Improving the Prognostic Performance of SUVmax in 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Using Tumor-to-Liver and Tumor-to-Blood Standard Uptake Ratio for Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. 18F-FDG PET/CT Image Acquisition
2.4. Image Interpretation and PET Data Analyses
2.5. Clinical Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Clinical Features and Treatment Outcomes
3.2. Cutoff Value of Metabolic Parameters
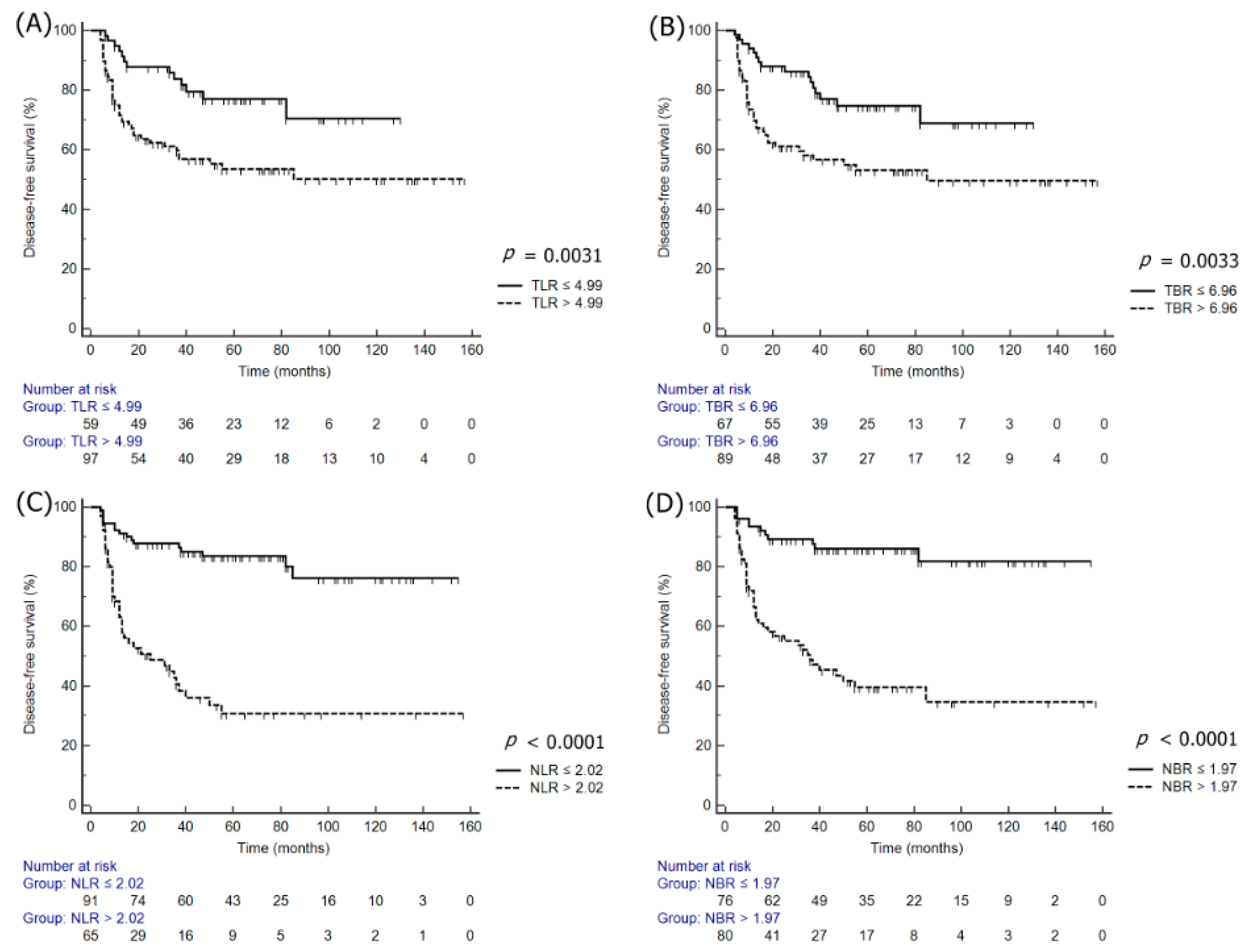
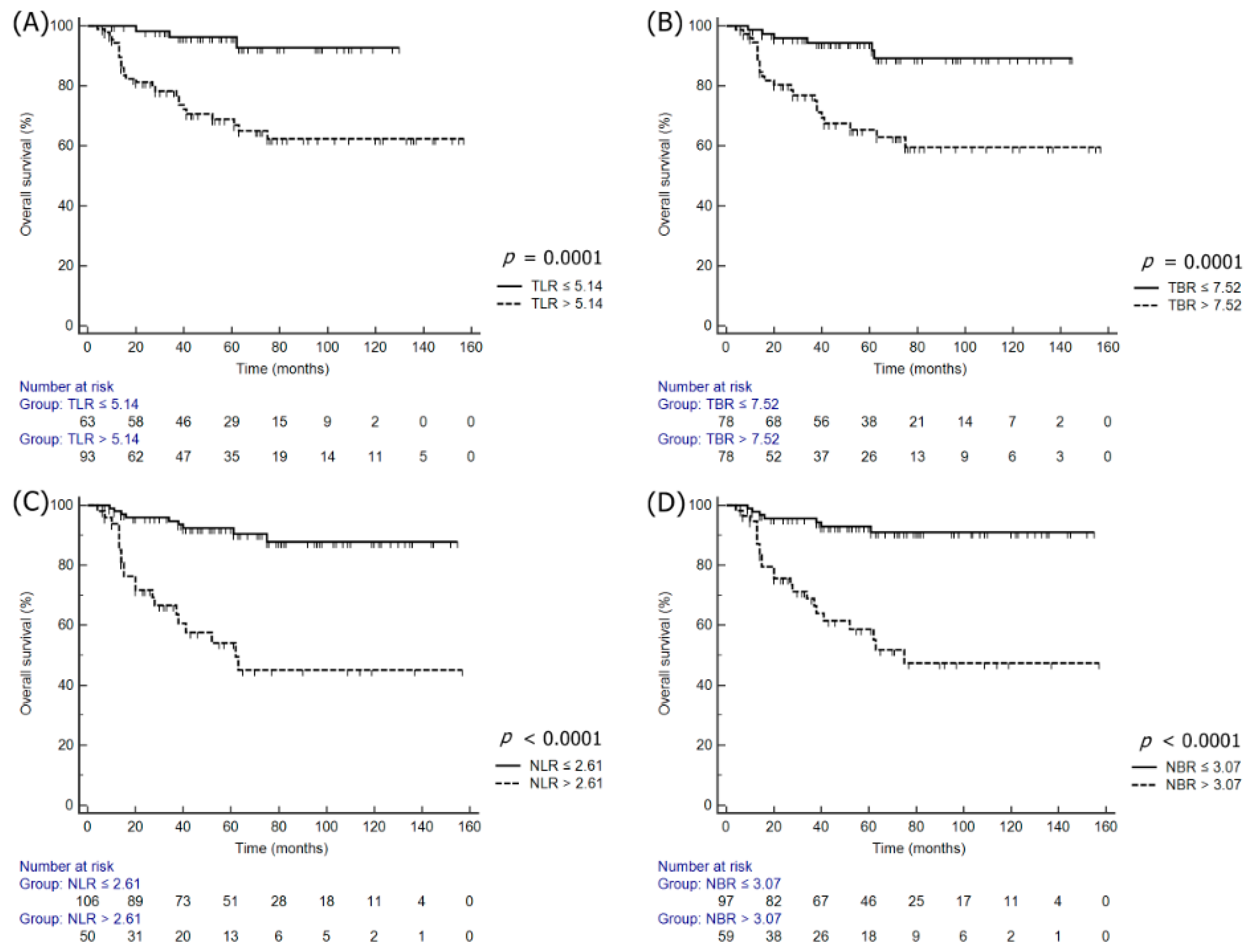
3.3. Univariate and Multivariate Survival Analyses
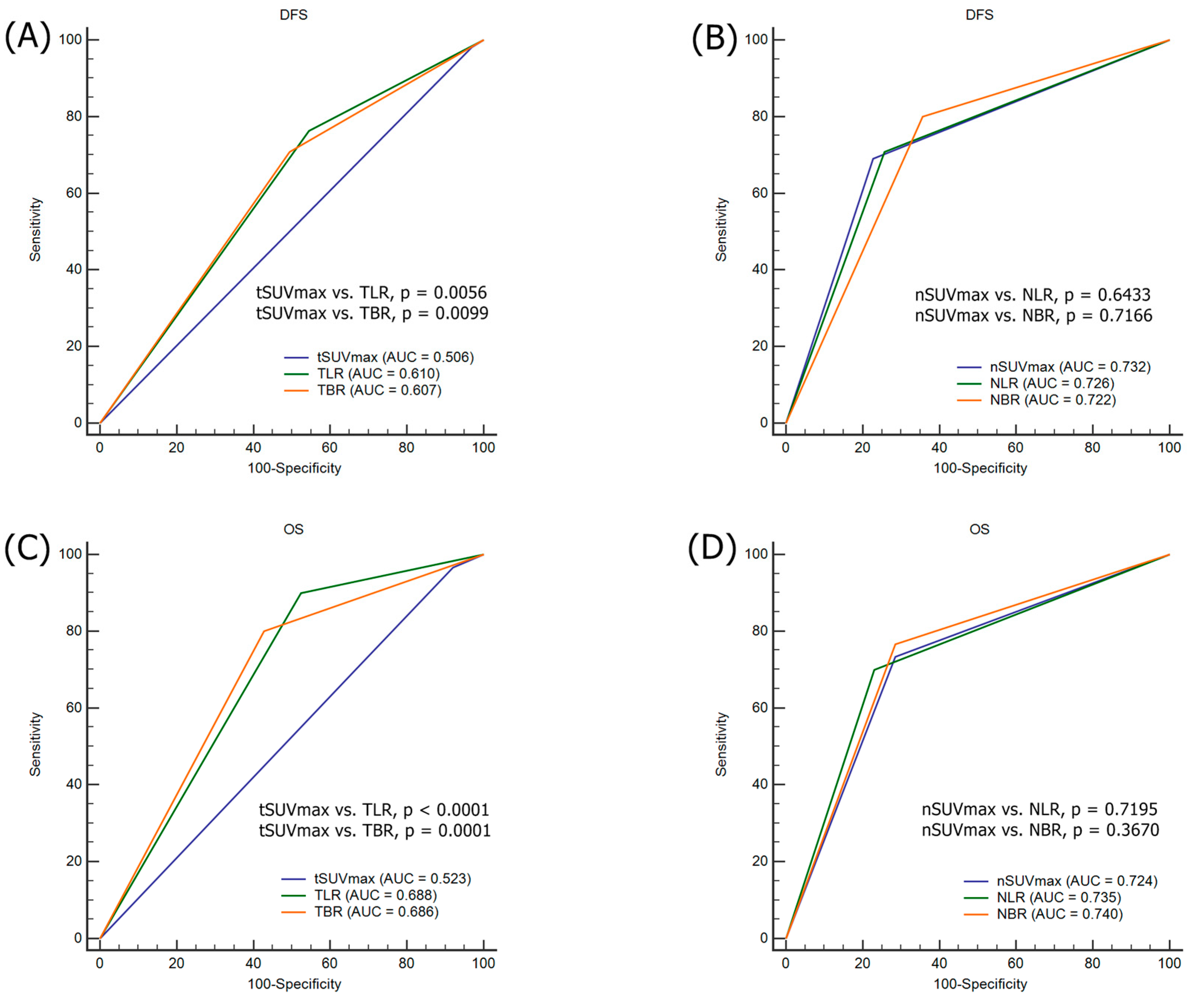
3.4. Comparison ROC for the Prediction of Tumor Recurrence and Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCRT | concurrent chemoradiotherapy |
| CI | confidence interval |
| DFS | disease-free survival |
| 18F-FDG PET/CT | F-18 fluorodeoxyglucose positron emission tomography/computed tomography |
| FIGO | Advanced International Federation of Gynecology and Obstetrics |
| HF | heterogeneity factor |
| HR | hazard ratio |
| SUVmax | maximum standardized uptake value |
| MTV | metabolic tumor volume |
| ROC | receiver operating characteristic |
| TLG | total lesion glycolysis |
| WB | whole body |
References
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001, 358, 781–786. [Google Scholar] [CrossRef]
- Whitney, C.W.; Sause, W.; Bundy, B.N.; Malfetano, J.H.; Hannigan, E.V.; Fowler, W.C., Jr.; Clarke-Pearson, D.L.; Liao, S.Y. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: A Gynecologic Oncology Group and Southwest Oncology Group study. J. Clin Oncol. 