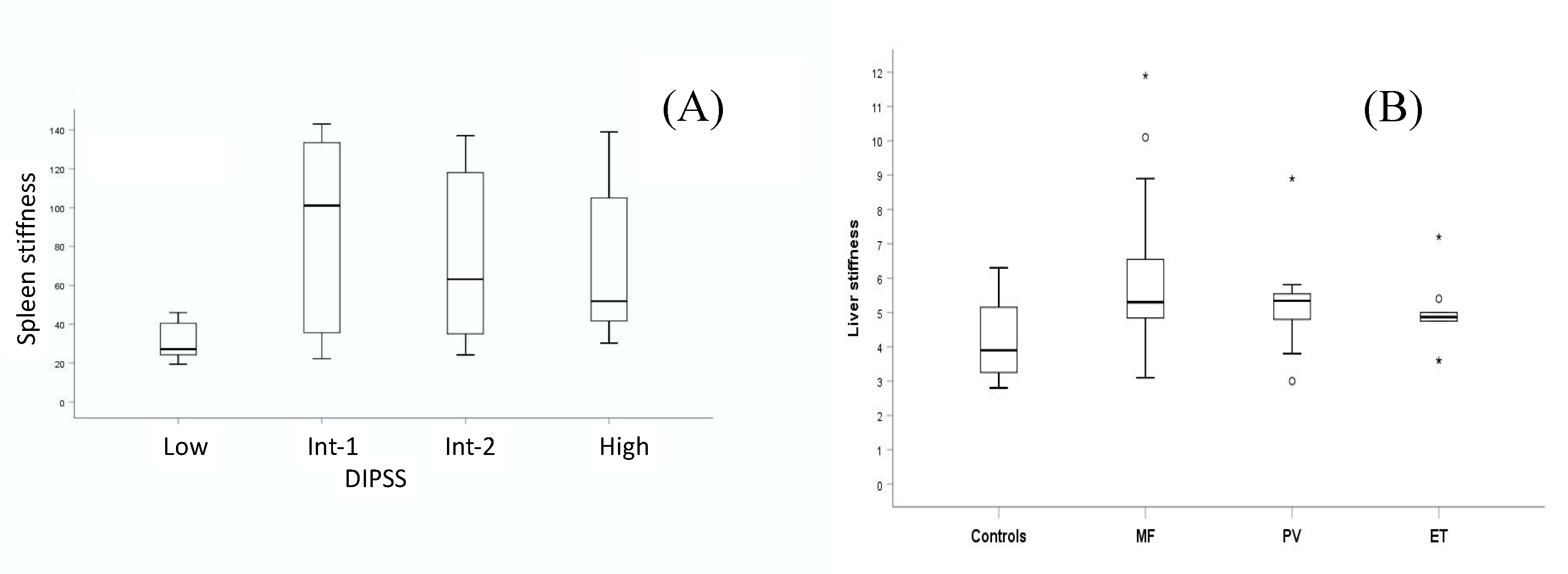
3.3. Spleen Stiffness and Disease Progression
3.4. Spleen Stiffness and Ruxolilitinib
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Cervantes, F.; Dupriez, B.; Pereira, A.; Passamonti, F.; Reilly, J.T.; Morra, E.; Vannucchi, A.M.; Mesa, R.A.; Demory, J.-L.; Barosi, G.; et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009, 113, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Cervantes, F.; Vannucchi, A.M.; Morra, E.; Rumi, E.; Cazzola, M.; Tefferi, A. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood 2010, 116, 2857–2858. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Caramazza, D.; Vaidya, R.; George, G.; Begna, K.; Schwager, S.; Van Dyke, D.; Hanson, C.; Wu, W.; Pardanani, A.; et al. DIPSS plus: A refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J. Clin. Oncol. 2011, 29, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, A.T.; Talati, C.; Padron, E.; Sweet, K.; Sallman, D.; List, A.F.; Lancet, J.E.; Komrokji, R.S. Genetically inspired prognostic scoring system (GIPSS) outperforms dynamic international prognostic scoring system (DIPSS) in myelofibrosis patients. Am. J. Hematol. 2019, 94, 87–92. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Lasho, T.L.; Rotunno, G.; Mudireddy, M.; Mannarelli, C.; Nicolosi, M.; Pacilli, A.; Pardanani, A.; Rumi, E.; Rosti, V.; et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients with Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 310–318. [Google Scholar] [CrossRef]
- Tefferi, A.; Guglielmelli, P.; Lasho, T.L.; Gangat, N.; Ketterling, R.P.; Pardanani, A.; Vannucchi, A.M. MIPSS70+ Version 2.0: Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis. J. Clin. Oncol. 2018, 36, 1769–1770. [Google Scholar] [CrossRef]
- Vener, C.; Fracchiolla, N.S.; Gianelli, U.; Calori, R.; Radaelli, F.; Iurlo, A.; Caberlon, S.; Gerli, G.; Boiocchi, L.; Deliliers, G.L. Prognostic implications of the European consensus for grading of bone marrow fibrosis in chronic idiopathic myelofibrosis. Blood 2008, 111, 1862–1865. [Google Scholar] [CrossRef][Green Version]
- Gianelli, U.; Vener, C.; Bossi, A.; Cortinovis, I.; Iurlo, A.; Fracchiolla, N.S.; Savi, F.; Moro, A.; Grifoni, F.; De Philippis, C.; et al. The European Consensus on grading of bone marrow fibrosis allows a better prognostication of patients with primary myelofibrosis. Mod. Pathol. 2012, 25, 1193–1202. [Google Scholar] [CrossRef]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Ophir, J.; Cespedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K. Acoustic Radiation Force Impulse (ARFI) Imaging: A Review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, M.; Kojima, T.; Itoh, Y.; Harano, Y.; Fujii, K.; Nakajima, T.; Kato, T.; Takeda, N.; Okuda, J.; Ida, K.; et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 2007, 102, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Giuffrè, M.; Macor, D.; Masutti, F.; Abazia, C.; Tinè, F.; Patti, R.; Buonocore, M.R.; Colombo, A.; Visintin, A.; Campigotto, M.; et al. Evaluation of spleen stiffness in healthy volunteers using point shear wave elastography. Ann. Hepatol. 