Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives
Abstract
1. Introduction
2. Methods
3. Results
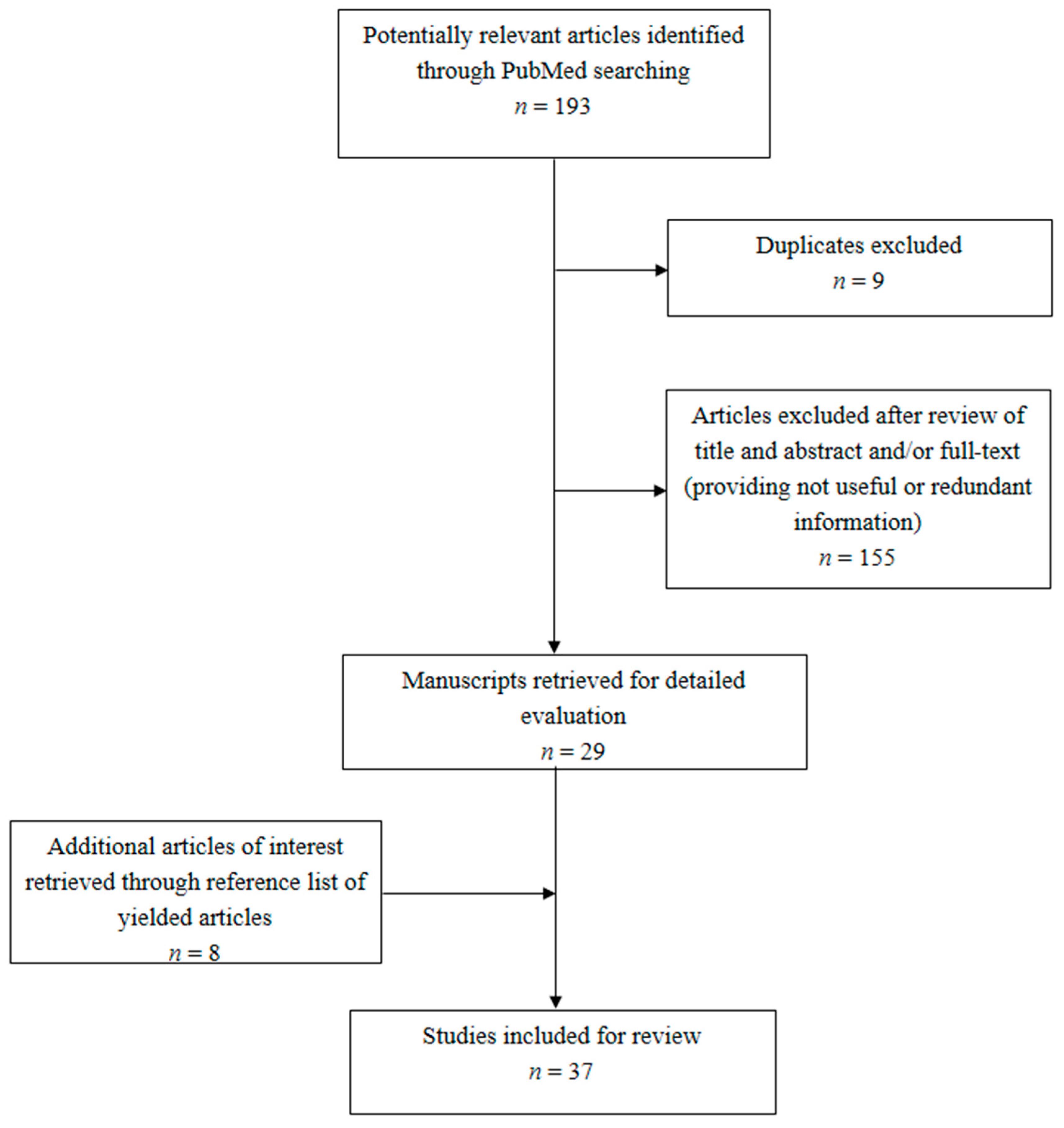
3.1. Literature Search
3.2. Lopinavir/Ritonavir: An Overview
3.3. Current Evidence for LPV/r in SARS
3.4. Current Evidence for LPV/r in MERS
3.5. Current Evidence for LPV/r in COVID-19
3.6. Guideline Recommendations for LPV/r in COVID-19
3.7. Ongoing Interventional Trials for LPV/r in COVID-19
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, T.T.; Qian, J.D.; Zhu, W.Y.; Wang, Y.; Wang, G.Q. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J. Med. Virol. 2020, 92, 556–563. [Google Scholar] [CrossRef]
- Ford, N.; Vitoria, M.; Rangaraj, A.; Norris, S.L.; Calmy, A.; Doherty, M. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: Initial assessment. J. Int. AIDS Soc. 2020, 23, e25489. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, Y.; Zhang, Z.L.; Liu, Y.X.; Le, K.J.; Cui, M.; Yu, Y.T.; Gu, Z.C.; Gao, Y.; Lin, H.W. Efficacy and safety of current therapeutic options for COVID-19 -lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol. Res. 2020, 157, 104872. [Google Scholar] [CrossRef] [PubMed]
- Lu, H. Drug treatment options for the 2019-new Coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Kupferschmidt, K.; Cohen, J. Race to find COVID-19 treatments accelerates. Science 2020, 367, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- “Solidarity” Clinical Trial for COVID-19 Treatments. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments (accessed on 6 May 2020).
