Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure
Abstract
1. Introduction
2. Methods
2.1. Patients and Protocol
2.2. Offline Data Analysis
2.3. Statistical Analysis
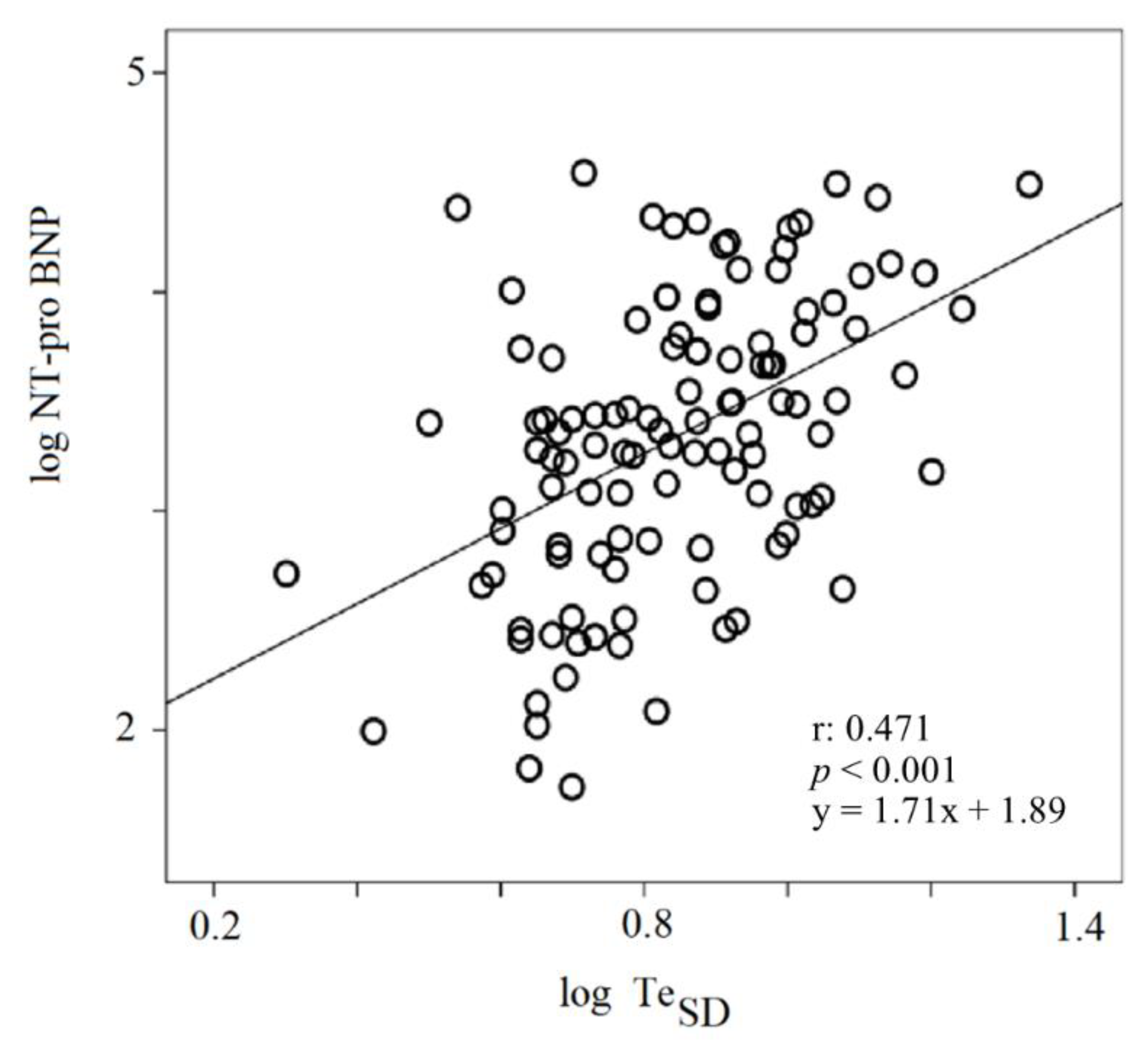
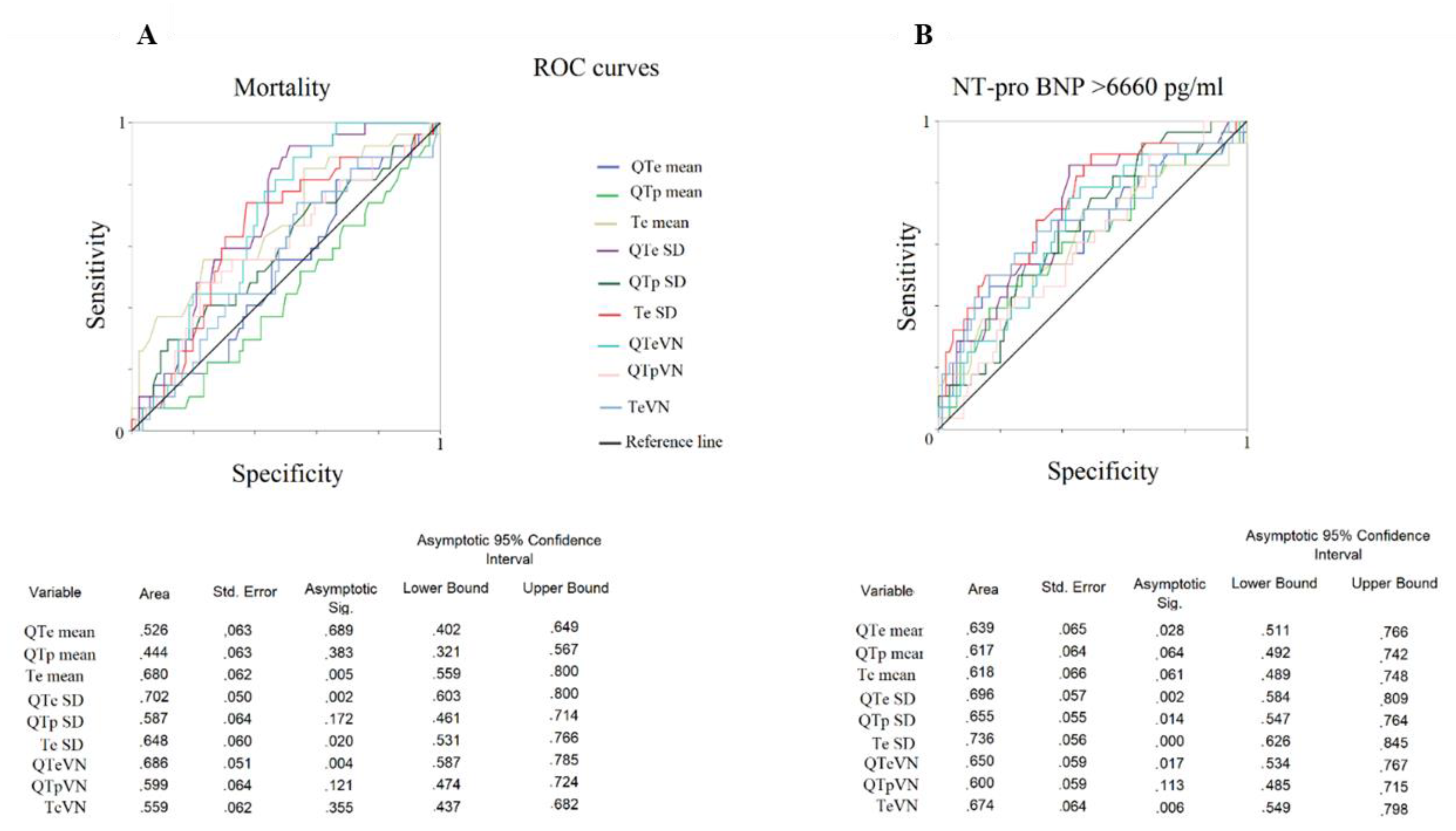
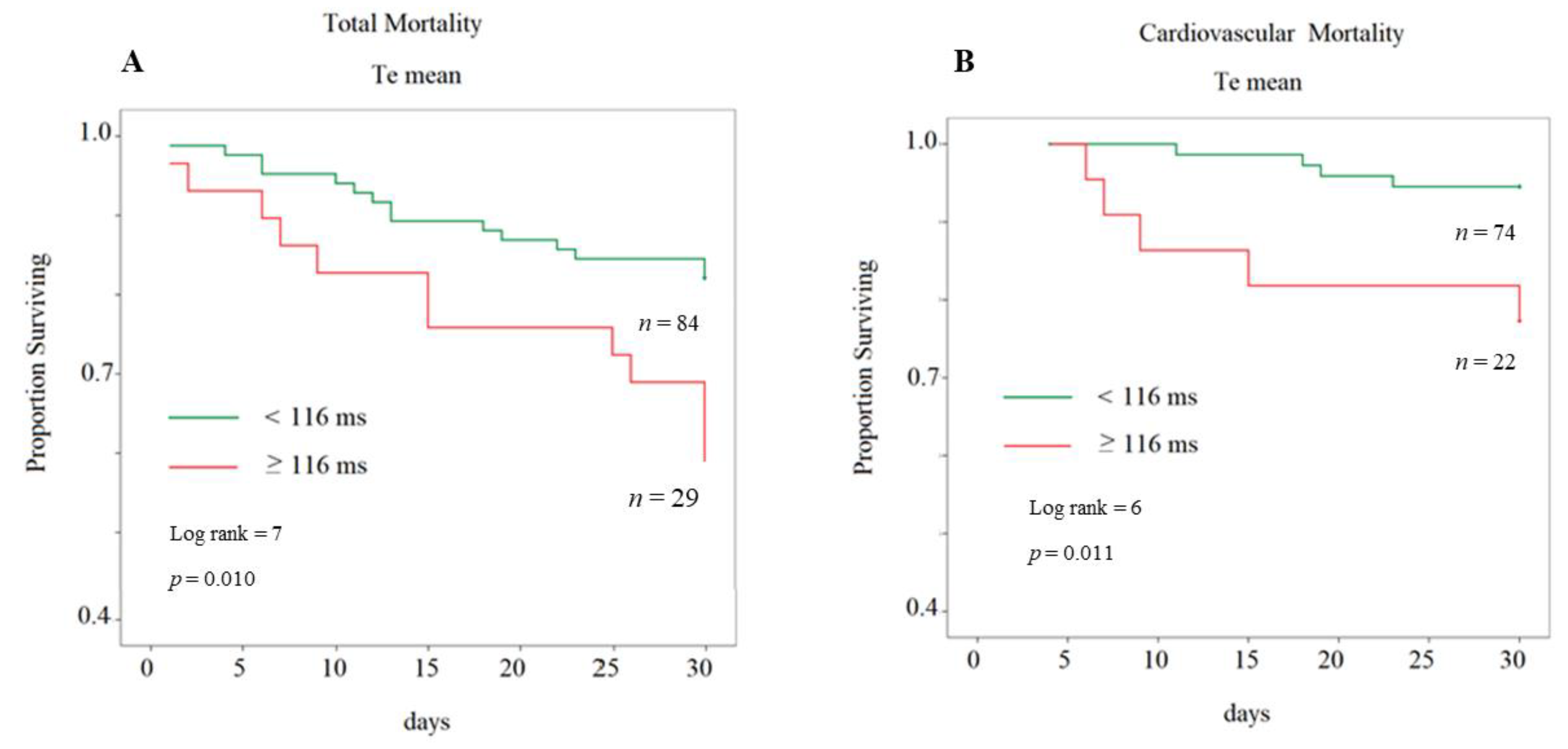
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Tereshchenko, L.G.; Cygankiewicz, I.; McNitt, S.; Bayes-Genis, A.; Han, L.; Sur, S.; Couderc, J.P.; Berger, R.D.; de Luna, A.B.; Zareba, W. Predictive value of beat-to-beat QT variability index across the continuum of left ventricular dysfunction: Competing risks of noncardiac or cardiovascular death and sudden or nonsudden cardiac death. Circ. Arrhythmia Electrophysiol. 2012, 5, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Magrì, D.; Matera, S.; Magnanti, M.; Torrini, A.; Pasquazzi, E.; Schifano, E.; Velitti, S.; Marigliano, V.; Quaglione, R.; et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: A prospective study. Eur. Heart J. 2007, 28, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Haigney, M.C.; Zareba, W.; Gentlesk, P.J.; Goldstein, R.E.; Illovsky, M.; McNitt, S.; Andrews, M.L.; Moss, A.J.; Multicenter Automatic Defibrillator Implantation Trial II investigators. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J. Am. Coll. Cardiol. 2004, 44, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Baumert, M.; Porta, A.; Vos, M.A.; Malik, M.; Couderc, J.P.; Laguna, P.; Piccirillo, G.; Smith, G.L.; Tereshchenko, L.G.; Volders, P.G. QT interval variability in body surface ECG: Measurement, physiological basis, and clinical value: Position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016, 18, 925–944. [Google Scholar]
- Tyack, P.L.; Calambokidis, J.; Friedlaender, A.; Goldbogen, J.; Southall, B. First Long-Term Behavioral Records from Cuvier’s Beaked Whales (Ziphiuscavirostris) Reveal Record-Breaking Dives. PLoS ONE 2014, 9, e92633. [Google Scholar]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef]
