Mesenchymal Stromal Cell-Based Therapy—An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials
Abstract
1. Introduction
1.1. Osteoarthritis
1.2. Mechanisms of Action behind MSCs
- (1)
- The initially favoured theory, that MSCs mainly differentiate into cells of a specific mesodermal lineage and replace damaged or missing cells has lost its popularity in recent years. However, MSCs seem to share the ability to induce tissue regeneration through the stimulation of local endogenous cells [13].
- (2)
- Today, the major potential of MSCs is seen in their secretion of paracrine factors (“bystander effect”), that allows an immunomodulation of the local pro-inflammatory environment, which plays a key role in cartilage degeneration in OA [14,15]. Whether paracrine effects are long-lasting or merely a “hit and run” phenomenon is currently debated [16].
1.3. First MSC Trials and Cartilage Repair
1.4. OA as an Inflammatory Disease and Immunomodulatory Properties of MSCs
1.5. Oncological Safety Profile of MSCs
1.6. Preparation of MSCs
- (1)
- MSC concentrates are the product of harvested fluids or tissues, which have been concentrated in order to increase the number of MSCs per unit of applied suspension. Bone-marrow aspirate concentrates (BMAC) include high concentrations of growth factors and high levels of anti-inflammatory IL-1 receptor antagonist next to MSCs [58].
- (2)
- Culture-expanded MSCs can be administered without scaffolds or seeded onto scaffolds after having been isolated and cultured. Traditionally two-dimensional (2-D) plastic culture plates are in use for cell expansion, but three-dimensional (3-D) techniques have evolved in recent years. 3-D cultures are able to mimic in vivo conditions and provide high density and expansion potential [59]. New additions like highly elastic culture dishes, automatically adapting the dishes’ surface to growing cell numbers, optimize the expansion process before MSCs are ready for application [60]. Increased complexity and non-standardized expansion protocols, alongside higher costs should be taken into consideration, when applying 3-D expansion techniques. However, reports about increased immunomodulatory and chondrogenic potential of MSCs, cultured in 3-D compared to conventional 2-D cultures, point towards promising technical developments in the field of tissue engineering for OA treatment [61,62].
- (3)
- The stromal vascular fraction (SVF) is a combination of ATMSCs, endothelial cells, growth factors, precursor cells, macrophages, t-regulatory cells, lymphocytes and others. The SVF is derived from lipoaspirates by mechanical or enzymatic isolation [63]. The SVF can be injected into the joint, after a purification process, often within the same visit, as there is no need for expansion or culturing of cells.
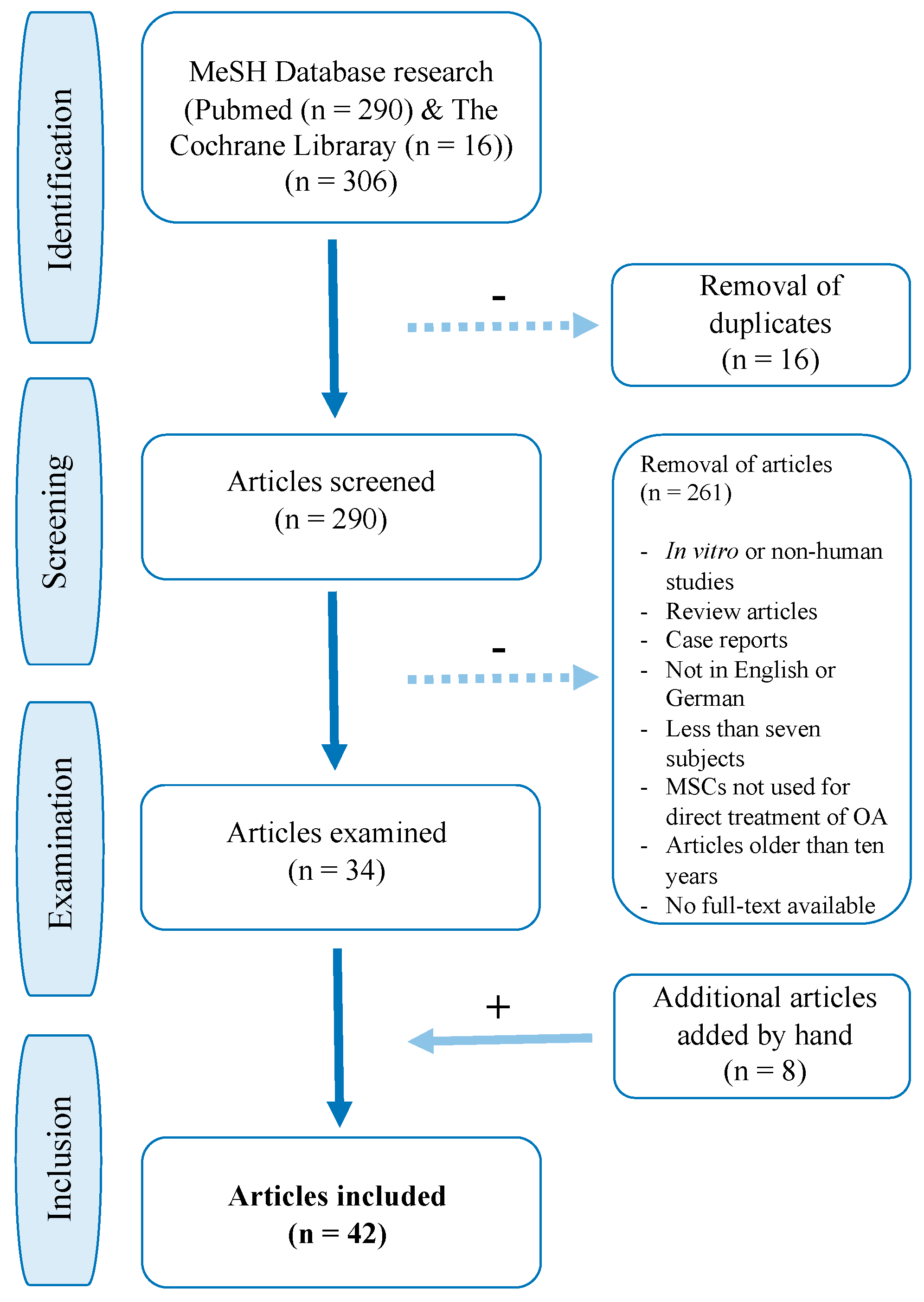
2. Methods
- (1)
- PubMed MeSH search terms: “mesenchymal stem cell transplantation” [Mesh] AND “osteoarthritis” [Mesh]: 290 results
- (2)
- Cochrane Library MeSH search terms: #1 MeSH (“mesenchymal stem cell transplantation”) explode all trees AND #2 MeSH (“osteoarthritis”) explode all trees: 16 results
- (3)
- ClinicalTrials.gov search terms: “mesenchymal stem cells” OR “mesenchymal stromal cells” AND “osteoarthritis”: 96 results
3. Study Designs and Route of Cell Delivery
4. Safety
5. Duration of Therapeutic Effects
6. Quality of Life and Mental Health
7. Radiological and Arthroscopic Outcome Evaluations
8. Cell Dose
9. Tissue Origin
10. Independent Outcome Predictors
11. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of Persons with Symptomatic Knee Osteoarthritis in the Us: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res. (Hoboken) 2016, 68, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of Total Joint Replacement in the United States: Future Projections to 2020–2040 Using the National Inpatient Sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.J.; Gold, G.E. Diagnosis of Osteoarthritis: Imaging. Bone 2012, 51, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef]
- Fuchs, J.; Rabenberg, M.; Scheidt-Nave, C. Prevalence of Selected Musculoskeletal Conditions in Germany: Results of the German Health Interview and Examination Survey for Adults (Degs1). Bundesgesundheitsblatt Gesundh. Gesundh. 2013, 56, 678–686. [Google Scholar] [CrossRef]
- Wilson, M.G.; Michet, C.J., Jr.; Ilstrup, D.M.; Melton, L.J., 3rd. Idiopathic Symptomatic Osteoarthritis of the Hip and Knee: A Population-Based Incidence Study. Mayo Clin. Proc. 1990, 65, 1214–1221. [Google Scholar] [CrossRef]
- Ayhan, E.; Kesmezacar, H.; Akgun, I. Intraarticular Injections (Corticosteroid, Hyaluronic Acid, Platelet Rich Plasma) for the Knee Osteoarthritis. World J. Orthop. 2014, 5, 351–361. [Google Scholar] [CrossRef]
- Gothesen, O.; Espehaug, B.; Havelin, L.; Petursson, G.; Lygre, S.; Ellison, P.; Hallan, G.; Furnes, O. Survival Rates and Causes of Revision in Cemented Primary Total Knee Replacement: A Report from the Norwegian Arthroplasty Register 1994-2009. Bone Joint J. 2013, 95, 636–642. [Google Scholar] [CrossRef]
- Badawy, M.; Espehaug, B.; Indrekvam, K.; Engesaeter, L.B.; Havelin, L.I.; Furnes, O. Influence of Hospital Volume on Revision Rate after Total Knee Arthroplasty with Cement. J. Bone Joint Surg. Am. 2013, 95, e131. [Google Scholar] [CrossRef]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The Effect of Patient Age at Intervention on Risk of Implant Revision after Total Replacement of the Hip or Knee: A Population-Based Cohort Study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Meehan, J.P.; Danielsen, B.; Kim, S.H.; Jamali, A.A.; White, R.H. Younger Age Is Associated with a Higher Risk of Early Periprosthetic Joint Infection and Aseptic Mechanical Failure after Total Knee Arthroplasty. J. Bone Joint Surg. Am. 2014, 96, 529–535. [Google Scholar] [CrossRef]
- Vinatier, C.; Guicheux, J. Cartilage Tissue Engineering: From Biomaterials and Stem Cells to Osteoarthritis Treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem Cell Therapy in a Caprine Model of Osteoarthritis. Arthritis Rheumatol. 2003, 48, 3464–3474. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Why Are Mscs Therapeutic? New Data: New Insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Feisst, V.; Meidinger, S.; Locke, M.B. From Bench to Bedside: Use of Human Adipose-Derived Stem Cells. Stem Cells Cloning 2015, 8, 149–162. [Google Scholar] [PubMed]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal Stem Cell-Based Therapy of Osteoarthritis: Current Knowledge and Future Perspectives. Biomed. Pharmacother. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal Stem Cells in Joint Disease and Repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Ashton, B.A.; Allen, T.D.; Howlett, C.R.; Eaglesom, C.C.; Hattori, A.; Owen, M. Formation of Bone and Cartilage by Marrow Stromal Cells in Diffusion Chambers in Vivo. Clin. Orthop. Relat. Res. 1980, 151, 294–307. [Google Scholar] [CrossRef]
- Mokbel, A.N.; El Tookhy, O.S.; Shamaa, A.A.; Rashed, L.A.; Sabry, D.; El Sayed, A.M. Homing and Reparative Effect of Intra-Articular Injection of Autologus Mesenchymal Stem Cells in Osteoarthritic Animal Model. BMC Musculoskelet. Disord. 2011, 12, 259. [Google Scholar] [CrossRef]
- Mak, J.; Jablonski, C.L.; Leonard, C.A.; Dunn, J.F.; Raharjo, E.; Matyas, J.R.; Biernaskie, J.; Krawetz, R.J. Intra-Articular Injection of Synovial Mesenchymal Stem Cells Improves Cartilage Repair in a Mouse Injury Model. Sci. Rep. 2016, 6, 23076. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Roussignol, G.; Toupet, K.; Penarier, G.; Bentz, I.; Teixeira, S.; Oustric, D.; Jung, M.; Lepage, O.; Steinberg, R.; et al. Utility of a Mouse Model of Osteoarthritis to Demonstrate Cartilage Protection by Ifngamma-Primed Equine Mesenchymal Stem Cells. Front. Immunol. 2016, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In Vitro Chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Kafienah, W.; Cheung, F.L.; Sims, T.; Martin, I.; Miot, S.; Von Ruhland, C.; Roughley, P.J.; Hollander, A.P. Lumican Inhibits Collagen Deposition in Tissue Engineered Cartilage. Matrix Biol. 2008, 27, 526–534. [Google Scholar] [CrossRef]