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Available online: https://pubmed.ncbi.nlm.nih.gov/31643176 (accessed on 21 May 2020).
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. SARS-CoV-2: Recent reports on antiviral therapies based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Corbellino, M.; Clementi, E.; Cattaneo, D.; Gervasoni, C. Lopinavir/ritonavir in COVID-19 patients: Maybe yes, but at what dose? J. Antimicrob. Chemother. 2020, dkaa190. [Google Scholar] [CrossRef]
- Wu, C.Y.; Jan, J.T.; Ma, S.H.; Kuo, C.J.; Juan, H.F.; Cheng, Y.S.; Hsu, H.H.; Huang, H.C.; Wu, D.; Brik, A.; et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef]
- Yousefifard, M.; Zali, A.; Mohamed Ali, K.; Madani Neishaboori, A.; Zarghi, A.; Hosseini, M.; Safari, S. Antiviral therapy in management of COVID-19: A systematic review on current evidence. Arch. Acad. Emerg. Med. 2020, 8, e45. [Google Scholar]
- Chan, K.S.; Lai, S.T.; Chu, C.M.; Tsui, E.; Tam, C.Y.; Wong, M.M.L.; Tse, M.W.; Que, T.L.; Peiris, J.S.M.; Sung, J.; et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: A multicentre retrospective matched cohort study. Hong Kong Med. J. 2003, 9, 399–406. [Google Scholar]
- Chu, C.M.; Cheng, V.C.; Hung, I.F.; Wong, M.M.; Chan, K.H.; Chan, K.S.; Kao, R.Y.; Poon, L.L.; Wong, C.L.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic review of treatment effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; Van Nieuwkoop, S.; Bestebroer, T.M.; Van Den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef]
- Choi, W.S.; Kang, C.I.; Kim, Y.; Choi, J.P.; Joh, J.S.; Shin, H.S.; Kim, G.; Peck, K.R.; Chung, D.R.; Kim, H.O.; et al. Clinical presentation and outcomes of Middle East Respiratory Syndrome in the Republic of Korea. Infect. Chemother. 2016, 48, 118–126. [Google Scholar] [CrossRef]
- Chan, J.F.; Yao, Y.; Yeung, M.L.; Deng, W.; Bao, L.; Jia, L.; Li, F.; Xiao, C.; Gao, H.; Yu, P.; et al. Treatment with Lopinavir/Ritonavir or Interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015, 212, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Alothman, A.; Balkhy, H.H.; Al-Dawood, A.; AlJohani, S.; Al Harbi, S.; Kojan, S.; Al Jeraisy, M.; Deeb, A.M.; Assiri, A.M.; et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): Study protocol for a randomized controlled trial. Trials 2018, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Treatment of MERS-CoV: Information for Clinicians. Clinical Decision-Making Support for Treatment of MERS-CoV Patients (v 4.0). Available online: https://www.gov.uk/government/publications/mers-cov-clinical-decision-making-support-for-treatment (accessed on 15 May 2020).