1991, 17, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Collaboration Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and metaanalysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, Y.T.; Kim, J.H.; Kim, S.; Kim, S.W.; Nam, E.J.; Kim, J.W. Clinical significance of tumor volume and lymph node involvement assessed by MRI in stage IIB cervical cancer patients treated with concurrent chemoradiation therapy. J. Gynecol. Oncol. 2010, 21, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Endo, D.; Todo, Y.; Okamoto, K.; Ninobe, S.; Kato, H.; Nishiyama, N. Prognostic factors for patients with cervical cancer treated with concurrent chemoradiotherapy: A retrospective analysis in a Japanese cohort. J. Gynecol. Oncol. 2015, 26, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Grigsby, P.W. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer 2010, 116, 1469–1475. [Google Scholar] [CrossRef]
- Onal, C.; Guler, O.C.; Reyhan, M.; Yapar, A.F. Prognostic value of 18F-fluorodeoxyglucose uptake in pelvic lymph nodes in patients with cervical cancer treated with definitive chemoradiotherapy. Gynecol. Oncol. 2015, 137, 40–46. [Google Scholar] [CrossRef]
- Bollineni, V.R.; Kramer, G.; Liu, Y.; Melidis, C.; deSouza, N.M. A literature review of the association between diffusion-weighted MRI derived apparent diffusion coefficient and tumour aggressiveness in pelvic cancer. Cancer Treat. Rev. 2015, 41, 496–502. [Google Scholar] [CrossRef]
- Chong, G.O.; Jeong, S.Y.; Park, S.H.; Lee, Y.H.; Lee, S.W.; Hong, D.G.; Kim, J.C.; Lee, Y.S.; Cho, Y.L. Comparison of the Prognostic Value of F-18 Pet Metabolic Parameters of Primary Tumors and Regional Lymph Nodes in Patients with Locally Advanced Cervical Cancer Who Are Treated with Concurrent Chemoradiotherapy. PLoS ONE 2015, 10, e0137743. [Google Scholar] [CrossRef]
