Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study
Abstract
1. Introduction
2. Experimental Section
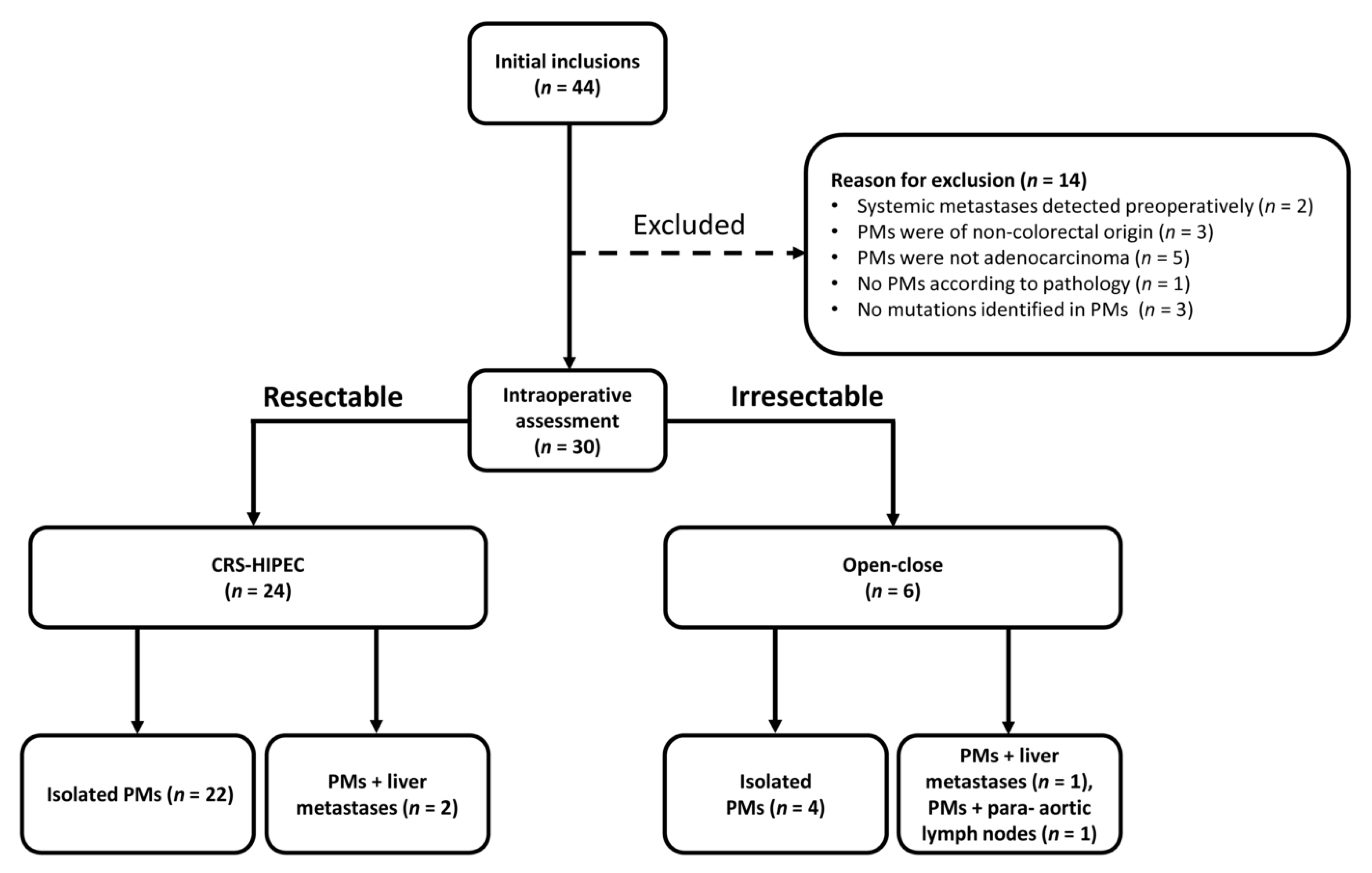
2.1. Study Design and Patients
2.2. Ethics Approval and Consent to Participate
2.3. Blood and Tumor Tissue Collection
2.4. DNA Isolation and Mutation Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
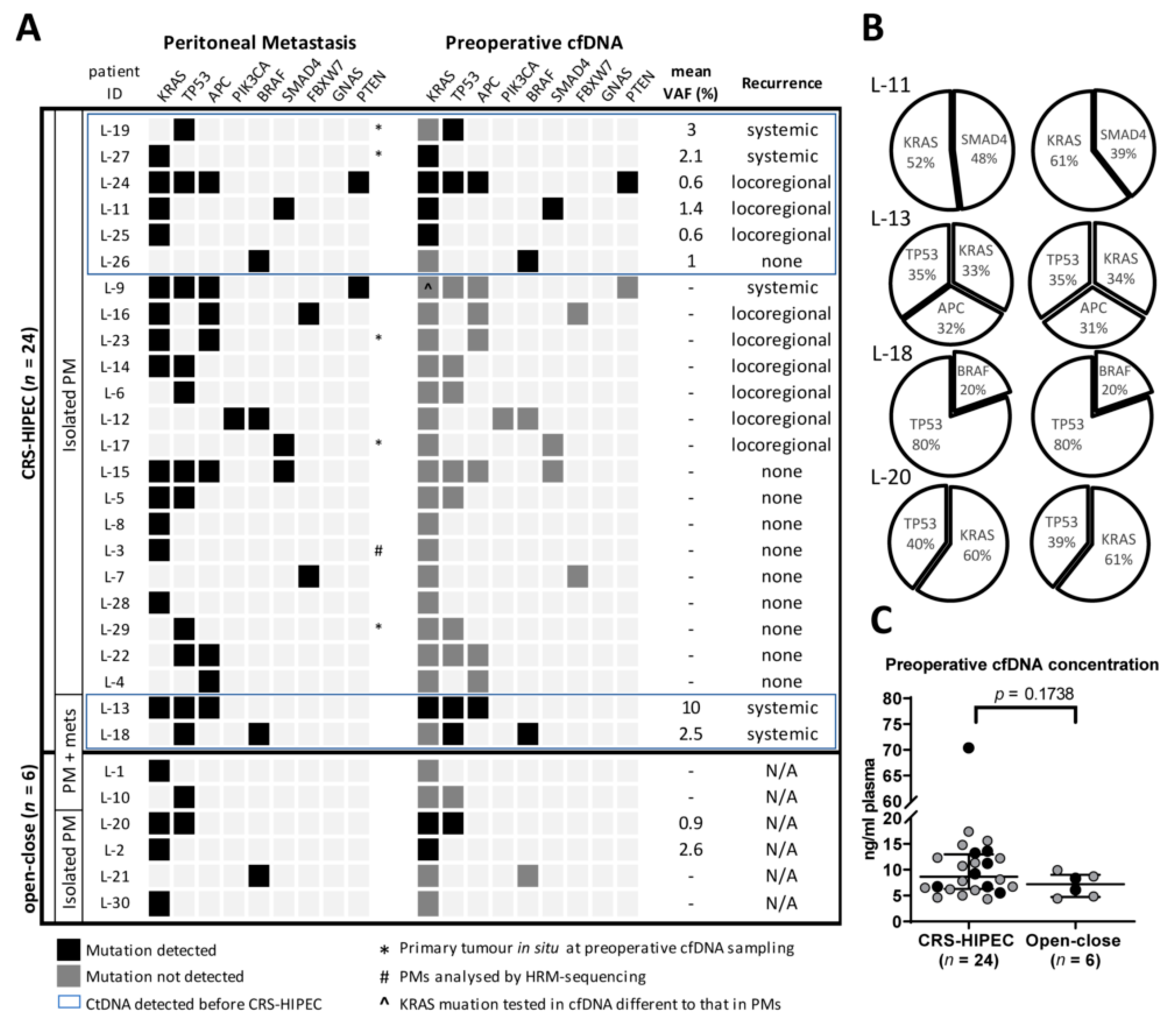
3.2. Accuracy of ctDNA Analysis for Detection of PMs
3.3. Preoperative ctDNA as a Prognostic Marker of Recurrence
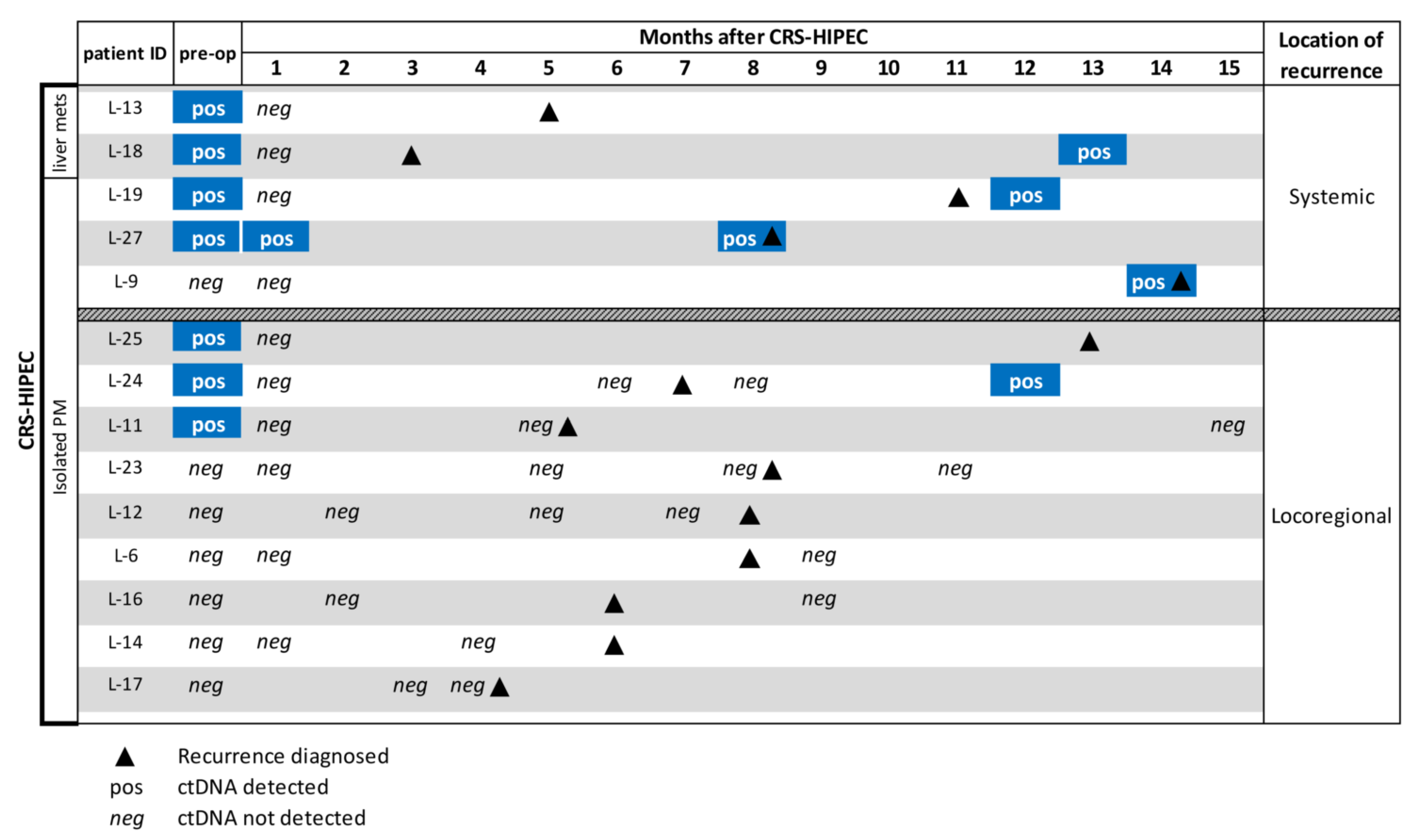
3.4. ctDNA to Support Recurrence Diagnosis During Follow-up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Hugen, N.; van de Velde, C.J.; de Wilt, J.H.; Nagtegaal, I.D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 2014, 25, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Bruin, S.; Boot, H.; van Slooten, G.; van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J., 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef]
- Cashin, P.H.; Mahteme, H.; Spang, N.; Syk, I.; Frodin, J.E.; Torkzad, M.; Glimelius, B.; Graf, W. Cytoreductive surgery and intraperitoneal chemotherapy versus systemic chemotherapy for colorectal peritoneal metastases: A randomised trial. Eur. J. Cancer 2016, 53, 155–162. [Google Scholar] [CrossRef]
- Esquivel, J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: Survival outcomes and patient selection. J. Gastrointest. Oncol. 