- Yin, H.; Wang, Y.; Sun, Z.; Sun, X.; Xu, Y.; Li, P.; Meng, H.; Yu, X.; Xiao, B.; Fan, T.; et al. Induction of Mesenchymal Stem Cell Chondrogenic Differentiation and Functional Cartilage Microtissue Formation for in Vivo Cartilage Regeneration by Cartilage Extracellular Matrix-Derived Particles. Acta Biomater. 2016, 33, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human Autologous Culture Expanded Bone Marrow Mesenchymal Cell Transplantation for Repair of Cartilage Defects in Osteoarthritic Knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced Chondrogenic and Adipogenic Activity of Mesenchymal Stem Cells from Patients with Advanced Osteoarthritis. Arthritis Rheumatol. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Garcia-Alvarez, F.; Alegre-Aguaron, E.; Desportes, P.; Royo-Canas, M.; Castiella, T.; Larrad, L.; Martinez-Lorenzo, M.J. Chondrogenic Differentiation in Femoral Bone Marrow-Derived Mesenchymal Cells (Msc) from Elderly Patients Suffering Osteoarthritis or Femoral Fracture. Arch. Gerontol. Geriatr. 2011, 52, 239–242. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of Aging on the Regenerative Properties of Bone Marrow-, Muscle-, and Adipose-Derived Mesenchymal Stem/Stromal Cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef]
- Koobatian, M.T.; Liang, M.S.; Swartz, D.D.; Andreadis, S.T. Differential Effects of Culture Senescence and Mechanical Stimulation on the Proliferation and Leiomyogenic Differentiation of Msc from Different Sources: Implications for Engineering Vascular Grafts. Tissue Eng. Part A 2015, 21, 1364–1375. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced Chondrocyte Proliferation and Mesenchymal Stromal Cells Chondrogenesis in Coculture Pellets Mediate Improved Cartilage Formation. J. Cell Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Qing, C.; Wei-ding, C.; Wei-min, F. Co-Culture of Chondrocytes and Bone Marrow Mesenchymal Stem Cells in Vitro Enhances the Expression of Cartilaginous Extracellular Matrix Components. Braz. J. Med. Biol. Res. 2011, 44, 303–310. [Google Scholar] [CrossRef]
- Jose, S.; Tan, S.W.; Ooi, Y.Y.; Ramasamy, R.; Vidyadaran, S. Mesenchymal Stem Cells Exert Anti-Proliferative Effect on Lipopolysaccharide-Stimulated Bv2 Microglia by Reducing Tumour Necrosis Factor-Alpha Levels. J. Neuroinflamm. 2014, 11, 149. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory Mediators in Osteoarthritis: A Critical Review of the State-of-the-Art, Current Prospects, and Future Challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Shariatzadeh, M.; Song, J.; Wilson, S.L. The Efficacy of Different Sources of Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Cell Tissue Res. 2019, 378, 399–410. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal Stem Cells Suppress Lymphocyte Proliferation in Vitro and Prolong Skin Graft Survival in Vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-Derived Mesenchymal Stem Cells Exert Antiinflammatory Effects on Chondrocytes and Synoviocytes from Osteoarthritis Patients through Prostaglandin E2. Arthritis Rheumatol. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Jang, I.K.; Lee, M.W.; Kim, H.E.; Yang, M.S.; Eom, Y.; Lee, J.E.; Kim, Y.J.; Yang, S.K.; Jung, H.L.; et al. Comparison of Immunomodulatory Properties of Mesenchymal Stem Cells Derived from Adult Human Tissues. Cell Immunol. 2009, 259, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yanez, R.; Lamana, M.L.; Garcia-Castro, J.; Colmenero, I.; Ramirez, M.; Bueren, J.A. Adipose Tissue-Derived Mesenchymal Stem Cells Have in Vivo Immunosuppressive Properties Applicable for the Control of the Graft-Versus-Host Disease. Stem Cells 2006, 24, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Du, L. The Role of Secreted Factors in Stem Cells-Mediated Immune Regulation. Cell. Immunol. 2018, 326, 24–32. [Google Scholar] [CrossRef]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyo, J.M.; Weimar, W.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Abrogate Plasmablast Formation and Induce Regulatory B Cells Independently of T Helper Cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, I.S. Double-Edged Sword of Mesenchymal Stem Cells: Cancer-Promoting Versus Therapeutic Potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef]
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal Stem Cells: Key Players in Cancer Progression. Mol. Cancer 2017, 16, 31. [Google Scholar] [CrossRef]
- Toyserkani, N.M.; Jorgensen, M.G.; Tabatabaeifar, S.; Jensen, C.H.; Sheikh, S.P.; Sorensen, J.A. Concise Review: A Safety Assessment of Adipose-Derived Cell Therapy in Clinical Trials: A Systematic Review of Reported Adverse Events. Stem Cells Transl. Med. 2017, 6, 1786–1794. [Google Scholar] [CrossRef]
- Ryan, A.E.; Lohan, P.; O’Flynn, L.; Treacy, O.; Chen, X.; Coleman, C.; Shaw, G.; Murphy, M.; Barry, F.; Griffin, M.D.; et al. Chondrogenic Differentiation Increases Antidonor Immune Response to Allogeneic Mesenchymal Stem Cell Transplantation. Mol. Ther. 2014, 22, 655–667. [Google Scholar] [CrossRef]
- Melero-Martin, J.M.; Santhalingam, S.; Al-Rubeai, M. Methodology for Optimal in Vitro Cell Expansion in Tissue Engineering. Adv. Biochem. Eng. Biotechnol. 2009, 112, 209–229. [Google Scholar] [PubMed]
- Tekkatte, C.; Gunasingh, G.P.; Cherian, K.M.; Sankaranarayanan, K. Humanized Stem Cell Culture Techniques: The Animal Serum Controversy. Stem Cells Int. 2011, 2011, 504723. [Google Scholar] [CrossRef] [PubMed]
- Skalska, U.; Kontny, E. Adipose-Derived Mesenchymal Stem Cells from Infrapatellar Fat Pad of Patients with Rheumatoid Arthritis and Osteoarthritis Have Comparable Immunomodulatory Properties. Autoimmunity 2016, 49, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Skalska, U.; Kuca-Warnawin, E.; Kornatka, A.; Janicka, I.; Musialowicz, U.; Burakowski, T.; Kontny, E. Articular and Subcutaneous Adipose Tissues of Rheumatoid Arthritis Patients Represent Equal Sources of Immunoregulatory Mesenchymal Stem Cells. Autoimmunity 2017, 50, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy May Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Mannava, S.; Cinque, M.E.; Geeslin, A.G.; Codina, D.; LaPrade, R.F. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc. Tech. 2017, 6, e441–e445. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and Challenges in Stem Cell Culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Majd, H.; Quinn, T.M.; Wipff, P.J.; Hinz, B. Dynamic Expansion Culture for Mesenchymal Stem Cells. Methods Mol. Biol. 2011, 698, 175–188. [Google Scholar]
- Mamidi, M.K.; Das, A.K.; Zakaria, Z.; Bhonde, R. Mesenchymal Stromal Cells for Cartilage Repair in Osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Robb, K.; Gómez-Aristizábal, A.; Gandhi, R.; Viswanathan, S. A Culture Engineering Strategy to Enhance Mesenchymal Stromal Cells for Treatment of Osteoarthritis. Osteoarthr. Cartil. 2019, 27, S427. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose Tissue-Derived Stromal Vascular Fraction in Regenerative Medicine: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Molto, F.; Nunez-Cordoba, J.M.; Sanchez-Echenique, C.; Bondia, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-Articular Injection of Two Different Doses of Autologous Bone Marrow Mesenchymal Stem Cells Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: Multicenter Randomized Controlled Clinical Trial (Phase I/Ii). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Molto, F.; Nunez-Cordoba, J.M.; Lopez-Elio, S.; Andreu, E.; Sanchez-Guijo, F.; Aquerreta, J.D.; Bondia, J.M.; et al. Intra-Articular Injection of Two Different Doses of Autologous Bone Marrow Mesenchymal Stem Cells Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: Long-Term Follow up of a Multicenter Randomized Controlled Clinical Trial (Phase I/Ii). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef]