- Antzelevitch, C. Drug-induced spatial dispersion of repolarization. Cardiol. J. 2008, 15, 100–121. [Google Scholar]
- Li, M.; Ramos, L.G. Drug-Induced QT Prolongation and Torsades de Pointes. Pharm. Ther. 2017, 42, 473–477. [Google Scholar]
- Piccirillo, G.; Moscucci, F.; Fabietti, M.; Di Iorio, C.; Mastropietri, F.; Sabatino, T.; Crapanzano, D.; Bertani, G.; Zaccagnini, G.; Lospinuso, I.; et al. Age, gender and drug therapy influences on Tpeak-tendinterval and on electrical risk score. J. Electrocardiol. 2020, 59, 88–92. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magnanti, M.; Matera, S.; Di Carlo, S.; De Laurentis, T.; Torrini, A.; Marchitto, N.; Ricci, R.; Magrí, D. Age and QT variability index during free breathing, controlled breathing and tilt in patients with chronic heart failure and healthy control subjects. Transl. Res. 2006, 148, 72–78. [Google Scholar] [CrossRef]
- Piccirillo, G.; Cacciafesta, M.; Lionetti, M.; Nocco, M.; Di Giuseppe, V.; Moisè, A.; Naso, C.; Marigliano, V. Influence of age, the autonomic nervous system and anxiety on QT-interval variability. Clin. Sci. (Lond.) 2001, 101, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Pascucci, M.; Pappadà, M.A.; D’Alessandro, G.; Rossi, P.; Quaglione, R.; Di Barba, D.; Barillà, F.; Magrì, D. Influence of aging and chronic heart failure on temporal dispersion of myocardial repolarization. Clin. Interv. Aging 2013, 8, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Fabietti, M.; Parrotta, I.; Mastropietri, F.; Di Iorio, C.; Sabatino, T.; Crapanzano, D.; Vespignani, G.; Mariani, M.V.; et al. Arrhythmic Risk in Elderly Patients Candidates to Transcatheter Aortic Valve Replacement: Predictive Role of Repolarization Temporal Dispersion. Front. Physiol. 2019, 10, 991. [Google Scholar] [CrossRef]