- Kostakoglu, L.; Chauvie, S. Metabolic Tumor Volume Metrics in Lymphoma. Metabolic Tumor Volume Metrics in Lymphoma. Semin. Nucl. Med. 2018, 48, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Brecht-Krauss, D.; Werner, M.; Hartwig, E.; Sarkar, M.R.; Keppler, P.; Kotzerke, J.; Guhlmann, A.; Delling, G.; Reske, S.N. Evaluation of neoadjuvant therapy response of osteogenic sarcoma using FDG PET. J. Nucl. Med. 1999, 40, 1637–1643. [Google Scholar] [PubMed]
- Chiaravalloti, A.; Danieli, R.; Abbatiello, P.; Di Pietro, B.; Travascio, L.; Cantonetti, M.; Guazzaroni, M.; Orlacchio, A.; Simonetti, G.; Schillaci, O. Factors affecting intrapatient liver and mediastinal blood pool ¹⁸F-FDG standardized uptake value changes during ABVD chemotherapy in Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chang, K.J.; Seo, Y.S.; Byun, B.H.; Choi, J.H.; Moon, H.; Lim, I.; Kim, B.I.; Choi, C.W.; Lim, S.M. Tumor SUVmax Normalized to Liver Uptake on (18)F-FDG PET/CT Predicts the Pathologic Complete Response After Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Nucl. Med. Mol. Imaging 2014, 48, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Pak, K.; Kim, I.J.; Kim, B.S.; Kim, S.J. Prognostic Value of Tumor-to-Blood Standardized Uptake Ratio in Patients with Resectable Non-Small-Cell Lung Cancer. Nucl. Med. Mol. Imaging 2017, 51, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Lee, W.K.; Chong, G.O.; Jeong, S.Y.; Lee, H.J.; Park, S.; Ryu, J.M.; Choi, Y.S.; Kang, S.; Koo, Y.; Lee, D.H.; et al. Prognosis-Predicting Model Based on [18F]fluorodeoxyglucose PET Metabolic Parameters in Locally Advanced Cervical Cancer Patients Treated with Concurrent Chemoradiotherapy: Multi-Center Retrospective Study. J. Clin. Med. 2020, 9, 427. [Google Scholar] [CrossRef]