- Wang, J. Fast identification of possible drug treatment of Coronavirus Disease -19 (COVID-19) through computational drug repurposing study. J. Chem. Inf. Model. 2020, 60, 3277–3286. [Google Scholar] [CrossRef]
- Nutho, B.; Mahalapbutr, P.; Hengphasatporn, K.; Pattaranggoon, N.C.; Simanon, N.; Shigeta, Y.; Hannongbua, S.; Rungrotmongkol, T. Why are Lopinavir and Ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry 2020, 59, 1769–1779. [Google Scholar] [CrossRef]
- Choy, K.T.; Wong, A.Y.; Kaewpreedee, P.; Sia, S.F.; Chen, D.; Hui, K.P.; Chu, D.K.; Chan, M.C.; Cheung, P.P.; Huang, X.; et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, K.H.; Chung, J.H.; Shin, K.C.; Choi, E.Y.; Jin, H.J.; Jang, J.G.; Lee, W.; Ahn, J.H. Clinical Features and Outcomes of 98 Patients Hospitalized with SARS-CoV-2 Infection in Daegu, South Korea: A Brief Descriptive Study. Yonsei Med. J. 2020, 61, 431–437. [Google Scholar] [CrossRef]
- Piva, S.; Filippini, M.; Turla, F.; Catteneo, S.; Margola, A.; De Fulviis, S.; Nardiello, I.; Beretta, S.; Ferrari, L.; Trotta, R.; et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Brescia, Italy. J. Crit. Care 2020, 58, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, e200994. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.T.; Luo, Y.L.; Xia, S.C.; Sun, Q.F.; Ding, J.G.; Zhou, Y.; Chen, W.; Wang, X.F.; Zhang, W.W.; Du, W.J.; et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur. Rev. Med. Pharm. Sci. 2020, 24, 3390–3396. [Google Scholar]
- Lim, J.; Jeon, S.; Shin, H.Y.; Kim, M.J.; Seong, Y.M.; Lee, W.J.; Choe, K.W.; Kang, Y.M.; Lee, B.; Park, S.J. Case of the index patient who caused tertiary transmission of COVID-19 Infection in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 2020, 35, e79. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, W.; Zhang, L.; Wang, D.; Wan, Y. Discontinuation of antiviral drugs may be the reason for recovered COVID-19 patients testing positive again. Br. J. Hosp. Med. (Lond.) 2020, 81, 1–2. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Lee, Y.L.; Chen, C.P.; Lin, Y.C.; Liu, C.E.; Liao, C.H.; Cheng, S.H. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J. Microbiol. Immunol. Infect. 2020, 53, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, C.; Zeng, Q.; Liu, X.; Li, X.; Zhang, H.; Hong, Z.; Xia, J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef]
- Zhu, Z.; Lu, Z.; Xu, T.; Chen, C.; Yang, G.; Zha, T.; Xue, Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020, 81, e21–e23. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Corrao, S.; Natoli, G.; Cacopardo, B. A Trial of Lopinavir-Ritonavir in Covid-19. Letter. Available online: https://www.nejm.org/doi/full/10.1056/NEJMc2008043 (accessed on 21 May 2020).
- Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Castañón, E. A Trial of Lopinavir-Ritonavir in Covid-19. Letter. Available online: https://www.nejm.org/doi/full/10.1056/NEJMc2008043 (accessed on 21 May 2020).
- Cao, B.; Zhang, D.; Wang, C. A Trial of Lopinavir-Ritonavir in Covid-19. Reply. N. Engl. J. Med. 2020, 382, e68. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.; Lung, K.C.; Tso, E.Y.; Liu, R.; Chung, T.W.; Chu, M.Y.; Ng, Y.Y.; Lo, J.; Chan, J.; Tam, A.R.; et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- Zhang, J.J.Y.; Lee, K.S.; Ang, L.W.; Leo, Y.S.; Young, B.E. Risk Factors of Severe Disease and Efficacy of Treatment in Patients Infected with COVID-19: A Systematic Review, Meta-Analysis and Meta-Regression Analysis. Clin. Infect. Dis. 2020, ciaa576. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Rochwerg, B.; Wang, Y.; Adhikari, N.K.; Murthy, S.; Lamontagne, F.; Fowler, R.A.; Qiu, H.; Wei, L.; Sang, L.; et al. Treatment of patients with nonsevere and severe coronavirus disease 2019: An evidence-based guideline. CMAJ 2020, 192, e536–e545. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Crit. Care Med. 2020, 48, e440–e469. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Aliberti, S.; Barisione, E.; Centanni, S.; De Rosa, F.G.; Di Marco, F.; Gori, A.; Granata, G.; Mikulska, M.; et al. Italian Society of Anti-infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: Current position of the Italian Society of anti-infective therapy (SITA) and the Italian Society of Pulmonology (SIP). Clin. Microbiol. Infect. 2020, 26, 880–894. [Google Scholar]
- NIH COVID-19 Treatment Guidelines. Available online: https://covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation (accessed on 6 May 2020).