- Emadedin, M.; Labibzadeh, N.; Liastani, M.G.; Karimi, A.; Jaroughi, N.; Bolurieh, T.; Hosseini, S.E.; Baharvand, H.; Aghdami, N. Intra-Articular Implantation of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells to Treat Knee Osteoarthritis: A Randomized, Triple-Blind, Placebo-Controlled Phase 1/2 Clinical Trial. Cytotherapy 2018, 20, 1238–1246. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-Up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Varma, H.S.; Dadarya, B.; Vidyarthi, A. The New Avenues in the Management of Osteo-Arthritis of Knee--Stem Cells. J. Indian Med. Assoc. 2010, 108, 583–585. [Google Scholar]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.; Schott, V.; Balduino, A.; Bastos, R.; Oliveira, J.M.; Reis, R.L.; Rodeo, S.; et al. Intra-Articular Injection of Culture-Expanded Mesenchymal Stem Cells with or without Addition of Platelet-Rich Plasma Is Effective in Decreasing Pain and Symptoms in Knee Osteoarthritis: A Controlled, Double-Blind Clinical Trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-Articular Injections of Expanded Mesenchymal Stem Cells with and without Addition of Platelet-Rich Plasma Are Safe and Effective for Knee Osteoarthritis. Knee. Surg. Sports Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef]
- Mautner, K.; Bowers, R.; Easley, K.; Fausel, Z.; Robinson, R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl. Med. 2019, 8, 1149–1156. [Google Scholar] [CrossRef]
- Chahal, J.; Gomez-Aristizabal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar]
- Emadedin, M.; Liastani, M.G.; Fazeli, R.; Mohseni, F.; Moghadasali, R.; Mardpour, S.; Hosseini, S.E.; Niknejadi, M.; Moeininia, F.; Fanni, A.A.; et al. Long-Term Follow-up of Intra-Articular Injection of Autologous Mesenchymal Stem Cells in Patients with Knee, Ankle, or Hip Osteoarthritis. Arch. Iran Med. 2015, 18, 336–344. [Google Scholar]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; Lopez, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final Results of a Phase I-Ii Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef]
- Al-Najar, M.; Khalil, H.; Al-Ajlouni, J.; Al-Antary, E.; Hamdan, M.; Rahmeh, R.; Alhattab, D.; Samara, O.; Yasin, M.; Abdullah, A.A.; et al. Intra-Articular Injection of Expanded Autologous Bone Marrow Mesenchymal Cells in Moderate and Severe Knee Osteoarthritis Is Safe: A Phase I/Ii Study. J. Orthop. Surg. Res. 2017, 12, 190. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentis, J.; Sanchez, A.; Garcia-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells: A Pilot Study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells: Two-Year Follow-up Results. Transplantation 2014, 97, e66–e68. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and Safety of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells (Stempeucel(R)): Preclinical and Clinical Trial in Osteoarthritis of the Knee Joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Vega, A.; Martin-Ferrero, M.A.; Del Canto, F.; Alberca, M.; Garcia, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis with Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase Iib, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy with Platelet-Rich Plasma Alone or in Combination with Mesenchymal Stem Cell Treatment: A Prospective Study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-Articular Injection of Autologous Adipose-Derived Stromal Vascular Fractions for Knee Osteoarthritis: A Double-Blind Randomized Self-Controlled Trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, P.K.; Suh, D.S.; Heo, D.B.; Tak, D.H.; Koh, Y.G. Implantation of Mesenchymal Stem Cells in Combination with Allogenic Cartilage Improves Cartilage Regeneration and Clinical Outcomes in Patients with Concomitant High Tibial Osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 544–554. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.S.; Koh, Y.G. Mesenchymal Stem Cell Implantation in Osteoarthritic Knees: Is Fibrin Glue Effective as a Scaffold? Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef]
- Kim, Y.S.; Koh, Y.G. Comparative Matched-Pair Analysis of Open-Wedge High Tibial Osteotomy with Versus without an Injection of Adipose-Derived Mesenchymal Stem Cells for Varus Knee Osteoarthritis: Clinical and Second-Look Arthroscopic Results. Am. J. Sports Med. 2018, 46, 2669–2677. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J. Infrapatellar Fat Pad-Derived Mesenchymal Stem Cell Therapy for Knee Osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Kim, Y.S.; Koh, Y.G. Injection of Mesenchymal Stem Cells as a Supplementary Strategy of Marrow Stimulation Improves Cartilage Regeneration after Lateral Sliding Calcaneal Osteotomy for Varus Ankle Osteoarthritis: Clinical and Second-Look Arthroscopic Results. Arthroscopy 2016, 32, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef] [PubMed]
- Schiavone Panni, A.; Vasso, M.; Braile, A.; Toro, G.; De Cicco, A.; Viggiano, D.; Lepore, F. Preliminary Results of Autologous Adipose-Derived Stem Cells in Early Knee Osteoarthritis: Identification of a Subpopulation with Greater Response. Int. Orthop. 2019, 43, 7–13. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Koh, Y.G. Mesenchymal Stem Cell Implantation in Knee Osteoarthritis: An Assessment of the Factors Influencing Clinical Outcomes. Am. J. Sports Med. 2015, 43, 2293–2301. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J.; Kwon, O.R.; Kim, Y.S. Second-Look Arthroscopic Evaluation of Cartilage Lesions after Mesenchymal Stem Cell Implantation in Osteoarthritic Knees. Am. J. Sports Med. 2014, 42, 1628–1637. [Google Scholar] [CrossRef]
- Koh, Y.G.; Choi, Y.J.; Kwon, S.K.; Kim, Y.S.; Yeo, J.E. Clinical Results and Second-Look Arthroscopic Findings after Treatment with Adipose-Derived Stem Cells for Knee Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, Y.J.; Lee, S.W.; Kwon, O.R.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Assessment of Clinical and Mri Outcomes after Mesenchymal Stem Cell Implantation in Patients with Knee Osteoarthritis: A Prospective Study. Osteoarthr. Cartil. 2016, 24, 237–245. [Google Scholar] [CrossRef]
- Roato, I.; Belisario, D.C.; Compagno, M.; Lena, A.; Bistolfi, A.; Maccari, L.; Mussano, F.; Genova, T.; Godio, L.; Perale, G.; et al. Concentrated Adipose Tissue Infusion for the Treatment of Knee Osteoarthritis: Clinical and Histological Observations. Int. Orthop. 2019, 43, 15–23. [Google Scholar] [CrossRef]
- Pintat, J.; Silvestre, A.; Magalon, G.; Gadeau, A.P.; Pesquer, L.; Perozziello, A.; Peuchant, A.; Mounayer, C.; Dallaudiere, B. Intra-Articular Injection of Mesenchymal Stem Cells and Platelet-Rich Plasma to Treat Patellofemoral Osteoarthritis: Preliminary Results of a Long-Term Pilot Study. J. Vasc. Interv. Radiol. 2017, 28, 1708–1713. [Google Scholar] [CrossRef]
- Koh, Y.G.; Jo, S.B.; Kwon, O.R.; Suh, D.S.; Lee, S.W.; Park, S.H.; Choi, Y.J. Mesenchymal Stem Cell Injections Improve Symptoms of Knee Osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human Adipose-Derived Mesenchymal Stem Cells for Osteoarthritis: A Pilot Study with Long-Term Follow-up and Repeated Injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Spasovski, D.; Spasovski, V.; Bascarevic, Z.; Stojiljkovic, M.; Vreca, M.; Andelkovic, M.; Pavlovic, S. Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells in the Treatment of Knee Osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; Gonzalez, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (Mscs) for Knee Osteoarthritis: Repeated Msc Dosing Is Superior to a Single Msc Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/Ii Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Khalifeh Soltani, S.; Forogh, B.; Ahmadbeigi, N.; Kharazi, H.H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and Efficacy of Allogenic Placental Mesenchymal Stem Cells for Treating Knee Osteoarthritis: A Pilot Study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef]