- Han, S.; Kim, H.; Kim, Y.J.; Suh, C.H.; Woo, S. Prognostic Value of Volume-Based Metabolic Parameters of 18F-FDG PET/CT in Uterine Cervical Cancer: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2018, 211, 1112–1121. [Google Scholar] [CrossRef]
- Chu, K.P.; Murphy, J.D.; La, T.H.; Krakow, T.E.; Iagaru, A.; Graves, E.E.; Hsu, A.; Maxim, P.G.; Loo, B.; Chang, D.T.; et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1521–1527. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.K.; Lee, S.M.; Moon, S.H.; Kim, T. Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol. Imaging Biol. 2011, 13, 565–572. [Google Scholar] [CrossRef]
- Kidd, E.A.; Siegel, B.A.; Dehdashti, F.; Rader, J.S.; Mutch, D.S.; Powell, M.A.; Grigsby, P.W. Lymph node staging by positron emission tomography in cervical cancer: Relationship to prognosis. J. Clin. Oncol. 2010, 28, 2108–2113. [Google Scholar] [CrossRef]
- Ramlov, A.; Kroon, P.S.; Jürgenliemk-Schulz, I.M.; De Leeuw, A.C.; Gormsen, L.C.; Fokdal, L.U.; Tanderup, K.; Lindegaard, J.C. Impact of radiation dose and standardized uptake value of (18) FDG PET on nodal control in locally advanced cervical cancer. Acta Oncol. 2015, 54, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, S.H.; Kim, H.; Koh, Y.W.; Kim, S.; Choi, E.C.; Yun, M. Lymph Node With the Highest FDG Uptake Predicts Distant Metastasis-Free Survival in Patients With Locally Advanced Nasopharyngeal Carcinoma. Clin. Nucl. Med. 2018, 43, e220–e225. [Google Scholar] [CrossRef] [PubMed]
| Variables | All (n = 156) | No Recurrence (n = 101) | Recurrence (n = 55) | p-Value |
|---|---|---|---|---|
| Age (years) | 55.1 ± 12.9 | 55.5 ± 12.8 | 54.3 ± 13.0 | 0.5965 |
| Histology (n, %) | 0.1244 | |||
| SCC | 141 (90.4) | 94 (93.1) | 47 (85.5) | |
| AC/ASC | 15 (9.6) | 7 (6.9) | 8 (14.5) | |
| FIGO stage (n, %) | 0.0006 | |||
| IIB | 120 (76.9) | 87 (86.1) | 33 (60.0 | |