- WHO. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. Interim Guidance 13 March 2020. WHO REFERENCE NUMBER: WHO/2019-nCoV/clinical/2020.4. Available online: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed on 6 May 2020).
- German Recommendations for Critically Ill Patients with COVID-19. Med Klin Intensivmed Notfmed. Available online: https://link.springer.com/article/10.1007/s00063-020-00689-w (accessed on 6 May 2020).
- Wilson, K.C.; Chotirmall, S.H.; Bai, C.; Rello, I. COVID-19: Interim Guidance on Management Pending Empirical Evidence. From an American Thoracic Society-led International Task Force. Available online: https://www.thoracic.org/about/newsroom/press-releases/journal/2020/ats-publishes-new-guidance-on-covid-19-management.php (accessed on 6 May 2020).
- Bhimraj, A.; Morgan, R.L.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V.C.; Edwards, K.M.; Gandhi, R.; Muller, W.J.; O’Horo, J.C.; et al. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin. Infect. Dis. 2020, ciaa478. [Google Scholar] [CrossRef] [PubMed]
- National COVID-19 Clinical Evidence Taskforce. Management of Patients with Moderate to Severe COVID-19. Version 3.0. Available online: https://covid19evidence.net.au/wp-content/uploads/COVID-19-FLOW-CHART-1-MODERATE-SEVERE_V3.0.pdf (accessed on 6 May 2020).
- Jin, Y.H.; Cai, L.; Cheng, Z.S.; Cheng, H.; Deng, T.; Fan, Y.P.; Fang, C.; Huang, D.; Huang, L.Q.; Huang, Q.; et al. Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef]
- Kim, S.B.; Huh, K.; Heo, J.Y.; Joo, E.J.; Kim, Y.J.; Choi, W.S.; Kim, Y.J.; Seo, Y.B.; Yoon, Y.K.; Ku, N.S.; et al. Interim Guidelines on Antiviral Therapy for COVID-19. Infect. Chemother. 2020, 52, e18. [Google Scholar]
- Diagnosis and Treatment Protocol for COVID-19 (Trial Version 7) issued by the General Office of the National Health Commission and the Office of the National Administration of Traditional Chinese Medicine. Available online: http://www.chinacdc.cn/en/COVID19 (accessed on 6 May 2020).
- SIMIT Vademecum Per La Cura Delle Persone Con Malattia Da COVI-19. Versione 2.0, 13 marzo 2020. Available online: http://www.simit.org/IT/index.xhtml (accessed on 6 May 2020).