- Child, N.; Hanson, B.; Bishop, M.; Rinaldi, C.A.; Bostock, J.; Western, D.; Cooklin, M.; O’Neil, M.; Wright, M.; Razavi, R.; et al. Effect of mental challenge induced by movie clips on action potential duration in normal human subjects independent of heart rate. Circ. Arrhythmia Electrophysiol. 2014, 7, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Taggart, P.; Critchley, H.; van Duijvendoden, S.; Lambiase, P.D. Significance of neuro-cardiac control mechanisms governed by higher regions of the brain. Auton. Neurosci. 2016, 199, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Magrì, D.; Ogawa, M.; Song, J.; Chong, V.J.; Han, S.; Joung, B.; Choi, E.K.; Hwang, S.; Chen, L.S.; et al. Autonomic nervous system activity measured directly and QT interval variability in normal and pacing-induced tachycardia heart failure dogs. J. Am. Coll. Cardiol. 2009, 54, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Rossi, P.; Mitra, M.; Quaglione, R.; Dell’Armi, A.; Di Barba, D.; Maisto, D.; Lizio, A.; Barillà, F.; Magrì, D. Indexes of temporal myocardial repolarization dispersion and sudden cardiac death in heart failure: Any difference? Ann. Noninvasive Electrocardiol. 2013, 18, 130–139. [Google Scholar] [CrossRef]
- Piccirillo, G.; Magrì, D.; Pappadà, M.A.; Maruotti, A.; Ogawa, M.; Han, S.; Joung, B.; Rossi, P.; Nguyen, B.L.; Lin, S.F.; et al. Autonomic nerve activity and the short-term variability of the Tpeak-Tend interval in dogs with pacing-induced heart failure. Heart Rhythm 2012, 9, 2044–2050. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; D’Alessandro, G.; Pascucci, M.; Rossi, P.; Han, S.; Chen, L.S.; Lin, S.F.; Chen, P.S.; Magrì, D. Myocardial repolarization dispersion and autonomic nerve activity in a canine experimental acute myocardial infarction model. Heart Rhythm 2014, 11, 110–118. [Google Scholar] [CrossRef]
- Yehya, Y.M.; Hussein, A.M.; Ezam, K.; Eid, E.A.; Ibrahim, E.M.; Sarhan, M.A.F.E.; Elsayed, A.; Sarhan, M.E. Blockade of Renin Angiotensin System Ameliorates the Cardiac Arrhythmias and Sympathetic Neural Remodeling in Hearts of Type 2 DM Rat Model. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 464–478. [Google Scholar] [CrossRef]