| IIIA | 14 (9.0) | 8 (7.9) | 6 (10.9) | |
| IIIB | 16 (10.3) | 5 (5.0) | 11 (20.0) | |
| IVA | 6 (3.8) | 1 (1.0) | 5 (9.1) | |
| Tumor size (cm) | 4.6 ± 1.5 | 4.4 ± 1.4 | 5.1 ± 1.6 | 0.0080 |
| LN metastasis (n, %) | 102 (65.4) | 55 (54.5) | 47 (85.5) | 0.0001 |
| Paraaortic LN metastasis (n, %) | 25 (16.0) | 18 (17.8) | 7 (12.7) | 0.4088 |
| SCC antigen (ng/mL) | 20.8 ± 36.0 | 16.8 ± 35.6 | 28.1 ± 35.9 | 0.0715 |
| tSUVmax | 6.0 ± 2.5 | 6.0 ± 2.7 | 6.0 ± 2.1 | 0.9542 |
| TLR | 5.9 ± 4.4 | 6.8 ± 4.1 | 7.5 ± 4.8 | 0.3356 |
| TBR | 9.5 ± 6.0 | 9.2 ± 6.0 | 10.1 ± 6.1 | 0.3717 |
| nSUVmax | 3.3 ± 7.3 | 3.5 ± 5.1 | 7.9 ± 9.5 | 0.0003 |
| NLR | 2.3 ± 4.0 | 1.5 ± 2.5 | 3.8 ± 5.6 | 0.0008 |
| NBR | 3.1 ± 5.0 | 2.1 ± 3.4 | 5.0 ± 6.7 | 0.0005 |
| Variables | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Level | HR | 95% CI | p-Value | Level | HR | 95% CI | p-Value | |
| Age (years) | >49 | 1.00 | - | - | >49 | 1.00 | - | - |
| ≤49 | 1.25 | 0.70–2.23 | 0.4584 | ≤49 | 2.24 | 1.06–4.77 | 0.0355 | |
| Histology | SCC | 1.00 | - | - | SCC | 1.00 | - | - |
| AC/ASC | 2.04 | 0.78–4.98 | 0.1531 | AC/ASC | 1.57 | 0.46–5.35 | 0.4699 | |
| FIGO stage | IIB | 1.00 | - | - | IIB | 1.00 | - | - |
| >IIB | 4.64 | 2.31–9.32 | <0.0001 | >IIB | 7.30 | 2.85–18.69 | <0.0001 | |
| Tumor size | <4 cm | 1.00 | - | - | <4.2 cm | 1.00 | - | - |
| ≥4 cm | 2.48 | 1.41–4.34 | 0.0016 | ≥4.2 cm | 3.09 | 1.50–6.38 | 0.0023 | |
| LN metastases | Negative | 1.00 | - | - | Negative | 1.00 | - | - |
| Positive | 3.09 | 1.79–5.32 | <0.0001 | Positive | 2.96 | 1.41–6.18 | 0.0039 | |
| Paraaortic LN metastases | Negative | 1.00 | 0.71–2.85 | 0.3245 | Negative | 1.00 | 1.10–6.99 | 0.0305 |
| Positive | 1.42 | Positive | 2.78 | |||||
| Serum SCC | <19.3 | 1.00 | - | - | <19.3 | 1.00 | - | - |