2019, 18, 736–741. [Google Scholar] [CrossRef]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.-M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Niederau, C.; Sonnenberg, A.; Muller, J.E.; Erckenbrecht, J.F.; Scholten, T.; Fritsch, W.P. Sonographic measurements of the normal liver, spleen, pancreas, and portal vein. Radiology 1983, 149, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Lamb, P.M.; Lund, A.; Kanagasabay, R.R.; Martin, A.; Webb, J.A.W.; Reznek, R.H. Spleen size: How well do linear ultrasound measurements correlate with three-dimensional CT volume assessments? Br. J. Radiol. 2002, 75, 573–577. [Google Scholar] [CrossRef]
- Gaiani, S.; Gramantieri, L.; Venturoli, N.; Piscaglia, F.; Siringo, S.; D’Errico, A.; Zironi, G.; Grigioni, W.; Bolondi, L. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J. Hepatol. 1997, 27, 979–985. [Google Scholar] [CrossRef]
- Berzigotti, A.; Piscaglia, F. Ultrasound in portal hypertension--part 1. Ultraschall Med. 2011, 32, 548–571. [Google Scholar] [CrossRef]
- Castera, L.; Foucher, J.; Bernard, P.-H.; Carvalho, F.; Allaix, D.; Merrouche, W.; Couzigou, P.; de Ledinghen, V. Pitfalls of liver stiffness measurement: A 5-year prospective study of 13,369 examinations. Hepatology 2010, 51, 828–835. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Bamber, J.; Berzigotti, A.; Bota, S.; Cantisani, V.; Castera, L.; Cosgrove, D.; Ferraioli, G.; Friedrich-Rust, M.; Gilja, O.H.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017, 38, e16–e47. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Zheng, J.; Huang, Z.P.; Xiao, Y.; Song, D.; Zeng, J.; Zheng, H.R.; Zheng, R.Q. Influence of Measurement Depth on the Stiffness Assessment of Healthy Liver with Real-Time Shear Wave Elastography. Ultrasound Med. Biol. 2014, 40, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Sporea, I.; Sirli, R.L.; Deleanu, A.; Popescu, A.; Focsa, M.; Danila, M.; Tudora, A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011, 32, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.G.; Ferraioli, G.; Palmeri, M.L.; Goodman, Z.D.; Garcia-Tsao, G.; Rubin, J.; Garra, B.; Myers, R.P.; Wilson, S.R.; Rubens, D.; et al. Elastography Assessment of Liver Fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Ultrasound Q. 2016, 32, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Procopet, B.; Berzigotti, A.; Abraldes, J.G.; Turon, F.; Hernandez-Gea, V.; García-Pagán, J.C.; Bosch, J. Real-time shear-wave elastography: Applicability, reliability and accuracy for clinically significant portal hypertension. J. Hepatol. 2015, 62, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Lee, J.Y.; Yoon, J.H.; Kim, W.; Cho, B.; Choi, B.I. Comparison of the reliability of acoustic radiation force impulse imaging and supersonic shear imaging in measurement of liver stiffness1. Radiology 2015, 277, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Saftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef]
