Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Data Collection
2.3. Antinuclear Antibody Detection
2.4. Polymorphonuclear Neutrophils (PMN) Isolation and Stimulation
2.5. Visualization and Quantification of Neutrophil Extracellular Traps (NETs)
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
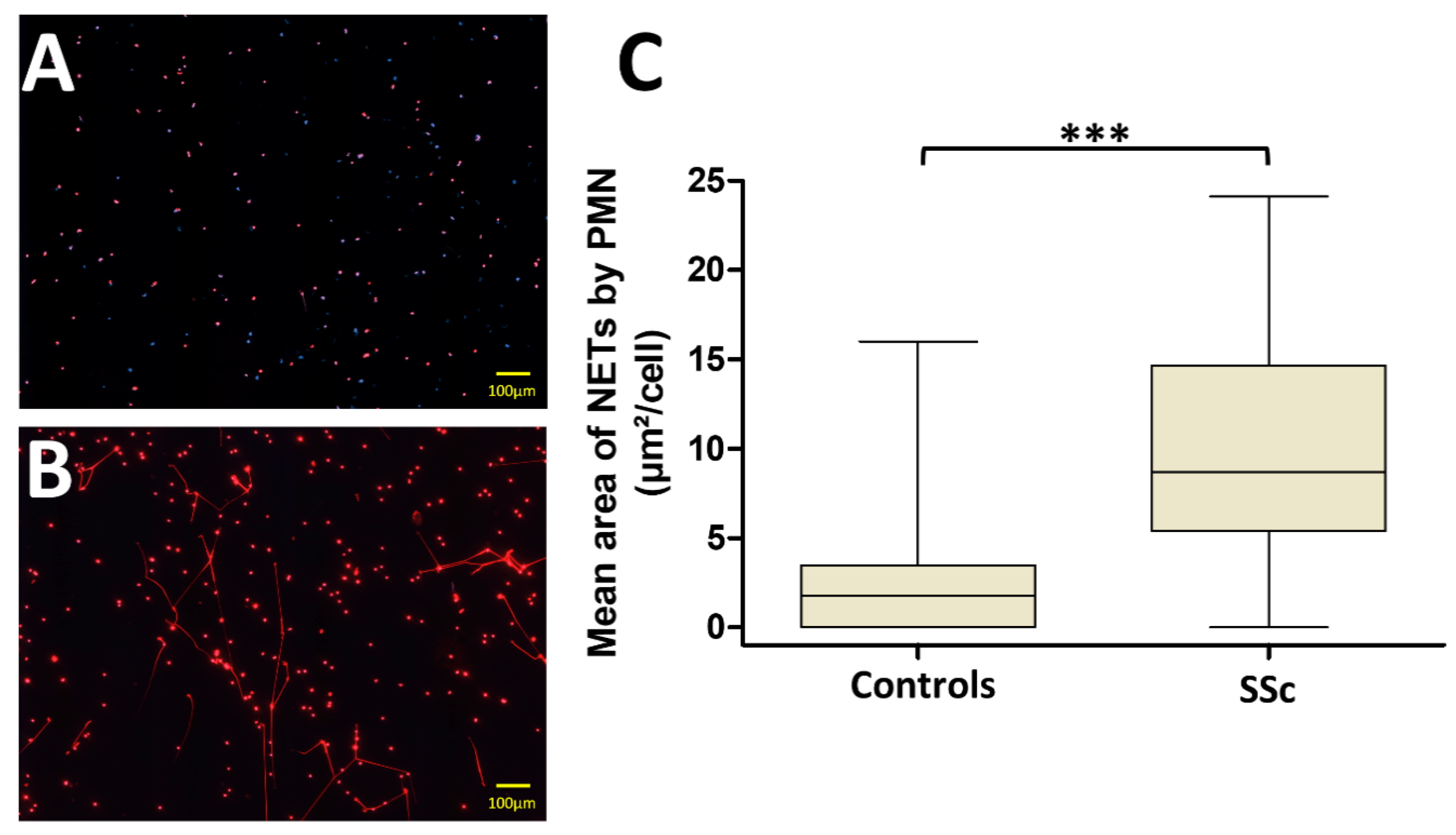
3.2. PMN from Systemic Sclerosis (SSc) Patients Generate More NETs Than Those from Controls
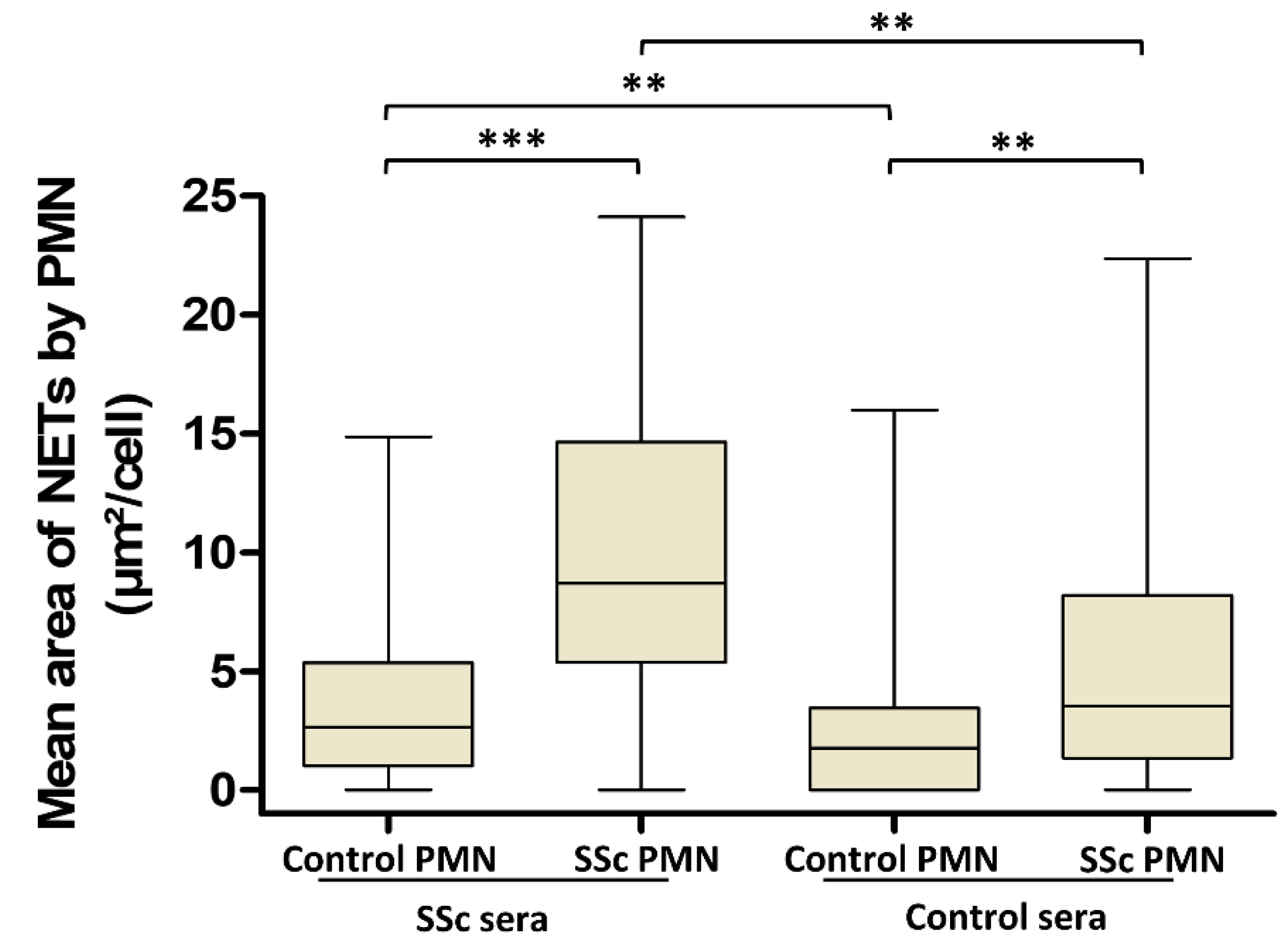
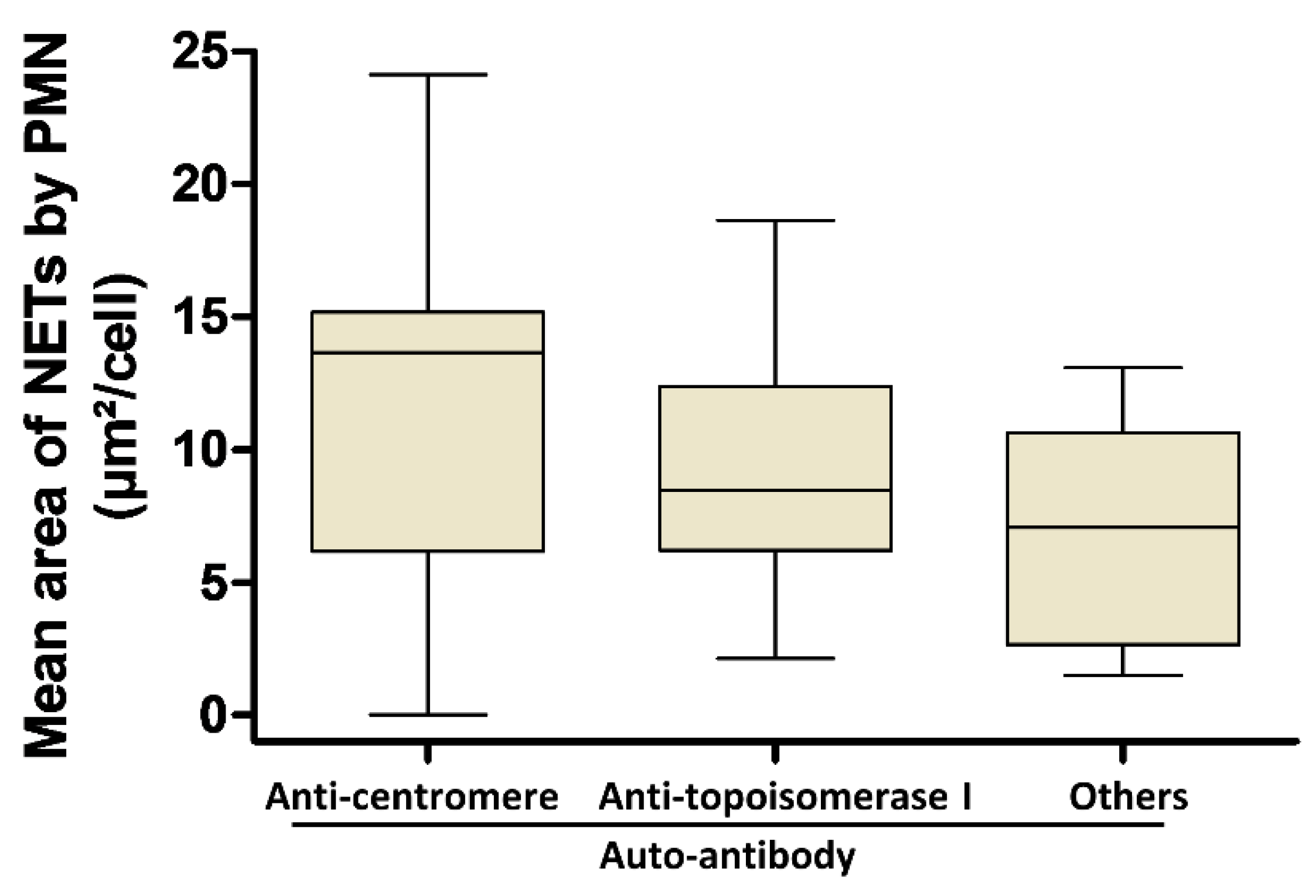
3.3. Involvement of Both PMN Activation Status and Serum Factors in the Level of NET Production Is Not Dependent on AAb Specificity in SSc
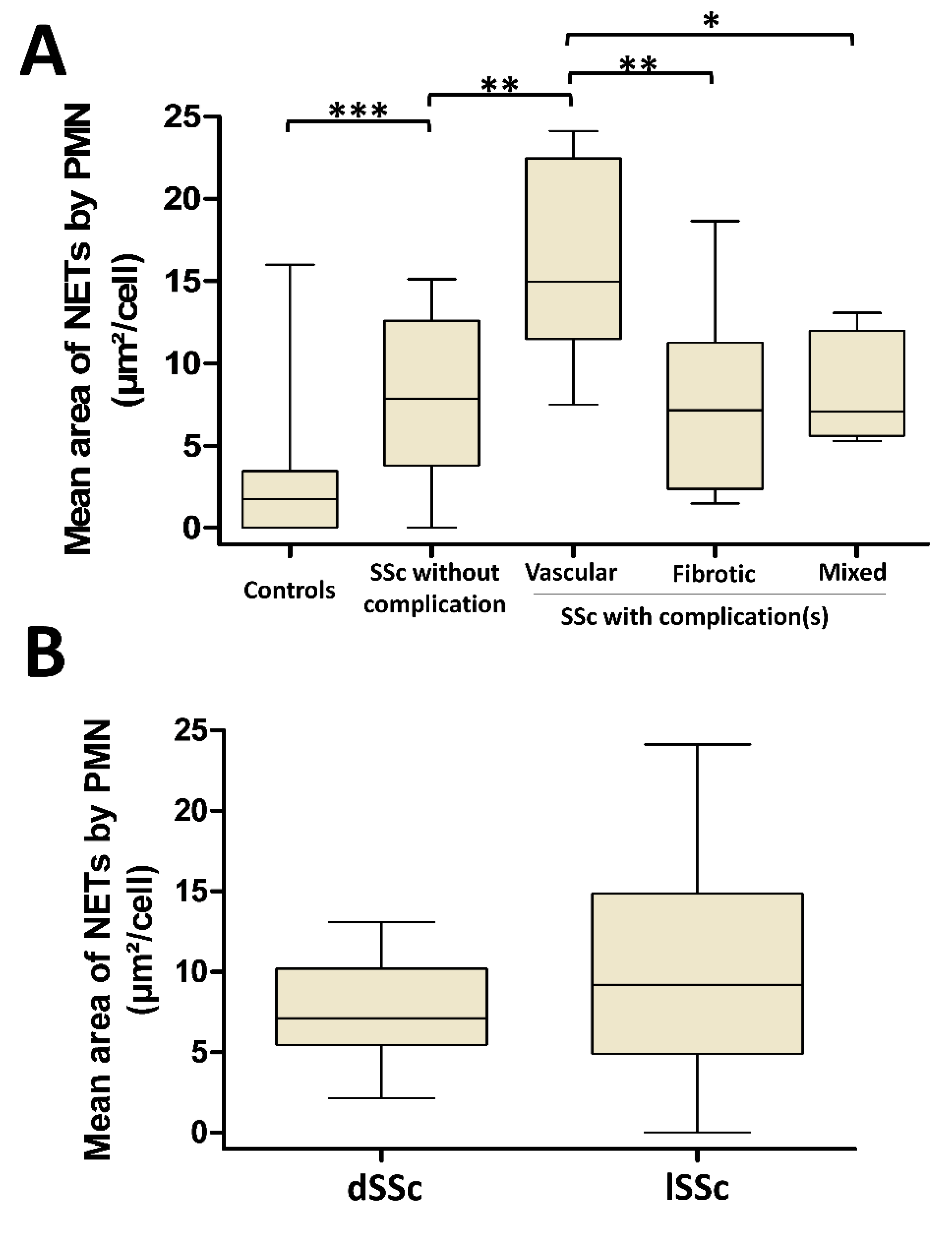
3.4. NETosis Is Higher in SSc Patients with Vascular Complications But Is Not Dependent on SSc Cutaneous Phenotype
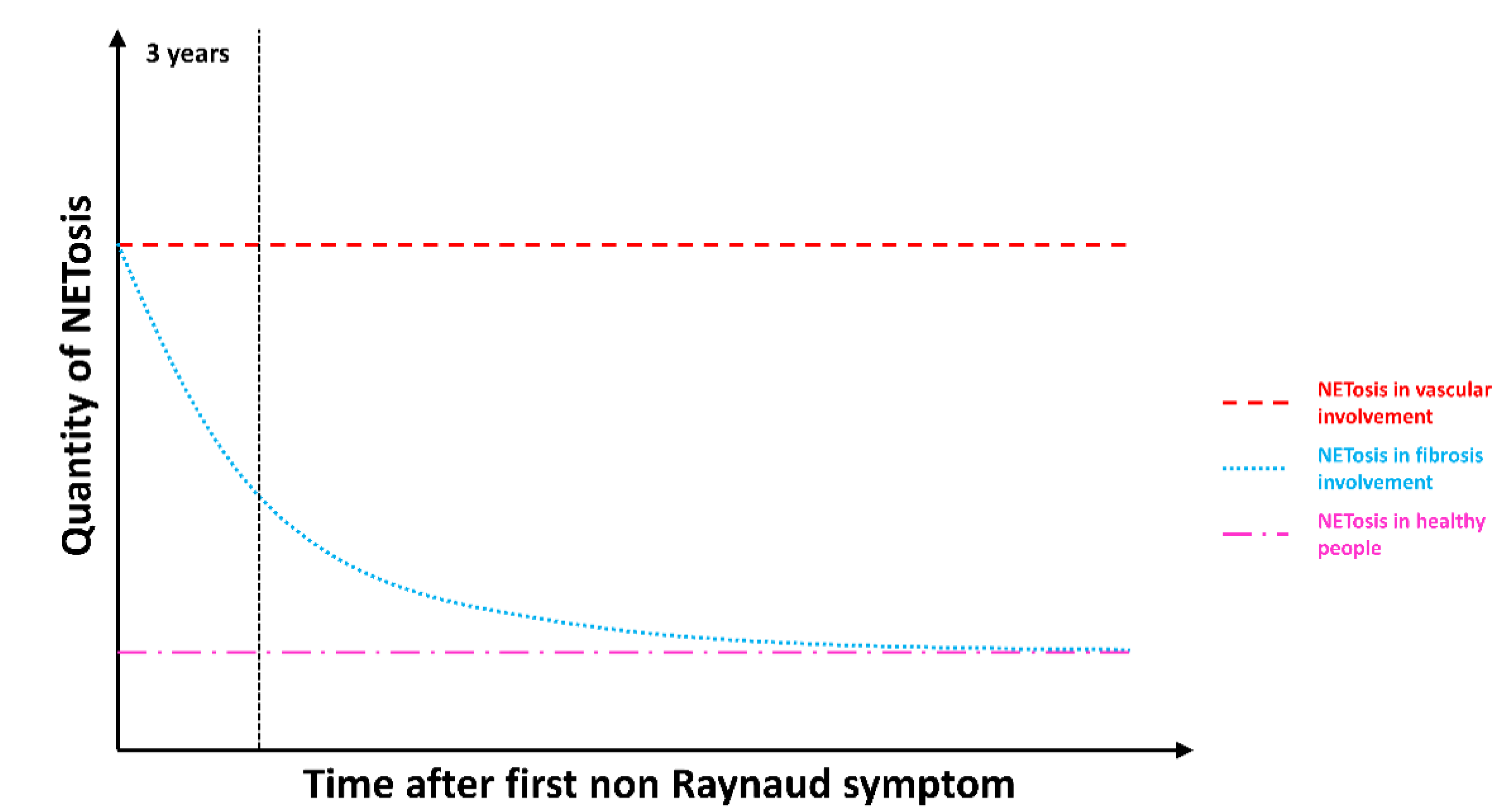
3.5. NETosis Is an Early Process Occurring in SSc That Lasts in SSc Patients with Vascular Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthr. Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Meune, C.; Boubaya, M.; Avouac, J.; Hachulla, E.; Balbir-Gurman, A.; Riemekasten, G.; Airò, P.; Joven, B.; Vettori, S.; et al. Mapping and predicting mortality from systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Tyndall, A.; Czirják, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Guiducci, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Mechanisms in the loss of capillaries in systemic sclerosis: Angiogenesis versus vasculogenesis. J. Cell Mol. Med. 2010, 14, 1241–1254. [Google Scholar] [CrossRef]
