The Predictive Value of the Cervical Consistency Index to Predict Spontaneous Preterm Birth in Asymptomatic Twin Pregnancies at the Second-Trimester Ultrasound Scan: A Prospective Cohort Study
Abstract
1. Introduction
2. Experimental Section
Image Acquisition and off-Line Analysis
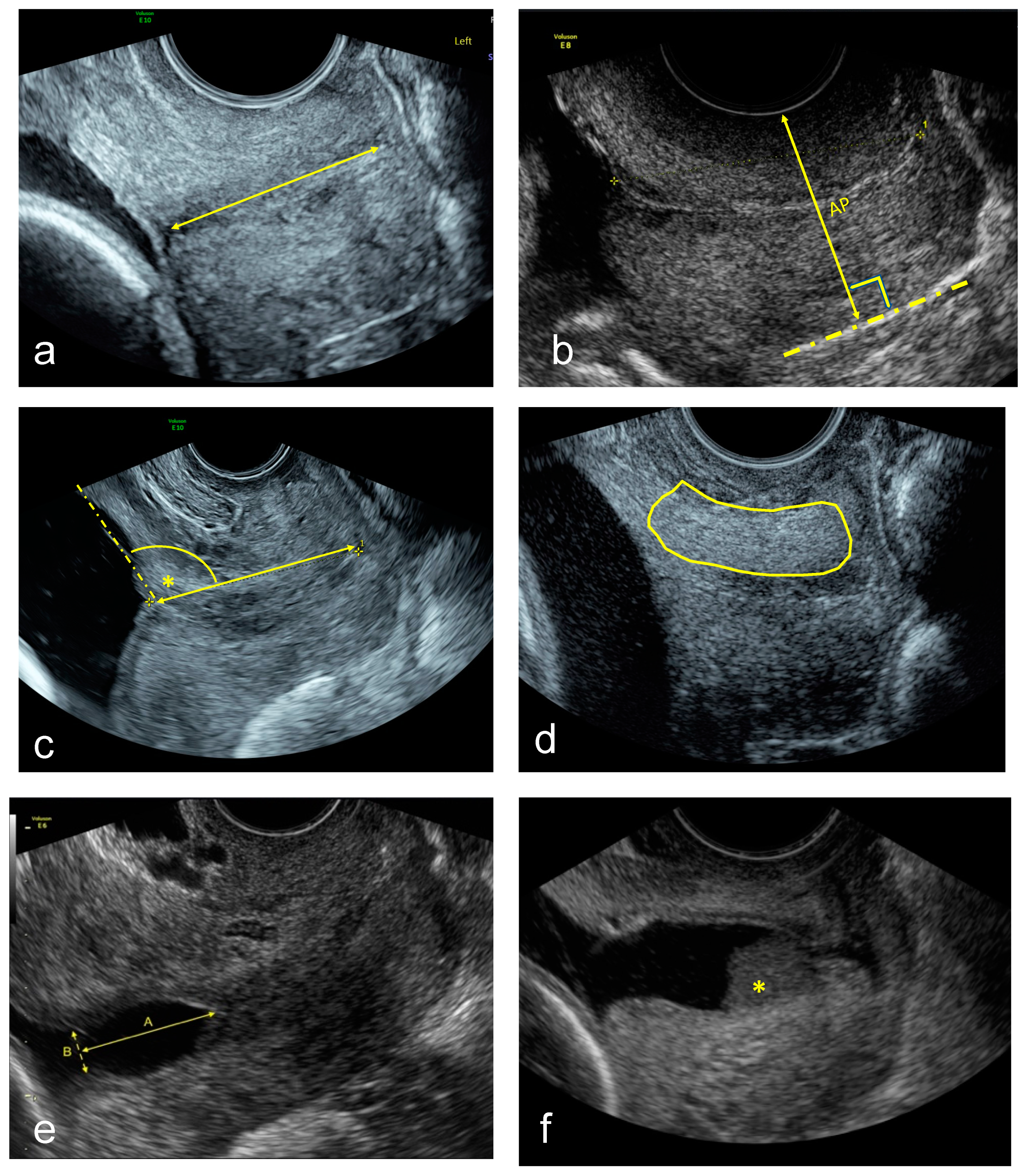
- Standard CL (straight-line) measurement in mm [21].