- Quintana, J.M.; Arostegui, I.; Escobar, A.; Azkarate, J.; Goenaga, J.I.; Lafuente, I. Prevalence of Knee and Hip Osteoarthritis and the Appropriateness of Joint Replacement in an Older Population. Arch. Intern. Med. 2008, 168, 1576–1584. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Rheumatol. Dis. Clin. N. Am. 2008, 34, 515–529. [Google Scholar] [CrossRef]
- Nguyen, C.; Rannou, F. The Safety of Intra-Articular Injections for the Treatment of Knee Osteoarthritis: A Critical Narrative Review. Expert Opin. Drug Saf. 2017, 16, 897–902. [Google Scholar] [CrossRef]
- Lee, C.; O’Connell, C.D.; Onofrillo, C.; Choong, P.F.M.; Di Bella, C.; Duchi, S. Human Articular Cartilage Repair: Sources and Detection of Cytotoxicity and Genotoxicity in Photo-Crosslinkable Hydrogel Bioscaffolds. Stem Cells Transl. Med. 2020, 9, 302–315. [Google Scholar] [CrossRef]
- Lee, D.K.; Wang, J.H.; Won, Y.; Min, Y.K.; Jaiswal, S.; Lee, B.H.; Kim, J.Y. Preoperative Latent Medial Laxity and Correction Angle Are Crucial Factors for Overcorrection in Medial Open-Wedge High Tibial Osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1411–1418. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, P.; Liu, L. Origin and Efficacy of Hyaluronan Injections in Knee Osteoarthritis: Randomized, Double-Blind Trial. Med. Sci. Monit. 2018, 24, 4728–4737. [Google Scholar] [CrossRef]
- Habib, G.S.; Saliba, W.; Nashashibi, M. Local Effects of Intra-Articular Corticosteroids. Clin. Rheumatol. 2010, 29, 347–356. [Google Scholar] [CrossRef]
- Hawker, G.; Melfi, C.; Paul, J.; Green, R.; Bombardier, C. Comparison of a Generic (Sf-36) and a Disease Specific (Womac) (Western Ontario and Mcmaster Universities Osteoarthritis Index) Instrument in the Measurement of Outcomes after Knee Replacement Surgery. J. Rheumatol. 1995, 22, 1193–1196. [Google Scholar]
- Centeno, C.J.; Busse, D.; Kisiday, J.; Keohan, C.; Freeman, M.; Karli, D. Increased Knee Cartilage Volume in Degenerative Joint Disease Using Percutaneously Implanted, Autologous Mesenchymal Stem Cells. Pain Physician 2008, 11, 343–353. [Google Scholar]
- Kim, S.H.; Ha, C.W.; Park, Y.B.; Nam, E.; Lee, J.E.; Lee, H.J. Intra-Articular Injection of Mesenchymal Stem Cells for Clinical Outcomes and Cartilage Repair in Osteoarthritis of the Knee: A Meta-Analysis of Randomized Controlled Trials. Arch. Orthop. Trauma Surg. 2019, 139, 971–980. [Google Scholar] [CrossRef]
- Kanamiya, T.; Naito, M.; Hara, M.; Yoshimura, I. The Influences of Biomechanical Factors on Cartilage Regeneration after High Tibial Osteotomy for Knees with Medial Compartment Osteoarthritis: Clinical and Arthroscopic Observations. Arthroscopy 2002, 18, 725–729. [Google Scholar] [CrossRef]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A Dose Response Analysis of a Specific Bone Marrow Concentrate Treatment Protocol for Knee Osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 258. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Zeng, L. Adipose Tissue or Bone Marrow, Store for Purchasing Mesenchymal Stem Cells? Circ. J. 2011, 75, 2060–2061. [Google Scholar] [CrossRef]
- Reinisch, A.; Etchart, N.; Thomas, D.; Hofmann, N.A.; Fruehwirth, M.; Sinha, S.; Chan, C.K.; Senarath-Yapa, K.; Seo, E.Y.; Wearda, T.; et al. Epigenetic and in Vivo Comparison of Diverse Msc Sources Reveals an Endochondral Signature for Human Hematopoietic Niche Formation. Blood 2015, 125, 249–260. [Google Scholar] [CrossRef]
- Ragni, E.; Orfei, C.P.; De Luca, P.; Colombini, A.; Vigano, M.; De Girolamo, L. Secreted Factors and Ev-Mirnas Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1582. [Google Scholar] [CrossRef]
- Tofino-Vian, M.; Guillen, M.I.; Del Caz, M.D.P.; Silvestre, A.; Alcaraz, M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell Physiol. Biochem. 2018, 47, 11–25. [Google Scholar] [CrossRef]
- Khatab, S.; Van Osch, G.J.; Kops, N.; Bastiaansen-Jenniskens, Y.M.; Bos, P.K.; Verhaar, J.A.; Bernsen, M.R.; Van Buul, G.M. Mesenchymal Stem Cell Secretome Reduces Pain and Prevents Cartilage Damage in a Murine Osteoarthritis Model. Eur. Cells Mater. 2018, 36, 218–230. [Google Scholar] [CrossRef]
- Huskisson, E.C. Measurement of Pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef]
- Roldan, M.; Macias-Gonzalez, M.; Garcia, R.; Tinahones, F.J.; Martin, M. Obesity Short-Circuits Stemness Gene Network in Human Adipose Multipotent Stem Cells. FASEB J. 2011, 25, 4111–4126. [Google Scholar] [CrossRef]
- Wu, C.L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-Induced Obesity Alters the Differentiation Potential of Stem Cells Isolated from Bone Marrow, Adipose Tissue and Infrapatellar Fat Pad: The Effects of Free Fatty Acids. Int. J. Obes. (Lond.) 2013, 37, 1079–1087. [Google Scholar] [CrossRef]
| Indication | Number of Patients | Source of MSCs | Intervention | Control | Study Phase | Location and Study Identifier |
|---|---|---|---|---|---|---|
| Double-blinded randomized controlled trials | ||||||
| Knee OA | 260 | Autologous ATMSCs | Single intra-articular injection of ATMSCs (108 cells) vs. control | Saline | III | Cheongju-si, Daegu, Jeonju, Jinju-si, Seoul, all: Republic of Korea (South) (NCT03990805) |
| Knee OA | 153 | Autologous ATMSCs | Single intra-articular injection of ATMSCs (1 or 2 × 107 cells) vs. control | Saline | II | Montpellier, France (NCT02838069) |
| Knee OA | 60 | Allogenic UCMSCs | Single intra-articular injection of allogenic UCMSCs (107 cells) + PRP (5 mL) vs. control | HA | I/II | Liaocheng, Shandong, China (NCT03166865) |
| Knee OA | 60 | Autologous BMMSCs | Repeated (three) intra-articular injections of autologous BMMSCs + PRP (3 mL) vs. control over six months | PRP | II | Yantai, Shandong, China (NCT03969680) |
| Knee OA | 40 | Autologous ATMSCs | Single intra-articular injection of autologous ATMSCs vs. control | GC | III | Stanford, California, USA (NCT03467919) |
| Knee OA | 24 | BMMSCs | Single intra-articular injection of BMMSCs (108 cells) vs. control | Saline | I/II | Seoul, Republic of Korea (South) (NCT04240873) |
| Randomized controlled trials | ||||||
| Knee OA | 60 | Dental pulp mesenchymal stem cells | Single intra-articular injection of low dose dental pulp MSCs vs. high dose dental pulp MSCs vs. control | HA | I | Shanghai, China (NCT04130100) |
| Knee OA | 60 | Autologous ATMSCs | Repeated (three) intra-articular injections of autologous ATMSCs + PRP (3 mL) vs. control over six months | PRP | II | Yantai, Shandong, China (NCT04212728) |
| Knee OA | 48 | Autologous ATMSCs | Single intra-articular injection of autologous ATMSCs vs. control | GC | III | Beirut, Lebanon (NCT04230902) |
| Knee OA | 30 | Allogeneic ATMSCs | Single intra-articular injection of allogeneic ATMSCs (6.7 × 106 or 4 × 107) vs. control | HA | I/II | Hsinchu, Taiwan (NCT03943576) |
| Knee OA | 30 | Autologous ATMSCs | HTO followed by intra-articular autologous ATMSC injection vs. control, one month post osteotomy | HA | I/II | Jinan, Shandong, China (NCT03955497) |
| Knee OA | 9 | Allogenic UCMSCs | Repeated (three) intra-articular injections of allogenic UCMSCs (107 cells) + HA (2 mL) + recombinant human somatropin (8 IU) vs. allogenic UCMSCs + HA vs. control weekly over three weeks | HA | I | Jakarta, Indonesia (NCT03800810) |
| Prospective uncontrolled trials | ||||||
| Knee, hip, glenohumeral OA | 100 | Autologous ATMSCs | Repeated (three) intra-articular injections of autologous ATMSCs every three months over 12 months (≥ 106 cells per injection) | - | I/II | Warsaw, Poland (NCT03869229) |
| Knee, hip, glenohumeral OA | 100 | Allogenic WJMSCs | Repeated (three) intra-articular injections of allogenic WJMSCs every three months over 12 months (≥ 106 cells per injection) | - | I/II | Warsaw, Poland (NCT03866330) |