2016, 7, 72–78. [Google Scholar]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Kuijpers, A.M.; Mirck, B.; Aalbers, A.G.; Nienhuijs, S.W.; de Hingh, I.H.; Wiezer, M.J.; van Ramshorst, B.; van Ginkel, R.J.; Havenga, K.; Bremers, A.J.; et al. Cytoreduction and HIPEC in the Netherlands: Nationwide long-term outcome following the Dutch protocol. Ann. Surg. Oncol. 2013, 20, 4224–4230. [Google Scholar] [CrossRef] [PubMed]
- Mirnezami, R.; Mehta, A.M.; Chandrakumaran, K.; Cecil, T.; Moran, B.J.; Carr, N.; Verwaal, V.J.; Mohamed, F.; Mirnezami, A.H. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br. J. Cancer 2014, 111, 1500–1508. [Google Scholar] [CrossRef]
- Sluiter, N.R.; Rovers, K.P.; Salhi, Y.; Vlek, S.L.; Coupe, V.M.H.; Verheul, H.M.W.; Kazemier, G.; de Hingh, I.; Tuynman, J.B. Metachronous Peritoneal Metastases After Adjuvant Chemotherapy are Associated with Poor Outcome After Cytoreduction and HIPEC. Ann. Surg. Oncol. 2018, 25, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Quenet, F.; Elias, D.; Roca, L.; Goere, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J. Clin. Oncol. 2018, 36, LBA3503. [Google Scholar] [CrossRef]
- Feferman, Y.; Solomon, D.; Bhagwandin, S.; Kim, J.; Aycart, S.N.; Feingold, D.; Sarpel, U.; Labow, D.M. Sites of Recurrence After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Carcinomatosis from Colorectal and Appendiceal Adenocarcinoma: A Tertiary Center Experience. Ann. Surg. Oncol. 2019, 26, 482–489. [Google Scholar] [CrossRef]
- Braam, H.J.; van Oudheusden, T.R.; de Hingh, I.H.; Nienhuijs, S.W.; Boerma, D.; Wiezer, M.J.; van Ramshorst, B. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J. Surg. Oncol. 2014, 109, 841–847. [Google Scholar] [CrossRef]