- Kuwana, M.; Kaburaki, J.; Okano, Y.; Tojo, T.; Homma, M. Clinical and prognostic associations based on serum antinuclear antibodies in Japanese patients with systemic sclerosis. Arthr. Rheum. 1994, 37, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pellar, R.E.; Pope, J.E. Evidence-based management of systemic sclerosis: Navigating recommendations and guidelines. Semin. Arthr. Rheum. 2017, 46, 767–774. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Maureen, D.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; et al. Myeloablative autologous stem-cell transplantation for severe scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef]
- Didier, K.; Robbins, A.; Antonicelli, F.; Pham, B.N.; Giusti, D.; Servettaz, A. Updates in systemic sclerosis pathogenesis: Toward new therapeutic opportunities. La Rev. De Med. Interne 2019, 40, 654–663. [Google Scholar] [CrossRef]
- Didier, K.; Bolko, L.; Giusti, D.; Toquet, S.; Robbins, A.; Antonicelli, F.; Servettaz, A. Autoantibodies associated with connective tissue diseases: What meaning for clinicians? Front. Immunol. 2018, 9, 541. [Google Scholar] [CrossRef]
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Vona, R.; Giovannetti, A.; Gambardella, L.; Malorni, W.; Pietraforte, D.; Straface, E. Oxidative stress in the pathogenesis of systemic scleroderma: An overview. J. Cell Mol. Med. 2018, 22, 3308–3314. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef]
- Leffler, J.; Gullstrand, B.; Jönsen, A.; Nilsson, J.-Å; Martin, M.; Blom, A.M.; Bengtsson, A.A. Degradation of neutrophil extracellular traps co-varies with disease activity in patients with systemic lupus erythematosus. Arthr. Res. Ther. 2013, 15, R84. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, C.; Ruiz-Limón, P.; Aguirre, M.A.; Jiménez-Gómez, Y.; Arias-de la Rosa, I.; Ábalos-Aguilera, M.C.; Rodriguez-Arizaa, A.; Castro-Villegasab, M.C.; Ortega-Castroab, R.; Segui, P. Diagnostic potential of NETosis-derived products for disease activity, atherosclerosis and therapeutic effectiveness in Rheumatoid Arthritis patients. J Autoimmun. 2017, 82, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef]
- Giusti, D.; Bini, E.; Terryn, C.; Didier, K.; Jan, S.L.; Gatouillat, G.; Durlach, A.; Nesmond, S.; Muller, C.; Bernard, P.; et al. NET formation in bullous pemphigoid patients with relapse is modulated by IL-17 and IL-23 interplay. Front. Immunol. 2019, 10, 701. [Google Scholar] [CrossRef]
- van der Linden, M.; van den Hoogen, L.L.; Westerlaken, G.H.A.; Fritsch-Stork, R.D.E.; van Roon, J.A.G.; Radstake, T.R.D.J.; Meyaard, L. Neutrophil extracellular trap release is associated with antinuclear antibodies in systemic lupus erythematosus and anti-phospholipid syndrome. Rheumatology 2018, 57, 1228–1234. [Google Scholar] [CrossRef]