| ≥19.3 | 3.71 | 1.94–7.12 | 0.0001 | ≥19.3 | 3.75 | 1.55–9.08 | 0.0035 | |
| tSUVmax | ≤4.81 | 1.00 | - | - | ≤6.43 | 1.00 | - | - |
| >4.81 | 1.39 | 0.26–7.30 | 0.6975 | >6.43 | 2.05 | 0.60–7.03 | 0.2528 | |
| TLR | ≤4.99 | 1.00 | - | - | ≤5.14 | 1.00 | - | - |
| >4.99 | 2.26 | 1.37–3.87 | 0.0031 | >5.14 | 4.23 | 2.05–8.70 | 0.0001 | |
| TBR | ≤6.96 | 1.00 | - | - | ≤7.52 | 1.00 | - | |
| >6.96 | 2.23 | 1.31–3.81 | 0.0033 | >7.52 | 4.26 | 2.07–8.79 | 0.0001 | |
| nSUVmax | ≤4.63 | 1.00 | - | - | ≤4.91 | 1.00 | - | - |
| >4.63 | 6.86 | 3.81–12.36 | < 0.0001 | >4.91 | 7.72 | 3.53–16.88 | < 0.0001 | |
| NLR | ≤2.02 | 1.00 | - | - | ≤2.61 | - | - | |
| >2.02 | 6.26 | 3.51–11.14 | < 0.0001 | >2.61 | 10.27 | 4.51–23.39 | <0.0001 | |
| NBR | ≤1.97 | 1.00 | - | - | ≤3.07 | 1.00 | - | - |
| >1.97 | 4.74 | 2.76–8.15 | < 0.0001 | >3.07 | 8.02 | 3.71–17.36 | <0.0001 | |
| Variables | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Level | HR | 95% CI | p-Value | Level | HR | 95% CI | p-Value | |
| tSUVmax | ≤4.81 | 1.00 | - | - | ≤6.43 | 1.00 | - | - |
| >4.81 | 0.29 | 0.04–2.38 | 0.2478 | >6.43 | 0.66 | 0.08–5.85 | 0.7117 | |
| TLR | ≤4.9885 | 1.00 | - | - | ≤5.1368 | 1.00 | - | - |
| >4.9885 | 1.48 | 0.76–2.87 | 0.2509 | >5.1368 | 4.16 | 1.19–14.50 | 0.0252 | |
| TBR | ≤6.9612 | 1.00 | - | - | ≤7.5235 | 1.00 | - | - |
| >1.9612 | 1.42 | 0.75–2.70 | 0.2864 | >7.5235 | 3.01 | 1.04–8.70 | 0.0415 | |
| nSUVmax | ≤4.63 | 1.00 | - | - | ≤4.91 | 1.00 | - | - |
| >4.63 | 4.06 | 1.74–9.44 | 0.0012 | >4.91 | 6.99 | 1.56–31.25 | 0.0109 | |
| LNR | ≤2.0021 | 1.00 | - | - | ≤2.6098 | 1.00 | - | - |
| >2.0021 | 3.54 | 1.53–8.19 | 0.0032 | >2.6098 | 4.84 | 1.58–14.81 | 0.0057 | |
| NBR | ≤1.9681 | 1.00 | - | - | ≤3.0705 | 1.00 | - | - |