- Eng, O.S.; Dumitra, S.; O’Leary, M.; Raoof, M.; Wakabayashi, M.; Dellinger, T.H.; Han, E.S.; Lee, S.J.; Paz, I.B.; Lee, B. Association of Fluid Administration With Morbidity in Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. JAMA Surg. 2017, 152, 1156–1160. [Google Scholar] [CrossRef]
- van Eden, W.J.; Elekonawo, F.M.K.; Starremans, B.J.; Kok, N.F.M.; Bremers, A.J.A.; de Wilt, J.H.W.; Aalbers, A.G.J. Treatment of Isolated Peritoneal Recurrences in Patients with Colorectal Peritoneal Metastases Previously Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2018, 25, 1992–2001. [Google Scholar] [CrossRef]
- Koh, J.L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic antigen as a marker for colorectal cancer: Is it clinically useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef]
- Verwaal, V.J.; Zoetmulder, F.A. Follow-up of patients treated by cytoreduction and chemotherapy for peritoneal carcinomatosis of colorectal origin. Eur. J. Surg. Oncol. 2004, 30, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin. Surg. Oncol. 1998, 14, 254–261. [Google Scholar] [CrossRef]
- Passot, G.; Dumont, F.; Goere, D.; Arvieux, C.; Rousset, P.; Regimbeau, J.M.; Elias, D.; Villeneuve, L.; Glehen, O.; Group, B.-R.S.W. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br. J. Surg. 2018, 105, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Goere, D.; Dumont, F.; Honore, C.; Dartigues, P.; Stoclin, A.; Malka, D.; Boige, V.; Ducreux, M. Role of hyperthermic intraoperative peritoneal chemotherapy in the management of peritoneal metastases. Eur. J. Cancer 2014, 50, 332–340. [Google Scholar] [CrossRef]