- CCI measurement expressed as a percentage [11]. A straight cervical length was traced to align with the longitudinal axis of the cervix at the posterior vaginal wall. Then the midpoint of this line was calculated, and the anteroposterior diameter was measured perpendicular to this line with (AP’) and without (AP) pressure. Then the cervical consistency index was calculated with the following formula:CCI = (AP′/AP) × 100
- UCA calculated by measuring the angle between the straight anterior myometrial wall line and cervical length tracing [15].
- CTx was done as described using a machine learning algorithm [18].
- TVU cervical funnel analysis with and without fundal pressure and defined as protrusion of the amniotic membranes into the cervical canal. The longest and widest funnel length were taken at the internal os [21].
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Heal. 2018, 7, 37–46. [Google Scholar] [CrossRef]
- Zeitlin, J.M.A.; Delnord, M.; Zhang, W.H. European Perinatal Health Report: Health and Care of Pregnant Women and Babies in Europe in 2010. Available online: https://www.tno.nl/media/1975/european_perinatal_health_report_2010.pdf (accessed on 13 May 2020).
- Brubaker, S.G.; Gyamfi, C. Prediction and Prevention of Spontaneous Preterm Birth in Twin Gestations. Semin. Perinatol. 2012, 36, 190–194. [Google Scholar] [CrossRef]
- Gynecologists Committee on Practice Bulletins-Obstetrics TACoO. Practice bulletin no. 130: Prediction and prevention of preterm birth. Obstet. Gynecol. 2012, 120, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Conde-Agudelo, A.; da Fonseca, E.; O’Brien, J.M.; Cetingoz, E.; Creasy, G.; Hassan, S.S.; Nicolaides, K.H. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: A meta-analysis of individual patient data. Am. J. Obstet. Gynecol. 2018, 218, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. Predictive accuracy of changes in transvaginal sonographic cervical length over time for preterm birth: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2015, 213, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Iams, J.D.; Goldenberg, R.L.; Meis, P.J.; Mercer, B.M.; Moawad, A.; Das, A.; Roberts, J.M. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N. Engl. J. Med. 1996, 334, 567–572. [Google Scholar] [CrossRef]
- Dodd, J.M.; Grivell, R.; Obrien, C.M.; Dowswell, T.; Deussen, A.R. Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. Am. J. Obstet. Gynecol. 2014, 211, 583–595. [Google Scholar] [CrossRef]
- Baños, N.; Julia, C.; Lorente, N.; Ferrero, S.; Cobo, T.; Gratacós, E.; Palacio, M. Mid-Trimester Cervical Consistency Index and Cervical Length to Predict Spontaneous Preterm Birth in a High-Risk Population. Am. J. Perinatol. Rep. 2018, 8, 43–50. [Google Scholar] [CrossRef]