- Tamargo, J.; Caballero, R.; Gómez, R.; Delpón, E. Cardiac electrophysiological effects of nitric oxide. Cardiovasc. Res. 2010, 87, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Sordillo, D.C.; Helson, L. Review: The Prolonged QT Interval: Role of Pro-inflammatory Cytokines, Reactive Oxygen Species and the Ceramide and Sphingosine-1 Phosphate Pathways. In Vivo 2015, 29, 619–636. [Google Scholar] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front. Cardiovasc. Med. 2015, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Orini, M.; Mincholé, A.; Monasterio, V.; Cygankiewicz, I.; Bayés de Luna, A.; Martínez, J.P.; Laguna, P.; Pueyo, E. Sudden cardiac death and pump failure death prediction in chronic heart failure by combining ECG and clinical markers in an integrated risk model. PLoS ONE 2017, 12, e0186152. [Google Scholar] [CrossRef]
- Elitok, A.; Emet, S.; Karaayvaz, E.B.; Erdogan, O.; Aydogan, M.; Engin, B.; Cevik, E.; Orta, H.; Okumus, G.; Bilge, A.K. The relationship between t-wave peak-to-end interval and hemodynamic parameters in patients with pulmonary arterial hypertension. Ann. Noninvasive Electrocardiol. 2020, 18, e12764. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [PubMed]
- Berger, R.D.; Kasper, E.K.; Baughman, K.L.; Marban, E.; Calkins, H.; Tomaselli, G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997, 96, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Persi, A.; Di Barba, D.; Pappadà, M.A.; Rossi, P.; Quaglione, R.; Nguyen, B.L.; Barillà, F.; Casenghi, M.; et al. Intra-QT spectral coherence as a possible noninvasive marker of sustained ventricular tachycardia. Biomed. Res. Int. 2014, 2014, 583035. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Ottaviani, C.; Fiorucci, C.; Petrocchi, N.; Moscucci, F.; Di Iorio, C.; Mastropietri, F.; Parrotta, I.; Pascucci, M.; Magrì, D. Transcranial direct current stimulation improves the QT variability index and autonomic cardiac control in healthy subjects older than 60 years. Clin. Interv. Aging 2016, 11, 1687–1695. [Google Scholar] [CrossRef][Green Version]