- Dresen, R.C.; De Vuysere, S.; De Keyzer, F.; Van Cutsem, E.; Prenen, H.; Vanslembrouck, R.; De Hertogh, G.; Wolthuis, A.; D’Hoore, A.; Vandecaveye, V. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, G.; Ylstra, B.; Brenton, J.D. Sequencing Structural Variants in Cancer for Precision Therapeutics. Trends Genet. TIG 2016, 32, 530–542. [Google Scholar] [CrossRef]
- Van Helden, E.J.; Angus, L.; Menke-van der Houven van Oordt, C.W.; Heideman, D.A.M.; Boon, E.; van Es, S.C.; Radema, S.A.; van Herpen, C.M.L.; de Groot, D.J.A.; de Vries, E.G.E.; et al. RAS and BRAF mutations in cell-free DNA are predictive for outcome of cetuximab monotherapy in patients with tissue-tested RAS wild-type advanced colorectal cancer. Mol. Oncol. 2019, 13, 2361–2374. [Google Scholar] [CrossRef]
- Bach, S.; Sluiter, N.R.; Beagan, J.J.; Mekke, J.M.; Ket, J.C.F.; van Grieken, N.C.T.; Steenbergen, R.D.M.; Ylstra, B.; Kazemier, G.; Tuynman, J.B. Circulating Tumor DNA Analysis: Clinical Implications for Colorectal Cancer Patients. A Systematic Review. JNCI Cancer Spectr. 2019, 3, pkz042. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Baumgartner, J.M.; Raymond, V.M.; Lanman, R.B.; Tran, L.; Kelly, K.J.; Lowy, A.M.; Kurzrock, R. Preoperative Circulating Tumor DNA in Patients with Peritoneal Carcinomatosis is an Independent Predictor of Progression-Free Survival. Ann. Surg. Oncol. 2018, 25, 2400–2408. [Google Scholar] [CrossRef]