- Barosi, G.; Mesa, R.; Finazzi, G.; Harrison, C.; Kiladjian, J.-J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; Vannucchi, A.M.; Besses, C.; et al. Revised response criteria for polycythemia vera and essential thrombocythemia: An ELN and IWG-MRT consensus project. Blood 2013, 121, 4778–4781. [Google Scholar] [CrossRef]
- Tefferi, A.; Cervantes, F.; Mesa, R.; Passamonti, F.; Verstovsek, S.; Vannucchi, A.M.; Gotlib, J.; Dupriez, B.; Pardanani, A.; Harrison, C.; et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013, 122, 1395–1398. [Google Scholar] [CrossRef]
- Pardanani, A.; Finke, C.; Abdelrahman, R.A.; Lasho, T.L.; Tefferi, A. Associations and prognostic interactions between circulating levels of hepcidin, ferritin and inflammatory cytokines in primary myelofibrosis. Am. J. Hematol. 2013, 88, 312–316. [Google Scholar] [CrossRef]
- Iurlo, A.; Cattaneo, D.; Giunta, M.; Gianelli, U.; Consonni, D.; Fraquelli, M.; Orofino, N.; Bucelli, C.; Bianchi, P.; Augello, C.; et al. Transient elastography spleen stiffness measurements in primary myelofibrosis patients: A pilot study in a single centre. Br. J. Haematol. 2015, 170, 890–892. [Google Scholar] [CrossRef]
- Emanuel, R.M.; Dueck, A.C.; Geyer, H.L.; Kiladjian, J.-J.; Slot, S.; Zweegman, S.; Te Boekhorst, P.A.W.; Commandeur, S.; Schouten, H.C.; Sackmann, F.; et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J. Clin. Oncol. 2012, 30, 4098–4103. [Google Scholar] [CrossRef] [PubMed]
- Savona, M.R. Are we altering the natural history of primary myelofibrosis? Leuk. Res. 2014, 38, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.S.S.; Bryant, R.V.; Dong, Y.; Maaser, C.; Kucharzik, T.; Maconi, G.; Asthana, A.K.; Blaivas, M.; Goudie, A.; Gilja, O.H.; et al. WFUMB Position Paper. Learning Gastrointestinal Ultrasound: Theory and Practice. Ultrasound Med. Biol. 2016, 42, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Eman, A.R.; Maha, M.E.; Alsebaey, A.; Elmazaly, M.A.; ElSayed, S.T.; Hanaa, M.B.; NermIne, A.E. Evaluation of the Role of Liver and Splenic Transient Elastography in Chronic Hepatitis C Related Fibrosis. J. Liver Dis. Transpl. 2016, 3. [Google Scholar]
- Sharma, P.; Kirnake, V.; Tyagi, P.; Bansal, N.; Singla, V.; Kumar, A.; Arora, A. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am. J. Gastroenterol. 2013, 108, 1101–1107. [Google Scholar] [CrossRef]
- Karatzas, A.; Konstantakis, C.; Aggeletopoulou, I.; Kalogeropoulou, C.; Thomopoulos, K.; Triantos, C. Νon-invasive screening for esophageal varices in patients with liver cirrhosis. Ann. Gastroenterol. 2018, 31, 305–314. [Google Scholar] [CrossRef]