| >1.9681 | 3.38 | 1.02–11.19 | 0.0457 | >3.0705 | 6.87 | 1.55–30.54 | 0.0113 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chong, G.O.; Jeong, S.Y.; Lee, Y.H.; Park, S.-H.; Lee, H.J.; Lee, S.-W.; Hong, D.G.; Lee, Y.S. Improving the Prognostic Performance of SUVmax in 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Using Tumor-to-Liver and Tumor-to-Blood Standard Uptake Ratio for Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy. J. Clin. Med. 2020, 9, 1878. https://doi.org/10.3390/jcm9061878
Chong GO, Jeong SY, Lee YH, Park S-H, Lee HJ, Lee S-W, Hong DG, Lee YS. Improving the Prognostic Performance of SUVmax in 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Using Tumor-to-Liver and Tumor-to-Blood Standard Uptake Ratio for Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy. Journal of Clinical Medicine. 2020; 9(6):1878. https://doi.org/10.3390/jcm9061878
Chicago/Turabian StyleChong, Gun Oh, Shin Young Jeong, Yoon Hee Lee, Shin-Hyung Park, Hyun Jung Lee, Sang-Woo Lee, Dae Gy Hong, and Yoon Soon Lee. 2020. "Improving the Prognostic Performance of SUVmax in 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Using Tumor-to-Liver and Tumor-to-Blood Standard Uptake Ratio for Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy" Journal of Clinical Medicine 9, no. 6: 1878. https://doi.org/10.3390/jcm9061878
APA StyleChong, G. O., Jeong, S. Y., Lee, Y. H., Park, S.-H., Lee, H. J., Lee, S.-W., Hong, D. G., & Lee, Y. S. (2020). Improving the Prognostic Performance of SUVmax in 18F-Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography Using Tumor-to-Liver and Tumor-to-Blood Standard Uptake Ratio for Locally Advanced Cervical Cancer Treated with Concurrent Chemoradiotherapy. Journal of Clinical Medicine, 9(6), 1878. https://doi.org/10.3390/jcm9061878