- Demuth, C.; Spindler, K.G.; Johansen, J.S.; Pallisgaard, N.; Nielsen, D.; Hogdall, E.; Vittrup, B.; Sorensen, B.S. Measuring KRAS Mutations in Circulating Tumor DNA by Droplet Digital PCR and Next-Generation Sequencing. Transl. Oncol. 2018, 11, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Scholer, L.V.; Thomsen, R.; Tobiasen, H.; Vang, S.; Nordentoft, I.; Lamy, P.; Kannerup, A.S.; Mortensen, F.V.; Stribolt, K.; et al. Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery. Gut 2016, 65, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.A.; Hachiya, T.; Iwaya, T.; Kume, K.; Matsuo, T.; Kawasaki, K.; Abiko, Y.; Akasaka, R.; Matsumoto, T.; Otsuka, K.; et al. Individualized Mutation Detection in Circulating Tumor DNA for Monitoring Colorectal Tumor Burden Using a Cancer-Associated Gene Sequencing Panel. PLoS ONE 2016, 11, e0146275. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Sloothen, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- De Cuba, E.M.; Kwakman, R.; Knol, D.L.; Bonjer, H.J.; Meijer, G.A.; Te Velde, E.A. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat. Rev. 2013, 39, 321–327. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; Witkamp, A.; Boot, H.; van Slooten, G.; Zoetmulder, F.A. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2005, 12, 65–71. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Jablonski, K.A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 1995, 221, 124–132. [Google Scholar] [CrossRef]
- Sluiter, N.R. Netherlands Trial Register, Trial NL6960 (NTR7148). Available online: https://www.trialregister.nl/trial/6960 (accessed on 2 June 2020).