- Parra, G.; Vergara, F.; Navarro, E.; Parra-Saavedra, M.; Gömez, L.; Barrero, A. Prediction of preterm birth using the cervical consistency index. Ultrasound Obstet. Gynecol. 2011, 38, 44–51. [Google Scholar] [CrossRef]
- Maurer, M.M.; Badir, S.; Pensalfini, M.; Bajka, M.; Abitabile, P.; Zimmermann, R.; Mazza, E. Challenging the in-vivo assessment of biomechanical properties of the uterine cervix: A critical analysis of ultrasound based quasi-static procedures. J. Biomech. 2015, 48, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Rosen, H.; Stratulat, V.; Aviram, A.; Melamed, N.; Barrett, J.; Glanc, P. Midtrimester cervical consistency index measurement and prediction of preterm birth before 34 and 37 weeks in twin pregnancies. Ultrasound Obstet. Gynecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Dziadosz, M.; Bennett, T.-A.; Dolin, C.; Honart, A.W.; Pham, A.; Lee, S.S.; Pivo, S.; Roman, A. Uterocervical angle: A novel ultrasound screening tool to predict spontaneous preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 376. [Google Scholar] [CrossRef] [PubMed]
- Sochacki-Wójcicka, N.; Wojcicki, J.; Bomba-Opoń, D.; Wielgos, M. Anterior cervical angle as a new biophysical ultrasound marker for prediction of spontaneous preterm birth. Ultrasound Obstet. Gynecol. 2015, 46, 377–378. [Google Scholar] [CrossRef]
- Sepúlveda-Martínez, A.; Diaz-Moreno, F.; Muñoz, H.; Valdés, E.; Parra-Cordero, M. Second-Trimester Anterior Cervical Angle in a Low-Risk Population as a Marker for Spontaneous Preterm Delivery. Fetal Diagn. Ther. 2016, 41, 220–225. [Google Scholar] [CrossRef]
- Knight, J.C.; Tenbrink, E.; Onslow, M.; Patil, A.S. Uterocervical Angle Measurement Improves Prediction of Preterm Birth in Twin Gestation. Am. J. Perinatol. 2018, 35, 648–654. [Google Scholar] [CrossRef][Green Version]
- Baños, N.; Perez-Moreno, A.; Julià, C.; Murillo-Bravo, C.; Coronado, D.; Gratacos, E.; Deprest, J.; Palacio, M. Quantitative analysis of cervical texture by ultrasound in mid-pregnancy and association with spontaneous preterm birth. Ultrasound Obstet. Gynecol. 2018, 51, 637–643. [Google Scholar] [CrossRef]
- Fiset, S.; Martel, A.; Glanc, P.; Barrett, J.; Melamed, N. Prediction of spontaneous preterm birth among twin gestations using machine learning and texture analysis of cervical ultrasound images. Univ. Toronto Med. J. 2019, 96. [Google Scholar]
- Gibson, J.L.; Castleman, J.S.; Meher, S.; Kilby, M.D. Updated guidance for the management of twin and triplet pregnancies from the National Institute for Health and Care Excellence guidance, UK: What is new that may improve perinatal outcomes? Acta Obstet. Gynecol. Scand. 2019, 99, 147–152. [Google Scholar] [CrossRef]
- Kagan, K.O.; Sonek, J. How to measure cervical length. Ultrasound Obstet. Gynecol. 2015, 45, 358–362. [Google Scholar] [CrossRef]