| Guidelines, Year [Ref] | Recommendation | Strenght and/or Quality of Evidence | Grading of Recommendation | Net Balance of Recommendation |
|---|---|---|---|---|
| AMMI, CEPCPA, HPPC, 2020 [39] | We suggest not using LPV/r in patients with non-severe and severe COVID-19 | Weak recommendation | GRADE approach | Against |
| SSC, 2020 [40] | In critically ill adults with COVID-19 we suggest against the routine use of LPV/r | Weak recommendation, low-quality evidence | GRADE approach | Against |
| SITA, SIP, 2020 [41] | At the present time, evidence from the first published RCT does not support off-label treatment with LPV/r in COVID-19 patients | One randomized trial | Consensus | Against/Uncertain |
| NIH, 2020 [42] | There are insufficient data to recommend either for or against the use of any antiviral or immunomodulatory therapy in patients with COVID-19 who have mild, moderate, severe, or critical illness (AIII). Except in the context of a clinical trial, the Panel recommends against the use of LPV/r because of unfavorable pharmacodynamics and negative clinical trial data (AI). | A: Strong recommendation I: One or more randomized trials III: Expert opinion | Consensus | Against/Uncertain |
| WHO, 2020 [43] | There is no current evidence to recommend any specific anti-COVID-19 treatment for patients with confirmed COVID-19. There are many ongoing clinical trials testing various potential antivirals. | Not indicated | Not indicated | Uncertain |
| DGIIN, DIVI, DGP, DGAI, 2020 [44] | We recommend to only use LPV/r (and other drugs) as part of compassionate use programs or approved study protocols after carefully evaluating risks and benefits for the individual patient | Not indicated | Consensus, multidisciplinary approach | Uncertain |
| ATS, 2020 [45] | For hospitalized patients with COVID-19 who have evidence of pneumonia, we make no suggestion either for or against treatment with LPV/r | 30% for intervention, 26% no suggestion, 43% against intervention | CORE process | Uncertain |
| IDSA, 2020 [46] | Among patients who have been admitted to the hospital with COVID-19, the IDSA guideline panel recommends the combination of LPV/r only in the context of a clinical trial | Knowledge gap | GRADE approach | Uncertain |
| Australian Task force, 2020 [47] | For people with moderate to severe to critical COVID-19, only administer LPV/r in the context of clinical trials with appropriate ethical approval. Living Guidance | Evidence-based recommendation | Not indicated | Uncertain |
| CPAM, 2020 [48] | LPV/r orally, 2 capsules each time, twice a day, can be considered | Weak recommendation, low level of evidence | GRADE approach | Pro |
| KSID, KSAT, KSPID, 2020 [49] | Antiviral therapy may be considered for patients with confirmed COVID-19 and can be considered for patients with confirmed COVID-19 with moderate to severe course including pneumonia, those with worsening clinical findings, and those who are likely to progress to severe COVID-19 disease (elderly, chronic diseases, immunocompromised patients) (CIII). Antiviral agents should be administered as early as possible (BIII). LPV/r 400 mg/100 mg can be used up to twice a day, when used alone (CIII). Generally, antiviral agents are recommended for 7–10 days | B: Should generally be offered C: Optional III: Expert opinion or descriptive studies | GRADE approach | Pro |
| CCDCP, 2020 [50] | Antiviral treatment: LPV/r 200 mg/50 mg 2 pills twice a day, not exceeding 10 days | Not indicated | Not indicated | Pro |
| SIMIT, 2020 [51] | Recommended early start in case of: (1) age >70 years, and/or with comorbidities (2) mild-moderate symptoms with chest x-ray positive for pneumonia | Low-quality evidence | Consensus | Pro |
| Study Title | ClinicalTrials.gov Identifier | Interventions (LPV/r vs. or LPV/r Plus.) | Locations |
|---|---|---|---|
| Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | NCT04307693 | -Hydroxychloroquine | Korea |
| OUTpatient Treatment of COVID-19 in Patients with Risk Factor for Poor Outcome (OUTCOV) | NCT04365582 | -Azithromycin -Hydroxychloroquine | France |
| Trial of Early Therapies During Non-Hospitalized Outpatient Window for COVID-19 (TREAT-NOW) | NCT04372628 | -Hydroxychloroquine | USA |
| Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO) | NCT04330690 | -Remdesivir -Hydroxychloroquine | Canada |