- Van Essen, H.F.; Ylstra, B. High-resolution copy number profiling by array CGH using DNA isolated from formalin-fixed, paraffin-embedded tissues. Methods Mol. Biol. 2012, 838, 329–341. [Google Scholar] [CrossRef]
- Sie, D.; Snijders, P.J.; Meijer, G.A.; Doeleman, M.W.; van Moorsel, M.I.; van Essen, H.F.; Eijk, P.P.; Grunberg, K.; van Grieken, N.C.; Thunnissen, E.; et al. Performance of amplicon-based next generation DNA sequencing for diagnostic gene mutation profiling in oncopathology. Cell. Oncol. 2014, 37, 353–361. [Google Scholar] [CrossRef]
- Heideman, D.A.; Thunnissen, F.B.; Doeleman, M.; Kramer, D.; Verheul, H.M.; Smit, E.F.; Postmus, P.E.; Meijer, C.J.; Meijer, G.A.; Snijders, P.J. A panel of high resolution melting (HRM) technology-based assays with direct sequencing possibility for effective mutation screening of EGFR and K-ras genes. Cell. Oncol. 2009, 31, 329–333. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Henriksen, T.V.; Christensen, E.; Sharma, S.; Salari, R.; Sethi, H.; Knudsen, M.; Nordentoft, I.; Wu, H.T.; Tin, A.S.; et al. Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019, 5, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.P.; Bracke, M.E. Peritoneal minimal residual disease in colorectal cancer: Mechanisms, prevention, and treatment. Lancet Oncol. 2009, 10, 72–79. [Google Scholar] [CrossRef]
- Vidal, J.; Muinelo, L.; Dalmases, A.; Jones, F.; Edelstein, D.; Iglesias, M.; Orrillo, M.; Abalo, A.; Rodriguez, C.; Brozos, E.; et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann. Oncol. 2017, 28, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Bando, H.; Kagawa, Y.; Kato, T.; Akagi, K.; Denda, T.; Nishina, T.; Komatsu, Y.; Oki, E.; Kudo, T.; Kumamoto, H.; et al. A multicentre, prospective study of plasma circulating tumour DNA test for detecting RAS mutation in patients with metastatic colorectal cancer. Br. J. Cancer 2019, 120, 982–986. [Google Scholar] [CrossRef]
- Ehlert, T.; Tug, S.; Brahmer, A.; Neef, V.; Heid, F.; Werner, C.; Jansen-Winkeln, B.; Kneist, W.; Lang, H.; Gockel, I.; et al. Establishing PNB-qPCR for quantifying minimal ctDNA concentrations during tumour resection. Sci. Rep. 2017, 7, 8876. [Google Scholar] [CrossRef]
- Tarazona, N.; Gimeno-Valiente, F.; Gambardella, V.; Zuñiga, S.; Rentero-Garrido, P.; Huerta, M.; Roselló, S.; Martinez-Ciarpaglini, C.; Carbonell-Asins, J.A.; Carrasco, F.; et al. Targeted next-generation sequencing of circulating-tumor DNA for tracking minimal residual disease in localized colon cancer. Ann. Oncol. 2019, 30, 1804–1812. [Google Scholar] [CrossRef]