- Sekhar, M.; Roselli, M.; PATCH, D.W.; Tripathi, D.; Danaee, A.; Li, J.; Kwong, J.; Yu, D.; Roccarina, D. Spleen Stiffness in Myeloproliferative Disease Related Splanchnic Vein Thrombosis. Blood 2016, 128, 5468. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Raso, S.; Contrino, A.; Casimiro, P.; Di Piazza, F.; Perez, A.; Russo, A.; Siragusa, S. Splenomegaly impacts prognosis in essential thrombocythemia and polycythemia vera: A single center study. Hematol. Rep. 2019, 11, 8281. [Google Scholar] [CrossRef]
- Barraco, D.; Cerquozzi, S.; Hanson, C.A.; Ketterling, R.P.; Pardanani, A.; Gangat, N.; Tefferi, A. Prognostic impact of bone marrow fibrosis in polycythemia vera: Validation of the IWG-MRT study and additional observations. Blood Cancer J. 2017, 7, e538. [Google Scholar] [CrossRef]
- Lekovic, D.; Gotic, M.; Perunicic-Jovanovic, M.; Vidovic, A.; Bogdanovic, A.; Jankovic, G.; Cokic, V.; Milic, N. Contribution of comorbidities and grade of bone marrow fibrosis to the prognosis of survival in patients with primary myelofibrosis. Med. Oncol. 2014, 31, 869. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Pardanani, A.; Bernard, O.A.; Finazzi, G.; Crispino, J.D.; Gisslinger, H.; Kralovics, R.; Odenike, O.; Bhalla, K.; Gupta, V.; et al. Unraveling the genetic underpinnings of myeloproliferative neoplasms and understanding their effect on disease course and response to therapy: Proceedings from the 6th International Post-ASH Symposium. Am. J. Hematol. 2012, 87, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Kvasnicka, M.; Thiele, J. Replacement of hematopoietic system by allogeneic stem cell transplantation in myelofibrosis patients induces rapid regression of bone marrow fibrosis. Fibrogenesis Tissue Repair 2012, 5, S25. [Google Scholar] [CrossRef] [PubMed]
- Silver, R.T.; Vandris, K.; Goldman, J.J. Recombinant interferon-alpha may retard progression of early primary myelofibrosis: A preliminary report. Blood 2011, 117, 6669–6672. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Mesa, R.A.; Gotlib, J.; Levy, R.S.; Gupta, V.; DiPersio, J.F.; Catalano, J.V.; Deininger, M.; Miller, C.; Silver, R.T.; et al. A Double-Blind, Placebo-Controlled Trial of Ruxolitinib for Myelofibrosis. N. Engl. J. Med. 2012, 366, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.; Vannucchi, A.M.; Kiladjian, J.-J.; Al-Ali, H.K.; Sirulnik, A.; Stalbovskaya, V.; McQuitty, M.; Hunter, D.S.; Levy, R.S.; Passamonti, F.; et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 2013, 122, 4047–4053. [Google Scholar] [CrossRef]

| Age | 68 (IQR 1: 53.5–76.0) |
| Sex | |
| Male | 35 |
| Female | 35 |
| Diagnosis | |
| Primary MF | 26 (41.3%) |
| Secondary MF | 17 (20.3%) |
| PV | 17 (24.3%) |
| ET | 10 (14.3%) |
| BMI (Body Mass Index) | 24 (IQR 23–26) |
| Mutational status | |
| JAK2 (V617F) | 49 (70%) |
| CALR | 11 (16%) |
| None | 10 (14%) |