| Glenohumeral OA | 30 | Autologous BMAC | Single intra-articular injection of autologous BMAC (9 mL) | - | - | Rozzano, Milano, Italy (NCT04308213) |
| Knee OA | 25 | Autologous BMAC | Single subchondral and intra-articular injection of autologous BMAC (6 mL) | - | - | Rozzano, Milano, Italy (NCT03790189) |
| Hip OA | 24 | Autologous ATMSCs | Single intra-articular injection of ATMSCs (3 × 107 cells) vs. two injections with one-month between injections | - | I | Rochester, Minnesota, United States (NCT03608579) |
| Knee OA | 24 | Allogenic UCMSCs | Single intra-articular injection of allogenic UCMSCs (2, 20 or 80 × 106 cells) | - | I | Santiago, Chile (NCT03810521) |
| Knee OA | 24 | Autologous ATMSCs | Single or monthly repeated (three) intra-articular injection(s) of autologous ATMSCs (5 or 10 × 107 cells) | - | I | Rochester, Minnesota, United States (NCT02805855) |
| Knee OA vs. focal chondral defect of the knee | 16 | Autologous BMMSCs | Single intra-articular injection of autologous BMMSCs (5 × 107 cells) | - | I | Cleveland, Ohio, United States (NCT03477942) |
| Knee OA | 15 | Allogenic BMMSCs | Single intra-articular injection of allogenic BMMSCs (1, 5 or 10 × 107 cells) | - | I/IIa | Taipei, Taiwan (NCT03589287) |
| Knee OA | 12 | Allogenic UCMSCs | Single intra-articular injection: low dose vs. mid dose vs. high dose (4, 10 or 20 × 106 cells / 2 mL) | - | I | Seoul, Jongno-gu, Republic of Korea (South) (NCT04037345) |
| Knee OA | 10 | Allogenic WJMSCs | Repeated (two) intra-articular injections of allogenic WJMSCs (5 × 107 cells in each dose) | - | I | Amman, Jordan (NCT02963727) |
| Knee OA | 10 | Autologous ATMSCs | Repeated (two) intra-articular injections of autologous ATMSCs (5 × 107 cells in each dose) | - | I | Amman, Jordan (NCT02966951) |
| Knee OA | 8 | Autologous ATMSCs | Single intra-articular injection of ATMSCs | - | - | Qilu, Shandong, China (NCT03956719) |
| References | OA Site | Severity of OA | Number of Patients | Follow-Up | Blin-Ding | Details of Treatment Arms and Mean Cell Numbers | Outcome Measures | Study Type | Control Arm | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Autologous BMMSCs/BMAC | ||||||||||
| Lamo-Espinosa et al., Journal of Translational Medicine (2016) [64] | Knee | K.L. ≥ 2 | 30 | 12 months | Double-blinded | Intra-articular injection of 107 cells (n = 10) mixed with 1.5 mL of saline vs. 108 cells (n = 10) mixed with of saline, both followed by an injection of 4 mL of HA (60 mg) vs. control | Pain VAS score, WOMAC score, range of motion, x-ray, MRI (WORMS) | Randomized, double-blinded, multicenter, placebo-controlled trial | Intra-articular injection of of HA (60 mg) (n = 10) | - No treatment-related SAEs or AEs. - Pain VAS score improved significantly in low- and high-dose treatment groups compared to baseline over time. WOMAC score improved significantly in the high-dose treatment group compared to baseline over time. The range of motion increased significantly in both treatment groups compared to baseline. X-ray images revealed joint space reduction in the control group but not in the high-dose treatment group (assessment in the low-dose group was not possible). MRI improvements were only seen in the high-dose treatment group. |
| Lamo-Espinosa et al., Journal of Translational Medicine (2018) [65] | “ | “ | 27 | 48 months | “ | “ | Pain VAS score, WOMAC score | Follow-up visit to Lamo-Espinosa et al., Journal of Translational Medicine (2016) [64] | “ | - No SAEs or AEs. - At the 4-year follow-up, significant improvements in pain VAS and WOMAC scores were observed for the low- and high dose group compared to the control group, which showed a progressive deterioration. |
| Emadedin et al., Cytotherapy (2018) [66] | Knee | K.L. 2–4 | 43 | Six months | Triple-blinded | Intra-articular injection of 4 × 107 cells in 5 mL of saline with 2% human serum albumin (n = 19) vs. control | Pain VAS score, WOMAC score, walking distance, standing time, range of motion | Randomized, triple-blinded, placebo-controlled trial | Intra-articular injection of of saline with 2% human serum albumin (n = 24) | - No significant AEs. - Significant improvement in painless walking distance and WOMAC pain sub-scale in the MSC group compared to placebo over time. |
| Wong et al., Arthroscopy (12/2013) [67] | Knee | - | 56 | 24 months | - | HTO, microfracturing and intra-articular injection of 1,46 × 107 cells mixed with 2 mL of HA (n = 28) vs. control | IKDC score, Tegner activity scale, Lysholm score, MRI (MOCART score) | Randomized controlled trial | HTO, microfracturing and intra-articular injection of 2 mL of HA (n = 28) | - No treatment-related SAEs. - The MSC group showed a significantly greater added improvement in all clinical scores, compared to the control group at the final follow-up. MRI showed significantly better results compared to the control group after one year. |
| Varma et al., Journal of the Indian Medical Association (2010) [68] | Knee | - | 50 | Six months | Double-blinded | Intra-articular injection of BMAC alongside arthroscopic debridement (n = 25) vs. control | Pain VAS score, KOOS | Randomized controlled trial | Arthroscopic debridement (n = 25) | - The MSC group showed a continuous decline of pain VAS score and KOOS at each follow-up compared to the control group. |
| Bastos et al., Knee Surgery, Sports Traumatology, Arthroscopy (2019) [69] | Knee | - | 47 | 12 months | Double-blinded | Intra-articular injection of MSCs alone (n = 16) vs. MSCs + PRP (n = 14) vs. control | KOOS, range of motion, intra-articular cytokines | Randomized controlled trial | Intra-articular injection of GC (4 mg of dexamethasone) (n = 17) | - Significant improvement in all three groups in most KOOS domains and global score after one month and in all KOOS domains and global score at the 12-month follow-up. MSC and MSC + PRP groups showed the highest percentage of improvement. |
| Bastos et al., Knee Surgery, Sports Traumatology, Arthroscopy (2018) [70] | Knee | Dejour grade I–IV | 18 | 12 months | Double-blinded | Intra-articular injection of MSCs + PRP (n = 9) vs. injection of MSCs alone (n = 9) | KOOS | Prospective randomized cohort study | - | - Two treatment-related SAEs (intense knee pain) and eight treatment-related AEs that resolved without any sequelae in five patients. - KOOS was significantly improved in both groups throughout a follow-up period of 12 months. No differences in clinical outcomes were observed between groups. |
| Mautner et al., Stem Cells Translational Medicine (2019) [71] | Knee | K.L. 1–4 | 76 | 13–22 months | - | Intra-articular injection of BMAC (n = 41) vs. micro fragmented adipose tissue (n = 35) | Pain VAS score, KOOS, EQOL, | Retrospective comparative study | - | - Significant improvement in KOOS, EQOL, and pain VAS score for both groups with no significant difference between groups. |
| Chahal et al., Stem Cells Translational Medicine (2019) [72] | Knee | K.L. ≥ 3 | 12 | 24 months | Radiologist blinded | Four cohorts; n = 3 in each of the first three cohorts received 1, 10 or 50 × 106 cells; in cohort four n = 3 received either 1, 10 or 50 × 106 cells | WOMAC score, KOOS, MRI (WORMS) | Prospective, uncontrolled trial | - | - No SAEs, four patients with minor transient AEs. - All four patients in the 50 × 106 group achieved the minimal important difference (MID) in all scores except for KOOS (2/4 patients showed improvements) and quality of life (3/4) at the 12-month follow up; two of four patients in the 1 x 106 group and one in the 10 × 106 group showed MID in all scores; the 50 × 106 cohort had the greatest number of patients achieving MID At higher MSC doses cartilage catabolic biomarkers and MRI-detected synovitis were significantly lower. |