- Söderberg, D.; Kurz, T.; Motamedi, A.; Hellmark, T.; Eriksson, P.; Segelmark, M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology 2015, 54, 2085–2094. [Google Scholar] [CrossRef]
- Maugeri, N.; Capobianco, A.; Rovere-Querini, P.; Ramirez, G.A.; Tombetti, E.; Valle, P.D.; Monno, A.; D’Alberti, V.; Gasparri, A.M.; Franchini, S.; et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci. Transl. Med. 2018, 10, eaao3089. [Google Scholar] [CrossRef]
- Brinkmann, V.; Laube, B.; Abu Abed, U.; Goosmann, C.; Zychlinsky, A. Neutrophil extracellular traps: How to generate and visualize them. J. Vis. Exp. 2010, 36, e1724. [Google Scholar] [CrossRef]
- Rebernick, R.; Fahmy, L.; Glover, C.; Bawadekar, M.; Shim, D.; Holmes, C.L.; Rademacher, N.; Potluri, H.; Bartels, C.M.; Shelef, M.A. DNA Area and NETosis Analysis (DANA): A high-throughput method to quantify neutrophil extracellular traps in fluorescent microscope images. Biol. Proced. Online 2018, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.-Y.; Park, Y.; Moon, H.; Kim, H.; Hyug, J.; Young, C.; Choid, S.; Shimd, S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kawasaki, T.; Shigematsu, K.; Kawamura, K.; Oka, N. Neutrophil extracellular traps in neuropathy with anti-neutrophil cytoplasmic autoantibody-associated microscopic polyangiitis. Clin. Rheumatol. 2017, 36, 913–917. [Google Scholar] [CrossRef]