- Piccirillo, G.; Moscucci, F.; Pofi, R.; D’Alessandro, G.; Minnetti, M.; Isidori, A.M.; Francomano, D.; Lenzi, A.; Puddu, P.E.; Alexandre, J.; et al. Changes in left ventricular repolarization after short-term testosterone replacement therapy in hypogonadal males. J. Endocrinol. Investig. 2019, 42, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Maestri, R.; Mortara, A.; Capomolla, S.; Febo, O.; Ferrari, R.; Franchini, M.; Gnemmi, M.; Opasich, C.; et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation 2003, 107, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F., Jr.; Uddin, N.; Patterson, J.H. Clinical predictors of in-hospital mortality in acutely decompensated heart failure-piecing together the outcome puzzle. Congest. Heart Fail. 2008, 14, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rahm, A.K.; Lugenbiel, P.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Role of ion channels in heart failure and channelopathies. Biophys. Rev. 2018, 10, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Tse, G.; Gong, M.; Wong, W.T.; Georgopoulos, S.; Letsas, K.P.; Vassiliou, V.S.; Chan, Y.S.; Yan, B.P.; Wong, S.H.; Wu, W.K.K.; et al. The Tpeak-Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: A systematic review and meta-analysis. Heart Rhythm 2017, 14, 1131–1137. [Google Scholar] [CrossRef]
- Gronda, E.; Vanoli, E.; Sacchi, S.; Grassi, G.; Ambrosio, G.; Napoli, C. Risk of heart failure progression in patients with reduced ejection fraction: Mechanisms and therapeutic options. Heart Fail. Rev. 2020, 25, 295–303. [Google Scholar] [CrossRef]
- Kurokawa, J.; Abriel, H. Neurohormonal regulation of cardiac ion channels in chronic heart failure. J. Cardiovasc. Pharmacol. 2009, 54, 98–105. [Google Scholar] [CrossRef]