| Emadedin et al., Archives of Iranian Medicine (2015) [73] | Knee (n = 6), ankle (n = 6), hip (n = 5) | K.L. 3/4 | 17 | 30 months | - | Intra-articular injection of 5 × 105 cells/kg bodyweight | Pain VAS score, WOMAC score, HHS, FAOS, walking distance, MRI (T2) | Prospective, uncontrolled trial | - | - No treatment-related SAEs and a limited number of local treatment-related AEs. - Significant improvements in WOMAC score and walking distance in all patients. Pain VAS score improved significantly at the six- and 12-month follow-up and increased again afterwards. Radiological improvements were mainly present in the first six months (descriptive only). |
| Soler et al., The Knee (2016) [74] | Knee | K.L. 2/3 | 15 | 12 months | - | Intra-articular injection of 40.9 × 106 cells (XCEL-M-ALPHA) | Pain VAS score, WOMAC score, Lequesne index, MRI (T2), aHAQ, SF-36 | Prospective, uncontrolled trial | - | - No treatment-related SAEs and 16 musculoskeletal and connective tissue AEs. - Significant improvement in pain VAS and WOMAC scores, aHAQ and Lequesne index were seen over time. No significant changes were observed regarding the social and emotional roles and the mental health part of the SF-36. MRI showed significant improvements (cartilage regeneration) over time. |
| Al-Najar et al., Journal of Orthopaedic Surgery and Research (2017) [75] | Knee | K.L. 2/3 | 13 | 24 months | - | Two intra-articular injections one month apart, totalling 6.1 × 107 ± 0.6 × 106 cells | KOOS, MRI (T2) | Prospective, uncontrolled trial | - | - No treatment-related SAEs. - Significant improvement of cartilage thickness at the 12-month follow up (MRI) and significant improvement of KOOS at six, 12 and 24 months. |
| Orozco et al., Transplantation (2013) [76] | Knee | K.L. 2–4 | 12 | 12 months | - | Intra-articular injection of 4 × 107 cells | Pain VAS score, WOMAC score, Lequesne index, MRI (T2, PCI), SF-36 | Prospective, uncontrolled trial | - | - No treatment-related SAEs and a few minor transient treatment-related AEs. - Significant improvement of pain VAS and WOMAC scores and Lequesne index over time. No significant changes in SF-36. MRI revealed a significant decrease in PCI at six and 12 months. A greater and faster pain relief was noted during sports performance. |
| Orozco et al., Transplantation (2014) [77] | “ | “ | “ | 24 months | “ | “ | Pain VAS score, WOMAC score, Lequesne index, MRI (T2, PCI), SF-36 | Follow-up visit to Orozco et al., Transplantation (2013) [76] | “ | - No treatment-related SAEs and AEs. - Pain improvement was maintained. Further significant cartilage improvements were seen in MRI (PCI) compared to baseline at the 24-month follow-up. |
| Allogenic BMMSCs | ||||||||||
| Gupta et al., Arthritis Research and Therapy Journal (2016) [78] | Knee | K.L. 2/3 | 60 | 12 months | Double-blinded | Intra-articular injection of 2.5 × 107 cells (n = 10) vs. 5 × 107 cells (n = 10) vs. 7.5 × 107 cells (n = 10) vs. 15 × 107 cells (n = 10) (Stempeucel®) mixed with 2–4 mL PLASMA-LYTE A followed by a 2 mL injection of HA (20 mg) vs. control | Pain VAS score, WOMAC score, ICOAP, MRI (WORMS) | Randomized, double-blinded, multicentric, placebo-controlled trial | Intra-articular injection of 2–4 mL of PLASMA-LYTE A (n = 20) | - One treatment-related SAE (synovial effusion) in the 150 × 106 cells group and nine treatment-related AEs among all cell-dose groups. - A decrease of pain VAS score and ICOAP in all treatment groups, except for patients who received 15 × 107 cells, over time, yet without significance when compared to placebo. WOMAC score decreased in all treatment groups, again without significance. Although not significant, the maximum pain reduction in all scores was observed in the low-dose group. No MRI changes were described. |
| Vega et al., Transplantation (2015) [79] | Knee | K.L. ≥ 2 | 30 | 12 months | Double-blinded | Intra-articular injection of 4 × 107 cells (n = 15) vs. control | Pain VAS score, WOMAC score, Lequesne index, MRI (T2, PCI), SF-12 | Randomized controlled trial | Intra-articular injection of 3 mL of HA (60 mg) (n = 15) | - No SAEs and few mild transient AEs, that affected both groups. - Significant pain reduction in the treatment group at six- and 12-month follow-ups (pain VAS score, WOMAC score, Lequesne index). The active control showed a significant improvement in pain VAS score only at the 12-month follow-up. The treatment group showed significant MRI improvements at the 12-month follow-up. |
| Autologous ATMSCs | ||||||||||
| Lee et al., Stem Cells Translational Medicine (2019) [80] | Knee | K.L. ≥ 2 | 24 | Six months | Double-blinded | Intra-articular injection of 108 cells (n = 12) vs. control | WOMAC score | Randomized, double-blinded, placebo-controlled trial | Intra-articular injection of saline (n = 12) | - MSC group with significant improvement of WOMAC score at six months; control group without significant changes in WOMAC score at the same follow-up. |
| Koh et al., Arthroscopy (2014) [81] | Knee | K.L. ≤ 3 | 44 | 24–25 months | Single-blinded | HTO and intra-articular injection of 4.11 × 106 cells (SVF) mixed with of PRP (n = 21) vs. control | Pain VAS score, KOOS, Lysholm score, Kanamiya grading (arthroscopic) | Randomized controlled trial | HTO and intra-articular injection of 3 mL of PRP (n = 23) | - Significantly greater improvements in KOOS sub-scales and pain VAS score in the HTO + MSC + PRP group compared to the HTO + PRP group. Lysholm score was significantly improved in both groups at the final follow-up. Second-look arthroscopy findings after a mean of 19.8 months showed significantly better cartilage healing in the HTO + MSC + PRP group. |
| Hong et al., International Orthopaedics (2019) [82] | Knee | K.L. 2/3 | 16 | 12 months | Double-blinded | Arthroscopic implantation of SVF into one joint (n = 16) vs. control implantation into the contralateral joint | Pain VAS score, WOMAC score, range of motion, MRI (WORMS, MOCART score) | Randomized controlled trial | Arthroscopic implantation of 4 mL of HA (n = 16) | - No SAEs, few local AEs that resolved within two weeks. - Pain and functional scores improved significantly over time in SVF-treated knees, whereas HA-treated knees worsened over time without statistical significance. Significantly improved WORMS and MOCART scores were also reported for SVF-treated knees compared to HA-treated controls. |
| Freitag et al., Regenerative Medicine (2019) [83] | Knee | K.L. 2/3 | 30 | 12 months | - | Single intra-articular injection of 108 cells (n = 10) vs. repeated (two) injections of 108 cells six months apart (n = 10) vs. control | WOMAC score, KOOS, numeric pain rating scale, MRI (MOAKS) | Randomized controlled trial | Conservative treatment (n = 10) | - No SAEs, few self-limiting mild and moderate AEs. - Significant improvements for both treatment groups in pain and clinical outcomes over time and compared to control. No difference between treatment groups. Cartilage improvements or no further cartilage loss in the repeated injection group seen in MRI. |
| Kim et al., Knee Surgery, Sports Traumatology, Arthroscopy (2020) [84] | Knee | K.L. ≥ 3 | 80 | 12–27 months | - | HTO and intra-articular injection of cells (n = 40) vs. HTO and cells with allogenic cartilage (n = 40) | KOOS, Lysholm score, Kanamiya grading (arthroscopic) | Prospective randomized cohort study | - | - Clinical outcomes improved significantly for both groups at second-look arthroscopy. From the second-look arthroscopy to the final follow-up only the MSC allogenic cartilage group improved. Kanamiya grading was significantly higher in the MSC group with allogenic cartilage than in the MSC group. |