- Lande, R.; Lee, E.Y.; Palazzo, R.; Marinari, B.; Pietraforte, I.; Santos, G.S.; Mattenberger, Y.; Spadaro, F.; Stefanantoni, K.; Iannace, N.; et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-α production in systemic sclerosis. Nat. Commun. 2019, 10, 1731. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Zhao, W.; Yalavarthi, S.; Kaplan, M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015, 74, 1417–1424. [Google Scholar] [CrossRef]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Álvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthr. Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef]
- Mozzini, C.; Garbin, U.; Fratta, P.A.M.; Cominacini, L. An exploratory look at NETosis in atherosclerosis. Intern. Emerg. Med. 2017, 12, 13–22. [Google Scholar] [CrossRef]
- Shand, L.; Lunt, M.; Nihtyanova, S.; Hoseini, M.; Silman, A.; Black, C.M.; Denton, C.P. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: Application of a latent linear trajectory model. Arthr. Rheum. 2007, 56, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; de Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Panda, R.; Krieger, T.; Hopf, L.; Renné, T.; Haag, F.; Röber, N.; Conrad, K.; Csernok, E.; Fuchs, T.A. Neutrophil extracellular traps contain selected antigens of anti-neutrophil cytoplasmic antibodies. Front. Immunol. 2017, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Dioni, I.; Tommasi, C.; Alcaro, M.C.; Paolini, I.; Barbetti, F.; Boscaro, F.; Panza, F.; Puxeddu, I.; Rovero, P.; et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann. Rheum. Dis. 2014, 73, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.C.; Anderson, M.E.; Edwards, S.W.; Moots, R.J. Neutrophil-derived reactive oxygen species in SSc. Rheumatology 2012, 51, 1166–1169. [Google Scholar] [CrossRef]
- Tourkina, E.; Richard, M.; Oates, J.; Hofbauer, A.; Bonner, M.; Gööz, P.; Visconti, R.; Zhang, J.; Znoyko, S.; Hatfield, C.M.; et al. Caveolin-1 regulates leucocyte behaviour in fibrotic lung disease. Ann. Rheum. Dis. 2010, 69, 1220–1226. [Google Scholar] [CrossRef]
- Hakkim, A.; Fuchs, T.A.; Martinez, N.E.; Hess, S.; Prinz, H.; Zychlinsky, A.; Waldmann, H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011, 7, 75–77. [Google Scholar] [CrossRef]
- Gourh, P.; Arnett, F.C.; Assassi, S.; Tan, F.K.; Huang, M.; Diekman, L.; Mayes, M.D.; Reveille, J.D.; Agarwal, S.K. Plasma cytokine profiles in systemic sclerosis: Associations with autoantibody subsets and clinical manifestations. Arthr. Res. Ther. 2009, 11, R147. [Google Scholar] [CrossRef]

| Main Features | SSc Patients | Controls | ||
|---|---|---|---|---|
| Whole SSc Population | Diffuse SSc | Limited SSc | ||
| Number of individuals | 34 | 8 | 26 | 26 |
| Age (mean, years) | 59.5 (±10.9) | 55.3 (±11.7) | 60.8 (±10.6) | 60.5 (±11.2) |