- Chan, T.O.; Zhang, X.Q.; Gao, E.; Song, J.; Koch, W.J.; Feldman, A.M.; Cheung, J.Y. Induced overexpression of Na+/Ca2+ exchanger transgene: Altered myocyte contractility, [Ca2+]i transients, SR Ca2+ contents, and action potential duration. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 590–601. [Google Scholar]
- Rossow, C.F.; Dilly, K.W.; Yuan, C.; Nieves-Cintrón, M.; Cabarrus, J.L.; Santana, L.F. NFATc3-dependent loss of I(to) gradient across the left ventricular wall during chronic beta adrenergic stimulation. J. Mol. Cell. Cardiol. 2009, 46, 249–256. [Google Scholar] [CrossRef]
- Domenighetti, A.A.; Boixel, C.; Cefai, D.; Abriel, H.; Pedrazzini, T. Chronic angiotensin II stimulation in the heart produces an acquired long QT syndrome associated with IK1 potassium current downregulation. J. Mol. Cell. Cardiol. 2007, 42, 63–70. [Google Scholar] [CrossRef]
- Rivard, K.; Paradis, P.; Nemer, M.; Fiset, C. Cardiac-specific overexpression of the human type 1 angiotensin II receptor causes delayed repolarization. Cardiovasc. Res. 2008, 78, 53–62. [Google Scholar] [CrossRef]
- Rougier, J.S.; Muller, O.; Berger, S.; Centeno, G.; Schütz, G.; Firsov, D.; Abriel, H. Mineralocorticoid receptor is essential for corticosteroid-induced up-regulation of L-type calcium currents in cultured neonatal cardiomyocytes. Pflugers Arch. 2008, 456, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Boixel, C.; Gavillet, B.; Rougier, J.S.; Abriel, H. Aldosterone increases voltage-gated sodium current in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 2257–2266. [Google Scholar] [CrossRef] [PubMed]

| <75th | ≥75th | ||
|---|---|---|---|
| of NT-proBNP | |||
| Group 1 | Group 2 | ||
| n: 85 | n: 28 | p | |
| Age, years | 82 ± 10 | 85 ± 11 | ns |
| Male/Female, n | 44/41 | 15/13 | ns |
| BMI, kg/m2 | 26 ± 5 | 26 ± 5 | ns |
| SBP, mm Hg | 127 ± 21 | 124 ± 20 | ns |
| DBP, mm Hg | 70 ± 11 | 65 ± 11 | ns |
| Heart Rate, b/m | 74 ± 14 | 76 ± 14 | ns |
| Left Ventricular Ejection Fraction, % | 46 ± 9 | 35 ± 9 | <0.001 |
| NT-proBNP, pg/mL | 1530 (2225) | 13,100 (11,780) | <0.001 |