| Kim et al., The American Journal of Sports Medicine (2014) [85] | Knee | K.L. 1/2 | 54 | 24–34 months | - | Arthroscopic implantation of 3.9 × 106 cells (SVF) without scaffold (n = 37) vs. arthroscopic implantation of 3.9 × 106 cells (SVF) mixed with fibrin glue as a scaffold. (n = 17) | IKDC score, Tegner activity scale, ICRS grade (arthroscopic) | Retrospective comparative study | - | - Significant improvements of IKDC score and Tegner activity scale in both groups after a mean of 12.3 months. Significantly improved arthroscopic ICRS grades in the implantation + scaffold group compared to the implantation without scaffold group with a significant correlation between clinical outcomes and ICRS grades. Significant predictors for poor clinical outcomes in the implantation without scaffold group were overweight (BMI > 25.5kg/m2) and large lesion size (>5.7cm2). A similar trend was observed in the implantation + scaffold group, yet without significance. |
| Kim and Koh, The American Journal of Sports Medicine (2018) [86] | Knee | K.L. 2/3 | 100 | 32 months | - | HTO and intra-articular injection of a mean of 4.26 × 106 cells (SVF) (n = 50) vs. control | IKDC score, Lysholm score, x-ray, ICRS grade (arthroscopic) | Comparative matched-pair study | HTO (n = 50) | - Significant clinical improvements in the HTO + MSC group compared to the HTO group at the final follow-up. ICRS grades correlated with clinical findings. Radiological outcomes improved compared to pre-operative findings, yet they did not correlate with clinical findings. |
| Koh and Choi, The Knee (2012) [87] | Knee | K-L. < 4 | 50 | 12–18 months | - | Arthroscopic debridement accompanied by intra-articular injection of 1.89 × 106 cells, mixed with 3 mL of PRP (n = 25) vs. control | Pain VAS score, Tegner activity scale, Lysholm score | Retrospective comparative matched-pair study | Arthroscopic debridement and intra-articular injection of 3 mL of PRP (n = 25) | - No SAEs, one patient experienced marked knee pain with swelling, following the injection which resolved spontaneously. Few patients reported slight knee pain in the first two or three days after the injection. - Significant improvements in pain VAS score, Tegner activity scale and Lysholm score at the final follow-up in both groups. No significant differences between the study and control group at the last follow-up. |
| Kim and Koh., Arthroscopy (2016) [88] | Ankle | - | 49 | 24–34 months | - | Lateral sliding calcaneal osteotomy with bone-marrow stimulation and intra-articular injection of 4.1 × 106 cells (SVF) (n = 26) vs. control | Pain VAS score, AOFAS score, x-ray, ICRS grade (arthroscopic) | Retrospective comparative study | Lateral sliding calcaneal osteotomy with bone-marrow stimulation (n = 23) | - Significant improvements in pain VAS and AOFAS scores for both groups at the final follow-up compared to preoperative findings. Clinical scores showed a significantly greater improvement in osteotomy + stimulation + injection group compared to the control group. ICRS grades correlated with clinical findings and showed significantly greater improvements in the osteotomy + stimulation + injection group compared to the control group at the final follow-up. X-ray imaging showed significant improvements in both groups at the final follow-up compared to preoperative findings with no significant difference between groups. |
| Kim et al., The American Journal of Sports Medicine (2015) [89] | Knee | K.L. 1/2 | 40 | 24–42 months | - | Arthroscopic injection of 4.07 × 106 cells (SVF) mixed with PRP (n = 20) vs. arthroscopic implantation of 3.96 × 106 cells (SVF) on a fibrin glue scaffold (n = 20) | IKDC score, Tegner activity scale, ICRS grade (arthroscopic) | Retrospective comparative matched-pair study | - | - Significant improvements of IKDC score and Tegner activity scale at the final follow-up in the implantation group with a significant difference between groups. A significant correlation between clinical outcomes and ICRS grading (second-look arthroscopy after a mean of 12.6 months) was seen, with significantly greater improvements of ICRS grading in the implantation group. |
| Schiavone Panni, International Orthopaedics (2019) [90] | Knee | K.L. < 3 | 52 | 24 months | - | Arthroscopic debridement followed by intra-articular injection of 10–15 mL of SVF | Pain VAS score, IKS score | Retrospective case series | - | - No SAEs, three AEs related to harvesting procedure. - Significant improvement of pain VAS and IKS score over time. Patients with a baseline pain VAS score > 8 showed greater clinical and functional improvements. |
| Kim et al., The American Journal of Sports Medicine (2015) [91] | Knee | K.L. 1/2 | 49 | 24–36 months | - | Arthroscopic implantation of 4.3 × 106 cells (SVF) on a fibrin glue scaffold | IKDC score, Tegner activity scale | Retrospective case series | - | - Significant improvements of IKDC score and Tegner activity scale at the final follow-up. A high prognostic significance was observed regarding age and lesion size. 60 years and a lesion size of 6.0 cm2 were observed as an optimum cut-off for poor clinical outcomes. |
| Koh et al., The American Journal of Sports Medicine (2014) [92] | Knee | K.L. 1/2 | 35 | 24–34 months | - | Intra-articular injection of 3.8 × 106 cells | IKDC score, Tegner activity scale, ICRS grade (arthroscopic) | Retrospective case series | - | - Significant improvements of IKDC score and Tegner activity scale at the final follow-up. Overweight patients (BMI >27.5 kg/m2) and patients with a large lesion size (>5.4 cm2) had a significantly worse outcome regarding IKDC score, Tegner activity scale and ICRS grade. Both, the Tegner activity scale and the IKDC score were negatively correlated with ICRS grades. Second-look arthroscopic surgery revealed healed chondral lesions after a mean of 12.7 months. |
| Koh et al., Knee Surgery, Sports Traumatology, Arthroscopy (2015) [93] | Knee | K.L. 2/3 | 30 | 24–26 months | - | Intra-articular injection of 4.04 × 106 cells (SVF) mixed with 3 mL of PRP combined with arthroscopic lavage | Pain VAS score, KOOS, Lysholm score, x-ray, ICRS grade (arthroscopic) | Prospective, uncontrolled trial | - | - No major complications, three patients complained of slight knee pain. - Significantly improved clinical scores at the 24-month follow-up compared to the 12-month follow-up. Second-look arthroscopy revealed 18.7% very positive, 43.8% positive, 25% neutral and 12.5% negative cartilage healing results. Age and mean improvement in KOOS sub-scales were associated. K.L. grade 2 was associated with a higher Lysholm score improvement. |
| Kim et al., Osteoarthritis and Cartilage (2016) [94] | Knee | K.L. 1/2 | 20 | 24 months | - | Arthroscopic implantation of 4.4 × 106 cells (SVF) on a fibrin glue scaffold | IKDC score, Tegner activity scale, MRI (MOAKS, MOCART score) | Prospective, uncontrolled trial | - | - Significant improvement of IKDC score and Tegner activity scale at the final follow-up. Significant improvements in MRI scores with a correlation between clinical and MRI outcomes at the final follow-up. |
| Roato et al., International Orthopaedics (2018) [95] | Knee | K.L. 1–3 | 20 | 18 months | - | Arthroscopic implantation of 35 mL of concentrated autologous adipose tissue | Pain VAS score, WOMAC score, MRI (T1, T2) | Prospective, uncontrolled trial | - | - No SAEs and few self-limiting AEs, two patients underwent arthroplasty before trial completion. - Significant pain VAS and WOMAC score improvements at all follow-ups. No MRI changes. Histological sections of MSC-treated knees (who underwent arthroplasty) showed new tissue formation (descriptive only). |
| Pintat et al., Journal of Vascular and Interventional Radiology (2017) [96] | Knee | - | 19 | 12 months | - | Intra-articular injection of cells (SVF) mixed with PRP | WOMAC score, MRI (lesion grade, surface, T2) | Prospective, uncontrolled trial | - | - No complications reported. - Significantly improved WOMAC score at the six- and 12-month follow-up compared to baseline. No significant MRI differences |
| Koh et al., Arthroscopy (04/2013) [97] | Knee | K.L. 3/4 | 18 | 24–26 months | - | Intra-articular injection of 1.18 × 106 cells mixed with 3 mL of PRP | Pain VAS score, WOMAC score, Lysholm score, MRI (WORMS) | Prospective, uncontrolled trial | - | - Significant reduced WOMAC score and improved Lysholm and pain VAS score wat the final follow-up. MRI score showed a significant improvement at final follow-up. |