- Romero, R.; Kusanovic, J.P.; Espinoza, J.; Gotsch, F.; Nhan-Chang, C.L.; Erez, O.; Kim, C.J.; Khalek, N.; Mittal, P.; Goncalves, L.F.; et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet. Gynecol. 2007, 30, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R.; Hassan, S.S.; Yeo, L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2010, 203, 128.e1–128.e12. [Google Scholar] [CrossRef] [PubMed]
- Hester, A.E.; Ankumah, N.-A.E.; Chauhan, S.P.; Blackwell, S.C.; Sibai, B.M. Twin transvaginal cervical length at 16–20 weeks and prediction of preterm birth. J. Matern. Neonatal Med. 2017, 32, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Baños, N.; Murillo-Bravo, C.; Julià, C.; Migliorelli, F.; Perez-Moreno, A.; Ríos, J.; Gratacos, E.; Valentin, L.; Palacio, M. Mid-trimester sonographic cervical consistency index to predict spontaneous preterm birth in a low-risk population. Ultrasound Obstet. Gynecol. 2018, 51, 629–636. [Google Scholar] [CrossRef]
- Parra-Saavedra, M.A.; Gonce, A.; Masoller, N.; Gratacos, E.; Palacio, M. OP08.03: Tranvaginal ultrasonographic measurement of cervical consistency index (CCI) throughout gestation in twin pregnancy. Ultrasound Obstet. Gynecol. 2012, 40, 79. [Google Scholar] [CrossRef]
- Sung, K.U.; Nam, S.M.; Kim, G.E.; Jung, Y.J.; Lee, J.H.; Kim, Y.-H.; Kwon, J.Y. OB08: A comparison analysis between cervical elasticity and cervical consistency index at mid-trimester. Sci. Cen. J. 2018, 104, 204. [Google Scholar]
- Rosen, H.; Stratulat, V.; Aviram, A.; Melamed, N.; Okby, R.; Barrett, J.; Glanc, P. 461: Prediction of preterm birth in twin pregnancies by single mid-trimester cervical consistency index measurement. Am. J. Obstet. Gynecol. 2018, 218, 278. [Google Scholar] [CrossRef]
- Silva, S.V.L.; Damião, R.; Fonseca, E.B.; Garcia, S.; Lippi, U.G. Reference ranges for cervical length by transvaginal scan in singleton pregnancies. J. Matern. Neonatal Med. 2010, 23, 379–382. [Google Scholar] [CrossRef]
- Crane, J.; Hutchens, D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: A systematic review. Ultrasound Obstet. Gynecol. 2008, 31, 579–587. [Google Scholar] [CrossRef]
- Pagani, G.; Stagnati, V.; Fichera, A.; Prefumo, F. Cervical length at mid-gestation in screening for preterm birth in twin pregnancy. Ultrasound Obstet. Gynecol. 2016, 48, 56–60. [Google Scholar] [CrossRef]
- Rechberger, T.; Uldbjerg, N.; Oxlund, H. Connective tissue changes in the cervix during normal pregnancy and pregnancy complicated by cervical incompetence. Obstet. Gynecol. 1988, 71. [Google Scholar]


|
Total n = 63 | TB n = 36 | sPTB < 34 w n = 16 | sPTB < 37 w n = 27 | p-Value † | |
|---|---|---|---|---|---|
| Maternal Age (Years) | 31.2 ± 4.9 | 31.5 ± 5.2 | 29.9 ± 2.8 | 31.4 ± 5.6 | p = 0.79 |
| Caucasian Ethnicity | 95.2% (60/63) | 94.4% (34/36) | 93.8% (15/16) | 96.3% (26/27) | p = 0.92 |
| BMI (kg/m2) | 23.7 ± 4.9 | 24.6 ± 5.8 | 22.3 ± 2.4 | 22.4 ± 2.4 | p = 0.01 |