| Serum Potassium, mmol/L | 4.1 ± 0.5 | 4.3 ± 0.6 | ns |
| Serum Calcium, mmol/L | 2.2 ± 0.2 | 2.1 ± 0.4 | ns |
| Creatinine Clearance, mL/m | 49 ± 27 | 38 ± 30 | ns |
| CHF with Depressed Systolic Function, n (%) | 44 (52) | 24 (86) | ns |
| CHF with Preserved Systolic Function, n (%) | 59 (69) | 13 (46) | ns |
| Hypertension, n (%) | 64 (75) | 19 (68) | ns |
| Hypercholesterolemia, n (%) | 33 (39) | 17 (61) | ns |
| Diabetes, n (%) | 31 (36) | 13 (46) | ns |
| Known Myocardial Ischemia History, n (%) | 24 (28) | 10 (36) | ns |
| Valve Diseases | 12 (14) | 4 (14) | ns |
| Premature Supraventricular Complexes, n (%) | 12 (14) | 5 (18) | ns |
| Premature Ventricular Complexes, n (%) | 22 (26) | 6 (21) | ns |
| Permanent Atrial fibrillation, n (%) | 29 (34) | 9 (32) | ns |
| Left Bundle Branch Block, n (%) | 11 (13) | 7 (25) | ns |
| Right Bundle Branch Block, n (%) | 15 (18) | 7 (25) | ns |
| Pacemaker-ICD, n (%) | 16 (19) | 10 (36) | ns |
| β-blockers, n (%) | 49 (58) | 24 (86) | ns |
| Furosemide, n (%) | 61 (72) | 26 (93) | ns |
| ACEi/ARB | 36 (42) | 9 (32) | ns |
| Aldosterone antagonists, n (%) | 11 (13) | 3 (11) | ns |
| Potassium, n (%) | 5 (6) | 2 (7) | ns |
| Nitrates, n (%) | 10 (12) | 4 (14) | ns |
| Ivabradine, n (%) | 3 (4) | 2 (7) | ns |
| Digoxin, n (%) | 4 (5) | 2 (7) | ns |
| Statins, n (%) | 22 (26) | 12 (43) | ns |
| Antiplatelet drugs, n (%) | 32 (38) | 6 (21) | ns |
| Oral Anticoagulants, n (%) | 21 (25) | 9 (32) | ns |
| Diltiazem or Verapamil, n (%) | 6 (7) | 0 (0) | ns |
| Dihydropyridine Calcium channel blockers, n (%) | 13 (15) | 2 (7) | ns |
| Propafenone, n (%) | 1 (1) | 0 (0) | ns |
| Amiodarone, n (%) | 1 (1) | 4 (14) | <0.05 |
| Ranolazine, n (%) | 5 (6) | 0 (0) | ns |
| Sacubitril/Valsartan, n (%) | 1 (1) | 0 (0) | ns |
| <75th | ≥75th | ||
|---|---|---|---|
| of NT-proBNP | |||
| Group 1 | Group 2 | ||
| n: 85 | n: 28 | p | |
| QTe mean, ms | 428 ± 62 | 463 ± 85 | 0.020 |
| QTe SD, ms | 5 (4) | 8 (5) | 0.002 |
| QTe SD, log ms | 0.69 ± 0.29 | 0.87 ± 0.24 | 0.003 |