| Kariotype | |
| Normal | 60 (87.1%) |
| Complex | 4 (5.7%) |
| Other | 6 (8.6%) |
| Bone Marrow blasts (biopsy) | 5% (3–5%) |
| BM fibrosis | |
| 0 | 3 (4.3%) |
| 1 | 29 (41.4%) |
| 2 | 19 (27.1%) |
| 3 | 19 (27.1%) |
| Symptoms | |
| none | 43 (61.4%) |
| Blood test | |
| WBC | |
| MF | 8.120 × 109/L (range 1.170–34.000) |
| ET | 7.000 × 109/L (range 5.000–15.700) |
| PV | 9.000 × 109/L (range 3.310–140.000) |
| Hemoglobin | |
| MF | 12 gr/dL (range 8–18) |
| ET | 12.9 gr/dL (range 10–15) |
| PV | 18 gr/dL (range 9–21) |
| Hematocrit | |
| MF | 36% (range 22–49) |
| ET | 40% (range 36–47) |
| PV | 53% (range 29–61) |
| Platelet | |
| MF | 400.000 × 109/L (range 55.000–900.000) |
| ET | 700.000 × 109/L (range 150–900.000) |
| PV | 450.000 × 109/L (range 90.000–913.000) |
| LDH | |
| MF | 462 U/L (range 200–2630) |
| ET | 243 U/L (100–550) |
| PV | 263 U/L (100–450) |
| Ferritin | |
| MF | 122 ng/mL (21–200) |
| ET | 75.5 ng/mL (21–129) |
| PV | 100 ng/mL (9–122) |
| Patients treated with Ruxolitinib | |
| Policitemia Vera | 4 (18%) |
| Myelofibrosis | 10 (82%) |
| Parameter | M Spleen LD | M Spleen CSA | M Splenic Stiffness | M Diameter of Splenic Vein | M Liver Stiffness | M Diameter of Portal Vein | Portal Vein Flow | Parameter |
|---|---|---|---|---|---|---|---|---|
| (in cm) | (in cm2) | (in kPa) | (in mm) | (in kPa) | (in mm) | M Vmax/M Vm | ||
| MPN | 16.7 | 91.5 | 53.8 | 8 | 5 (r 4.5–6.9) | 11 | 41.5/31.9 | MPN |
| (70 pts) | (r 13.8–19.7) | (r 67.4–121) | (r 19.4–143) | (r 7–19) | (r 4–11) | (r 34–42)/(r 24–34) | (70 pts) | |
| Healthy Controls | 11.1 | 34 | 27.5 | 6.9 | 3.9 | 10.1 | 31.6/32 | Healthy Controls |
| −20 | (r 7.9–12.5) | (r 21–55) | (r 22.5–42.3)/(r 21.5–39.9) | −20 | ||||
| MF | 53.96 | 5.3 | MF | |||||
| (43 pts) | (r 31–121) | (r 3.1–11.9) | (43 pts) | |||||
| MF-0 MF-1 | 30.3 | MF-0 MF-1 | ||||||
| (11 pts) | (r 19.4–132) | (11 pts) | ||||||
| MF-2 MF-3 | 99.7 | MF-2 MF-3 | ||||||
| (32 pts) | (r 24.2–164.3) | (32 pts) | ||||||
| PMF | 50.7 | PMF | ||||||
| (26 pts) | (r 19.4–143) | (26 pts) | ||||||
| SMF | 107 | SMF | ||||||
| (17 pts) | (r 24–141) | (17 pts) | ||||||
| E.T. | 25.6 | 4.95 | E.T. | |||||
| (10 pts) | (r 22–31) | (r 3.6–7.2) | (10 pts) | |||||
| P.V. | 90.1 | 5.3 | P.V. | |||||
| (17 pts) | (r 48.5–99.7) | (r 3–8.9) | (17 pts) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Rho or Median (IQR) | p-Value | RC | p-Value |
| Age | 0.163 | |||
| Range: 68 years (IQR 53.5–76) | 0.171 | |||
| PV | 0.388 | |||
| (1) yes | 90.1 (IQR 48.5–99.7) | |||
| (0) no | 46.5 (IQR 27.7–119.0) | |||
| ET | 0.076 | 0.121 | ||
| (1) yes | 25.6 (IQR 22.0–31.0) | −21.6 | ||
| (0) no | 76.6 (IQR 35–121) | |||
| MF | 0.141 | |||
| (1) yes | 53.9 (IQR 31.0–121.0) | |||
| (0) no | 45.5 (IQR 24.1–97.9) | |||
| BMI | 0.126 | |||
| Range: 24 (IQR 23–26) | 0.187 | |||
| Mutational status | 0.411 | |||
| (0) none | 30.7 (IQR 26.3–71.6) | |||