| Clinical Trial to Evaluate Efficacy of Three Types of Treatment in Patients With Pneumonia by COVID-19 (Covid-19HUF) | NCT04346147 | -Imanitib -Baricitinib -Hydroxychloroquine | Spain |
| Chemoprophylaxis of SARS-CoV-2 Infection (COVID-19) in Exposed Healthcare Workers (COVIDAXIS) | NCT04328285 | -Placebo -Hydroxychloroquine | France |
| COVID MED Trial: Comparison of Therapeutics for Hospitalized Patients Infected With SARS-CoV-2 (COVIDMED) | NCT04328012 | -Placebo -Hydroxychloroquine -Losartan | USA |
| Safety and Efficacy of Hydroxychloroquine + Favipiravir Drug Regimen in Comparison with Hydroxychloroquine + Kaletra on the Need for Intensive Care Unit Treatment in Patients with COVID-19 | NCT04376814 | -Favipiravir -Hydroxychloroquine | Iran |
| Effectiveness and Safety of Medical Treatment for SARS-CoV-2 (COVID-19) in Colombia | NCT04359095 | -Azithromycin -Hydroxychloroquine -Standard treatment | Colombia |
| Efficacy and Safety of Umifenovir as an Adjuvant Therapy Compared to the Control Therapeutic Regiment of Interferon Beta 1a, Lopinavir/Ritonavir, and a Single Dose of Hydroxychloroquine in Moderate to Severe COVID-19: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial | NCT04350684 | -Umifenovir -Interferon-β 1a -Hydroxychloroquine -Standards of Care | Iran |
| A Prospective/Retrospective, Randomized Controlled Clinical Study of Antiviral Therapy in the 2019-nCoV Pneumonia | NCT04255017 | -Abidol hydrochloride -Oseltamivir | China |
| COVID-19 Ring-Based Prevention Trial with Lopinavir/Ritonavir (CORIPREV-LR) | NCT04321174 | None | Canada |
| Efficacy of Pragmatic Same-day COVID-19 Ring Prophylaxis for Adult Individuals Exposed to SARS-CoV-2 in Switzerland (COPEP) | NCT04364022 | -Hydroxychloroquine | Switzerland |
| Treatment of Moderate to Severe Coronavirus Disease (COVID-19) in Hospitalized Patients | NCT04321993 | -Baricitinib -Hydroxychloroquine | Canada |
| Interferon Beta 1a in Hospitalized COVID-19 Patients (IB1aIC) | NCT04350671 | -Interferon Beta-1A -Hydroxychloroquine | Iran |
| Evaluation of Efficacy of Levamisole and Formoterol+Budesonide in Treatment of COVID-19 | NCT04331470 | -Levamisole + Budesonide + Formoterol inhaler -Hydroxychloroquine | Iran |
| Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | NCT04261907 | -ASC09/ritonavir | China |
| Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) (ACOVACT) | NCT04351724 | -Hydroxychloroquine -Candesartan -Clazakizumab -Placebo -Other treatments | Austria |
| Antiviral Therapy and Baricitinib for the Treatment of Patients with Moderate or Severe COVID-19 | NCT04373044 | -Baricitinib -Hydroxychloroquine -Remdesivir | USA |
| Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) | NCT04315948 | -Remdesivir -Interferon Beta-1A Hydroxychloroquine -Standard of care | France |
| Low Dose Anti-Inflammatory Radiotherapy for the Treatment of Pneumonia by COVID-19 | NCT04380818 | -Low-dose radiotherapy -Hydroxychloroquine -Tocilizumab -Azithromycin -Corticosteroid -LMWH | Spain |
| Lopinavir/Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | NCT04276688 | -Ribavirin -Interferon Beta-1B | China |
| Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID-19: A Randomized Control Trial (THDMS-COVID-19) | NCT04303299 | -Darunavir -Oseltamivir -Favipiravir -Hydroxychloroquine | Thailand |
| Randomised Evaluation of COVID-19 Therapy (RECOVERY) | NCT04381936 | -Corticosteroid -Hydroxychloroquine -Azithromycin -Convalescent plasma -Tocilizumab | United Kingdom |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meini, S.; Pagotto, A.; Longo, B.; Vendramin, I.; Pecori, D.; Tascini, C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. J. Clin. Med. 2020, 9, 2050. https://doi.org/10.3390/jcm9072050
Meini S, Pagotto A, Longo B, Vendramin I, Pecori D, Tascini C. Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. Journal of Clinical Medicine. 2020; 9(7):2050. https://doi.org/10.3390/jcm9072050
Chicago/Turabian StyleMeini, Simone, Alberto Pagotto, Benedetta Longo, Igor Vendramin, Davide Pecori, and Carlo Tascini. 2020. "Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives" Journal of Clinical Medicine 9, no. 7: 2050. https://doi.org/10.3390/jcm9072050
APA StyleMeini, S., Pagotto, A., Longo, B., Vendramin, I., Pecori, D., & Tascini, C. (2020). Role of Lopinavir/Ritonavir in the Treatment of Covid-19: A Review of Current Evidence, Guideline Recommendations, and Perspectives. Journal of Clinical Medicine, 9(7), 2050. https://doi.org/10.3390/jcm9072050