| Smoker | 7.9% (5/63) | 8.3% (3/36) | 6.3% (1/16) | 7.4% (2/27) | p = 0.95 |
| Spont. Conception | 57.1% (36/63) | 77.8% (28/36) | 62.5% (10/16) | 66.7% (18/27) | p = 0.57 |
| Nulliparous | 31.7% (20/63) | 30.8% (8/36) | 62.5% (10/16) | 44.4% (12/27) | p = 0.02 |
| Dichorionicity | 63.5% (40/63) | 72.2% (26/36) | 43.8% (7/16) | 51.9% (14/27) | p = 0.09 |
| GA at Scan (Days) | 138 ± 17 | 135 ± 24 | 140 ± 4 | 141 ± 5 | p = 0.48 |
| Cervical Length (mm) | 35.9 ± 5.7 | 37.3 ± 5.1 | 32.5 ± 6.6 | 34.2 ± 6.2 | p = 0.02 |
| CCI (%) | 69.6 ± 10.3 | 74.7 ± 8.6 | 60.8 ± 8.0 | 63.1 ± 8.5 | p < 0.01 |
| UCA (°) | 105.7 ± 16.8 | 98.5 ± 10.8 | 116.8 ± 20.3 | 114.7 ± 18.8 | p < 0.01 |
| CTx Score | −7.8 ± 3.2 | −6.9 ± 3.2 | −10.6 ± 3.6 | −8.9 ± 2.8 | p = 0.09 |
| Funnelling (%) | 47.6% (3/63) | 12.5% (2/16) | 11.1% (3/27) | p = 0.10 | |
| Sludge (%) | 3.2% (2/63) | 2.8% (1/36) | 6.3% (1/16) | 3.7% (1/27) | p = 0.83 |
| Antenatal Complications | |||||
| GHT/Preeclampsia | 1.6% (1/63) | 2.8% (1/36) | |||
| GDM | 3.2% (2/63) | 5.6% (2/36) | |||
| sIUGR | 4.8% (3/63) | 2.8% (1/36) | 12.5% (2/16) | 7.4% (2/27) | p = 0.39 |
| Received Progesterone | 4.8% (3/63) | 2.8% (1/36) | 6.3% (1/16) | 7.4% (2/27) | p = 0.68 |
| Received Betamethasone | 39.7% (25/63) | 16.7% (6/36) | 93.8% (15/16) | 70.4% (19/27) | p < 0.01 |
| Delivery Outcomes | |||||
| GA at Delivery (w d) | 35 w 2 d ± 25 d | 37 w 3 d ± 4 d | 30 w 1 d ± 24 d | 32 w 4 d ± 27 d | p < 0.01 |
| Birth Weight-Largest (g) | 2312 ± 623 | 2683 ± 298 | 1490 ± 459 | 1882 ± 629 | p < 0.01 |
| Birth Weight-Smallest (g) | 2131 ± 616 | 2506 ± 270 | 1333 ± 463 | 1711 ± 627 | p < 0.01 |
| Spontaneous Labor | 58.7% (37/63) | 27.8% (10/36) | 100% (16/16) | 100% (27/27) | p < 0.01 |
| Induced Labor | 20.6% (13/63) | 36.1% (13/36) | |||
| Vaginal Delivery | 52.4% (33/63) | 50.0% (18/36) | 50.0% (8/16) | 55.5% (15/27) | p = 0.89 |
| Elective Caesarean | 19.0% (12/63) | 33.3% (12/36) | |||
| Emergency Caesarean | 28.6% (18/63) | 16.7% (6/36) | 50.0% (8/16) | 44.4% (12/27) | p = 0.02 |
| Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | LR + (95% CI) | LR − (95%CI) | dOR (95% CI) | |
|---|---|---|---|---|---|---|
| CL | <29 mm (p = 10) | 31.3 (11.0–58.7) | 93.6 (82.5–98.7) | 4.90 (1.32–18.22) | 0.73 (0.52–1.03) | 6.7 (1.37–32.25) |
| <35 mm | 62.5 (35.4–84.8) | 70.2 (55.1–82.7) | 2.10 (1.17–3.75) | 0.53 (0.28–1.03) | 3.9 (1.19–12.9) | |
| CCI | <60% (p = 10) | 40.0 (16.3–67.7) | 91.5 (79.6–97.6) | 4.70 (1.53–14.46) | 0.66 (0.43–1.00) | 7.2 (1.67–30.78) |
| <64% | 86.7 (59.5–98.3) | 65.9 (50.7–79.1) | 2.055 (1.63–3.97) | 0.20 (0.05–0.75) | 12.6 (2.52–62.77) | |
| UCA | >130° (p = 10) | 40.0 (16.3–67.7) | 91.5 (79.6–97.6) | 4.70 (1.53–14.46) | 0.66 (0.43–1.00) | 6.7 (1.06–25.92) |
| >103° | 68.8 (41.3–88.9) | 73.8 (57.9–86.1) | 2.63 (1.43–4.81) | 0.42 (0.20–0.90) | 6.2(1.76–21.89) | |