| (1) JAK2 (V617F) | 80 (IQR 30.6–115.5) | |||
| (2) CALR or MPL | 41.7 (IQR 31.7–124.5) | |||
| Karyotype | 0.338 | |||
| (0) missing value | 92.1 (IQR 73.4–96.1) | |||
| (1) favourable | 47 (IQR 26.7–112) | |||
| (2) unfavorable | 108.3 (IQR 71.6–137) | |||
| Bone marrow blasts | 0.27 | |||
| Range: 5% (IQR 3–5%) | 0.136 | |||
| Bone marrow fibrosis | <0.0001 | <0.0001 | ||
| Score: 0–4 | 0.584 | 22.8 | ||
| Symptoms | 0.129 | |||
| (0) no | 41.7 (IQR 28.3–119) | |||
| (1) yes | 73.2 (IQR 31–121) | |||
| White cell count | 0.177 | |||
| Range: 8505/mcL (IQR 5000–12,200) | 0.166 | |||
| Hgb | 0.725 | |||
| Range: 12.4 g/dL (IQR 10.6–15) | −0.043 | |||
| HcT | 0.966 | |||
| Range: 38% (IQR 32.5–47) | −0.005 | |||
| PLT | 0.102 | |||
| Range: 450,000/mcL (IQR 180,000–623,000) | −0.197 | |||
| LDH | 0.187 | |||
| Range: 336 U/L (IQR 232–541) | 0.191 | |||
| Splenic LD | 0.211 | |||
| Range: 16.7 cm (IQR 13.8–19.7) | 1.154 | |||
| Splenic CSA | 0.080 | 0.095 | ||
| Range: 91.5 cm2 (IQR 67.4–121) | 0.211 | −0.21 | ||
| Liver LD | 0.001 | 0.067 | ||
| Range: 16 cm (IQR: 15–18) | 0.384 | 4.29 | ||
| Splenic vein | 0.368 | |||
| Range: 8 mm (IQR 7–9) | 0.111 | |||
| Portal vein | 0.308 | |||
| Range: 11 mm (IQR 9–11.7) | −0.125 | |||
| Mean velocity Range: 31.9 cm/s (IQR 24.5–34) | 0.156 | 0.17 | ||
| Maximum velocity | 0.454 | |||
| Range: 41.5 cm/s (IQR 34–42) | 0.092 | |||
| Liver S | 0.455 | <0.001 | −22.0 | 0.478 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, E.; Tavarozzi, R.; Morganti, R.; Bruno, B.; Bramanti, E.; Baratè, C.; Balducci, S.; Iovino, L.; Ricci, F.; Ricchiuto, V.; et al. Organ Stiffness in the Work-Up of Myelofibrosis and Philadelphia-Negative Chronic Myeloproliferative Neoplasms. J. Clin. Med. 2020, 9, 2149. https://doi.org/10.3390/jcm9072149
Benedetti E, Tavarozzi R, Morganti R, Bruno B, Bramanti E, Baratè C, Balducci S, Iovino L, Ricci F, Ricchiuto V, et al. Organ Stiffness in the Work-Up of Myelofibrosis and Philadelphia-Negative Chronic Myeloproliferative Neoplasms. Journal of Clinical Medicine. 2020; 9(7):2149. https://doi.org/10.3390/jcm9072149
Chicago/Turabian StyleBenedetti, Edoardo, Rita Tavarozzi, Riccardo Morganti, Benedetto Bruno, Emilia Bramanti, Claudia Baratè, Serena Balducci, Lorenzo Iovino, Federica Ricci, Vittorio Ricchiuto, and et al. 2020. "Organ Stiffness in the Work-Up of Myelofibrosis and Philadelphia-Negative Chronic Myeloproliferative Neoplasms" Journal of Clinical Medicine 9, no. 7: 2149. https://doi.org/10.3390/jcm9072149
APA StyleBenedetti, E., Tavarozzi, R., Morganti, R., Bruno, B., Bramanti, E., Baratè, C., Balducci, S., Iovino, L., Ricci, F., Ricchiuto, V., Buda, G., & Galimberti, S. (2020). Organ Stiffness in the Work-Up of Myelofibrosis and Philadelphia-Negative Chronic Myeloproliferative Neoplasms. Journal of Clinical Medicine, 9(7), 2149. https://doi.org/10.3390/jcm9072149