| QTp mean, ms | 326 ± 60 | 351 ± 69 | ns |
| QTp SD, ms | 5 (3) | 7 (4) | 0.014 |
| QTp SD, log ms | 0.74 ± 0.20 | 0.85 ± 0.16 | 0.009 |
| Te mean, ms | 101 ± 18 | 113 ± 32 | 0.023 |
| Te SD, ms | 6 (4) | 9 (5) | <0.001 |
| Te SD, ms | 0.80 ± 0.16 | 0.96 ± 0.18 | <0.001 |
| QTeVN | 0.15 (0.32) | 0.30 (0.41) | 0.017 |
| QTpVN | 0.29 (0.49) | 0.36 (0.56) | ns |
| TeVN | 4 (4) | 9 (11) | 0.006 |
| Variables | Χ2 | Univariable Analysis Hazard Ratio (95% CI) | p-Values |
|---|---|---|---|
| QTe SD | 7.60 | 1.10 (1.03–1.18) | 0.006 |
| Mean Te | 12.18 | 1.02 (1.01–1.04) | < 0.001 |
| Te SD | 4.65 | 1.12 (1.01–1.23) | 0.026 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Moscucci, F.; Bertani, G.; Lospinuso, I.; Mastropietri, F.; Fabietti, M.; Sabatino, T.; Zaccagnini, G.; Crapanzano, D.; Di Diego, I.; et al. Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure. J. Clin. Med. 2020, 9, 1879. https://doi.org/10.3390/jcm9061879
Piccirillo G, Moscucci F, Bertani G, Lospinuso I, Mastropietri F, Fabietti M, Sabatino T, Zaccagnini G, Crapanzano D, Di Diego I, et al. Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure. Journal of Clinical Medicine. 2020; 9(6):1879. https://doi.org/10.3390/jcm9061879
Chicago/Turabian StylePiccirillo, Gianfranco, Federica Moscucci, Gaetano Bertani, Ilaria Lospinuso, Fabiola Mastropietri, Marcella Fabietti, Teresa Sabatino, Giulia Zaccagnini, Davide Crapanzano, Ilaria Di Diego, and et al. 2020. "Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure" Journal of Clinical Medicine 9, no. 6: 1879. https://doi.org/10.3390/jcm9061879
APA StylePiccirillo, G., Moscucci, F., Bertani, G., Lospinuso, I., Mastropietri, F., Fabietti, M., Sabatino, T., Zaccagnini, G., Crapanzano, D., Di Diego, I., Corrao, A., Rossi, P., & Magrì, D. (2020). Short-Period Temporal Dispersion Repolarization Markers Predict 30-Days Mortality in Decompensated Heart Failure. Journal of Clinical Medicine, 9(6), 1879. https://doi.org/10.3390/jcm9061879