| CTx | −13.5 (p = 10) | 27.3 (6.0–60.9) | 88.2 (72.5–96.7) | 2.32 (0.61–8.80) | 0.82 (0.56–1.21) | 2.8 (0.52–15.2) |
| −11.0 | 72.7 (39.0–93.9) | 38.2 (22.2–56.4) | 1.18 (0.75–1.84) | 0.71 (0.25–2.05) | 1.65 (0.37–7.37) |
| Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | LR + (95% CI) | LR − (95%CI) | dOR (95% CI) | |
|---|---|---|---|---|---|---|
| CL | <29 mm (p = 10) | 22.2 (8.6–42.3) | 94.4 (81.3–99.3) | 4.00 (0.87–18.30) | 0.82 (0.66–1.02) | 4.9 (0.89–26.33) |
| <36 mm | 51.8 (31.9–71.3) | 72.2 (54.8–85.8) | 1.87 (0.98–3.54) | 0.67 (0.43–1.04) | 2.8 (0.98–7.99) | |
| CCI | <60% (p = 10) | 30.8 (14.3–51.8) | 94.4 (85.5–99.9) | 5.54 (1.28–23.97) | 0.73 (0.56–0.96) | 7.6 (1.45–39.40) |
| <68% | 76.9 (56.3–91.0) | 75.0 (57.8–87.8) | 3.08 (1.68–5.63) | 0.31 (0.15–0.64) | 10.0 (3.06–32.67) | |
| UCA | >130° (p = 10) | 23.1 (8.9–43.7) | 100 (89.1–100) | 0.77 (0.62–0.95) | ||
| >105° | 65.4 (44.3–82.8) | 84.4 (67.2–94.7) | 4.18 (1.78–9.81) | 0.41 (0.24–0.71) | 10.2(2.92–35.61) | |
| CTx | −11.3 (p = 10) | 20.0 (5.73–43.7) | 96.0 (79.7–99.9) | 5.00 (0.61–41.28) | 0.83 (0.66–1.05) | 6.0 (0.61–58.71) |
| −8.5 | 60.0 (36.1–80.9) | 64.0 (42.5–82.0) | 1.67 (0.88–3.14) | 0.62 (0.34–1.15) | 2.7 (0.79–8.95) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Merwe, J.; Couck, I.; Russo, F.; Burgos-Artizzu, X.P.; Deprest, J.; Palacio, M.; Lewi, L. The Predictive Value of the Cervical Consistency Index to Predict Spontaneous Preterm Birth in Asymptomatic Twin Pregnancies at the Second-Trimester Ultrasound Scan: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 1784. https://doi.org/10.3390/jcm9061784
van der Merwe J, Couck I, Russo F, Burgos-Artizzu XP, Deprest J, Palacio M, Lewi L. The Predictive Value of the Cervical Consistency Index to Predict Spontaneous Preterm Birth in Asymptomatic Twin Pregnancies at the Second-Trimester Ultrasound Scan: A Prospective Cohort Study. Journal of Clinical Medicine. 2020; 9(6):1784. https://doi.org/10.3390/jcm9061784
Chicago/Turabian Stylevan der Merwe, Johannes, Isabel Couck, Francesca Russo, Xavier P. Burgos-Artizzu, Jan Deprest, Montse Palacio, and Liesbeth Lewi. 2020. "The Predictive Value of the Cervical Consistency Index to Predict Spontaneous Preterm Birth in Asymptomatic Twin Pregnancies at the Second-Trimester Ultrasound Scan: A Prospective Cohort Study" Journal of Clinical Medicine 9, no. 6: 1784. https://doi.org/10.3390/jcm9061784
APA Stylevan der Merwe, J., Couck, I., Russo, F., Burgos-Artizzu, X. P., Deprest, J., Palacio, M., & Lewi, L. (2020). The Predictive Value of the Cervical Consistency Index to Predict Spontaneous Preterm Birth in Asymptomatic Twin Pregnancies at the Second-Trimester Ultrasound Scan: A Prospective Cohort Study. Journal of Clinical Medicine, 9(6), 1784. https://doi.org/10.3390/jcm9061784