| Women (%) | 29 (85.3) | 5 (62.5) | 24 (92.3) | 19 (73.1) |
| Disease’s duration (years) | 8.6 (±6) | 5 (± 3.5) | 9.8 (± 6.7) | - |
| Modified Rodnan score | 11.8 (±8.7) | 25.4 (± 6.2) | 7.6 (± 6) | - |
| Internal organ involvement | - | |||
| Interstitial lung disease | 9 (26.5) | 3 (37.5) | 6 (23.1) | - |
| Ischemic digital ulcers | 8 (23.5) | 3 (37.5) | 5 (19.2) | - |
| Pulmonary arterial hypertension | 3 (8.8) | 0 (0) | 3 (11.5) | - |
| Scleroderma renal crisis | 2 (5.9) | 2 (25) | 0 (0) | - |
| Auto-antibody | - | |||
| Anti-topoisomerase I | 8 (23.5) | 3 (37.5) | 5 (19.2) | - |
| Anti-centromere | 17 (50) | 0 (0) | 17 (65.4) | - |
| Anti-RNA polymerase III | 2 (5.9) | 2 (25) | 0 (0) | - |
| Anti-U1RNP | 1 (2.9) | 0 (0) | 1 (3.9) | - |
| Anti-U3RNP | 2 (5.9) | 2 (25) | 0 (0) | - |
| Anti-NOR90 | 1 (2.9) | 1 (12.5) | 0 (0) | - |
| Undetermined | 3 (8.8) | 0 (0) | 3 (11.5) | - |
| Treatment at the time of blood collection | - | |||
| Immunosuppressive drug (including corticosteroids) | 2 (5.9) | 2 (25) | 0 (0) | - |
| Vasodilator drug | 8 (23.5) | 1 (12.5) | 7 (26.9) | - |
| Characteristics | SSc Patients without Complication | SSc Patients with Fibrotic Complications | SSc Patients with Vascular Complications | SSc Patients with both Fibrotic and Vascular Complications |
|---|---|---|---|---|
| Number of patients | 12 | 9 | 8 | 5 |
| Mean of disease duration, year (SD) | 8 (±4) | 13.6 (±15.6) | 7.3 (±7.6) | 3.8 (4.2) |