| Song et al., Regenerative Medicine (2018) [98] | Knee | K.L. ≥ 2 | 18 | 22 months | - | Intra-articular injection of 1 × 107 cells (n = 6) vs. 2 × 107 cells (n = 6) vs. 5 × 107cells (n = 6) into each knee-joint, followed by a third injection of 5 × 107 cells after eleven months | WOMAC score, NRS-11, MRI (cartilage volume), SF-36 | Prospective, uncontrolled trial | - | - No SAEs and few AEs occurred equally distributed among groups. - WOMAC score and the NRS-11 significantly improved over time. SF-36 showed a significant reduction only at the three-month follow-up for the low-dose group and at the 22-month follow-up for the middle-dose group. MRI showed a significant increase in cartilage volume over time, compared to baseline. The high-dose group showed the greatest improvements overall. |
| Jo et al., Stem Cells (2014) [99] | Knee | K.L. ≥ 2 | 18 | Six months | - | Intra-articular injections of 1 × 107 cells (n = 3) vs. 5 × 107 cells (n = 3) vs. 1 × 108 (n = 12) cells mixed with saline | Pain VAS score, WOMAC score, KOOS, KSS, x-ray, MRI (size and depth of cartilage defect), ICRS grade (arthroscopic), histology (safranin O, immunohistochemistry) | Prospective, uncontrolled trial | - | - No treatment-related SAEs or AEs. - An improvement of pain VAS and WOMAC score at the six-month follow-up in the high-dose group. KSS improved significantly in the low- and high-dose group. MRI (size of cartilage defect) showed a significant improvement in the high-dose group. Second look arthroscopy revealed a significant reduction o cartilage defects and of the ICRS grade in the high-dose group. Histology showed regeneration of articular cartilage defects (descriptive only). |
| Jo et al., American Journal of Sports Medicine (2017) [100] | “ | “ | “ | 24 months | “ | “ | Pain VAS score, WOMAC score, KOOS, KSS, x-ray, MRI (size and depth of cartilage defect) | Follow-up visit to Jo et al., Stem Cells (2014) [99] | “ | - No treatment-related SAEs or AEs. - Improvement in WOMAC score, KSS, KOOS and reduced knee pain for up to 24 months were seen for any dose. Statistical significance was reached mainly in the high-dose group. Clinical outcomes (WOMAC score) declined after 12 months in the low- and mid-dose group, whereas a plateau was observed in the high-dose group until 24 months. Similar results were obtained for MRI evaluations. |
| Pers et al., Stem Cells Translational Medicine (2016) [101] | Knee | K.L. 3/4 | 18 | Six months | - | Intra-articular injection of 2 × 106 cells (SVF) (n = 6) vs. 10 × 106 cells (SVF) (n = 6) vs. 50 × 106 cells (SVF) (n = 6) | Pain VAS score, WOMAC score, KOOS, SAS, MRI (dGMERIC and T1rho), histology (protein S 100, CD 34, Ki 67) SF-36, PGA | Prospective, bicentric uncontrolled trial | - | - No treatment-related SAEs and five potentially treatment-related AEs. - Improvement in pain, function and mobility were observed, regardless of dose. Significantly improved pain levels and function were detected only in the low-dose group. No correlation between clinical changes and MRI. All but one patient refused a previously scheduled total knee arthroplasty. Histological findings varied in their description. No statistically significant differences between groups for SF-36. |
| Spasovski et al., The Journal of Gene Medicine [102] | Knee | IKDC B & D | nine | 18 months | - | Intra-articular injection of 0.5–1 × 107 cells | Pain VAS score, KSS, HSS-KS, Tegner-Lysholm score, X-ray, MRI (MOCART score) | Prospective, uncontrolled trial | - No SAEs and few AEs, that resolved within one week. - Significant improvements between baseline and 3-month follow-up and further improvements between 3- and 6-month follow-up in all scores. Scores remained improved at 12- and 18-month follow-up, without further significant development. A significant cartilage restoration (MOCART score) was observed at the final follow-up, compared to baseline. X-ray imaging showed neither improvement nor deterioration. | |
| Allogeneic UCMSCs | ||||||||||
| Matas et al., Stem Cells Translational Medicine (2019) [103] | Knee | K.L. 1–3 | 26 | 12 months | Triple-blinded | Repeated (two) intra-articular injections of 2 × 107 cells in 3 mL of saline with 5% plasma six months apart (n = 9) vs. single injection of 2 × 107 cells (n = 9), followed by 3 mL of saline with 5% plasma six months apart vs. control | Pain VAS score, WOMAC score, SF-36, Patient Global Assessment, OMERACT-OARSI Responder Index Criteria, MRI (WORMS) | Randomized controlled trial | Intra-articular injection of 3 mL of HA (n = 8) | - No SAEs, few transient AEs (acute synovitis and local pain). - Significant pain and functional improvements over time for MSC-treated patients compared to control. The single injection group stopped improving after month 9, while the repeated injections group continued improving until final the follow-up. All patients in the repeated injections group were found to be responders, according to the OMERACT-OARSI Responder Index Criteria. No changes in SF-36 and MRI. |
| Park et al., Stem Cells Translational Medicine (2017) [104] | Knee | K.L. 3 | seven | 98 months | - | Implantation of “Cartistem”, a composite of UCMSCs and HA hydrogel, into drill holes at two different dosages: 1.15–1.25 × 107 cells (n = 4) vs. 1.65–2 × 107 cells (n = 3) | Pain VAS score, IKDC score, MRI (dGMERIC), ICRS grade (arthroscopic), histology (Masson’s trichrome, safranin O, immunohisto-chemistry) | Prospective, uncontrolled trial | - | - No treatment-related SAEs and one treatment-related AE. - Pain VAS and IKDC score improved significantly over time and remained improved without significant deterioration at the seven-year follow-up. MRI showed high glycosaminoglycan contents in regenerated cartilage (qualitative). Hyaline-like cartilage was found at lesion sites at the one-year arthroscopic follow-up in two patients with histological findings of regenerated cartilage (descriptive only). |
| Allogenic PLMSCs | ||||||||||
| Khalifeh Soltani et al., Cytotherapy (2019) [105] | Knee | K.L. 2–4 | 20 | Six months | Double-blinded | Intra-articular injection of 5–6 × 107 cells in 1mL of saline (n = 10) vs. control | KOOS, range of motion, MRI (cartilage thickness) | Randomized, double-blinded, placebo-controlled trial | Intra-articular injection of 10 mL of saline (n = 10) | - No SAEs and four self-limiting AEs. - Significantly improved pain score and range of motion in the MSC group at week 8. Cartilage thickness improved in the MSC group only. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maleitzke, T.; Elazaly, H.; Festbaum, C.; Eder, C.; Karczewski, D.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal Stromal Cell-Based Therapy—An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials. J. Clin. Med. 2020, 9, 2062. https://doi.org/10.3390/jcm9072062
Maleitzke T, Elazaly H, Festbaum C, Eder C, Karczewski D, Perka C, Duda GN, Winkler T. Mesenchymal Stromal Cell-Based Therapy—An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials. Journal of Clinical Medicine. 2020; 9(7):2062. https://doi.org/10.3390/jcm9072062
Chicago/Turabian StyleMaleitzke, Tazio, Hisham Elazaly, Christian Festbaum, Christian Eder, Daniel Karczewski, Carsten Perka, Georg N. Duda, and Tobias Winkler. 2020. "Mesenchymal Stromal Cell-Based Therapy—An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials" Journal of Clinical Medicine 9, no. 7: 2062. https://doi.org/10.3390/jcm9072062
APA StyleMaleitzke, T., Elazaly, H., Festbaum, C., Eder, C., Karczewski, D., Perka, C., Duda, G. N., & Winkler, T. (2020). Mesenchymal Stromal Cell-Based Therapy—An Alternative to Arthroplasty for the Treatment of Osteoarthritis? A State of the Art Review of Clinical Trials. Journal of Clinical Medicine, 9(7), 2062. https://doi.org/10.3390/jcm9072062