| Diffuse cutaneous involvement (%) | - | 3 (33.3) | - | 5 (100) |
| Interstitial lung disease (%) | - | 8 (88.9) | - | 1 (20) |
| Ischemic digital ulcer(s) (%) | - | - | 5 (62.5) | 3 (60) |
| Pulmonary arterial hypertension (%) | - | - | 3 (37.5) | 0 (0) |
| Scleroderma renal crisis (%) | - | - | 0 (0) | 2 (40) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didier, K.; Giusti, D.; Le Jan, S.; Terryn, C.; Muller, C.; Pham, B.N.; Le Naour, R.; Antonicelli, F.D.; Servettaz, A. Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. J. Clin. Med. 2020, 9, 2136. https://doi.org/10.3390/jcm9072136
Didier K, Giusti D, Le Jan S, Terryn C, Muller C, Pham BN, Le Naour R, Antonicelli FD, Servettaz A. Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. Journal of Clinical Medicine. 2020; 9(7):2136. https://doi.org/10.3390/jcm9072136
Chicago/Turabian StyleDidier, Kevin, Delphine Giusti, Sebastien Le Jan, Christine Terryn, Celine Muller, Bach Nga Pham, Richard Le Naour, Frank D. Antonicelli, and Amelie Servettaz. 2020. "Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis" Journal of Clinical Medicine 9, no. 7: 2136. https://doi.org/10.3390/jcm9072136
APA StyleDidier, K., Giusti, D., Le Jan, S., Terryn, C., Muller, C., Pham, B. N., Le Naour, R., Antonicelli, F. D., & Servettaz, A. (2020). Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. Journal of Clinical Medicine, 9(7), 2136. https://doi.org/10.3390/jcm9072136