- Tie, J.; Cohen, J.D.; Wang, Y.; Li, L.; Christie, M.; Simons, K.; Elsaleh, H.; Kosmider, S.; Wong, R.; Yip, D.; et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut 2019, 68, 663–671. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Simkens, G.; Burger, J.W.A.; Nienhuijs, S.W.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parralel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar] [CrossRef]
- Dodson, R.M.; McQuellon, R.P.; Mogal, H.D.; Duckworth, K.E.; Russell, G.B.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. Quality-of-Life Evaluation After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 772–783. [Google Scholar] [CrossRef]
- Kato, S.; Schwaederle, M.C.; Fanta, P.T.; Okamura, R.; Leichman, L.; Lippman, S.M.; Lanman, R.B.; Raymond, V.M.; Talasaz, A.; Kurzrock, R. Genomic Assessment of Blood-Derived Circulating Tumor DNA in Patients With Colorectal Cancers: Correlation With Tissue Sequencing, Therapeutic Response, and Survival. JCO Precis. Oncol. 2019, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van’t Sant, I.; van Eden, W.J.; Engbersen, M.P.; Kok, N.F.M.; Woensdregt, K.; Lambregts, D.M.J.; Shanmuganathan, S.; Beets-Tan, R.G.H.; Aalbers, A.G.J.; Lahaye, M.J. Diffusion-weighted MRI assessment of the peritoneal cancer index before cytoreductive surgery. Br. J. Surg. 2019, 106, 491–498. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 30) | Underwent CRS-HIPEC (n = 24) | ||||
|---|---|---|---|---|---|
| Characteristics | |||||
| General | |||||
| Age in years | Mean/SD | 65.1 | 9.4 | 65.2 | 9.6 |
| Median/range | 67 | 37–81 | 66.5 | 36–81 | |
| Male gender | (n/%) | 17 | 56.7 | 14 | 58.3 |
| BMI | Mean/SD-all | 27.1 | 5.2 | 27 | 5.5 |
| male | 25.6 | 3.1 | 25.4 | 2.7 | |
| female | 29.1 | 6.7 | 29.2 | 7.6 | |
| Median/range-all | 26.8 | 21.3–49 | 26.8 | 21.3–49 | |
| male | 24.4 | 21.3–32.1 | 24.3 | 21.3–29.8 | |
| female | 28.4 | 22.0–49.0 | 27.9 | 22.0–49.0 | |
| ASA classification | I-II (n/%) | 22 | 73.3 | 17 | 70.8 |
| III (n/%) | 8 | 26.7 | 7 | 29.2 | |
| Primary Tumor | n | % | n | % | |
| Location | Colon | 27 | 90 | 22 | 91.7 |
| Rectum | 3 | 10 | 2 | 8.3 | |
| TNM-stage at diagnosis | II | 8 | 26.7 | 7 | 29.2 |
| III | 10 | 33.3 | 8 | 33.3 | |
| IV | 12 | 40 | 9 | 37.5 | |
| Differentiation grade | Good/moderate | 24 | 92.3 | 19 | 90.5 |
| Poor | 1 | 3.8 | 1 | 4.8 | |
| Signet cell | 1 | 3.8 | 1 | 4.8 | |
| Lymph invasion | 7 | 25.9 | 5 | 22.7 | |
| Venous invasion | 10 | 37 | 8 | 36.4 | |
| Tumor type | Adenocarcinoma | 21 | 70 | 17 | 70.8 |
| Mucinous adenocarcinoma | 8 | 26.7 | 6 | 25 | |
| Signet cell type | 1 | 3.3 | 1 | 4.2 | |
| Treatment | |||||
| Adjuvant chemotherapy primary | (n/%) | 11 | 36.7 | 9 | 37.5 |
| Primary tumor in situ at intended CRS-HIPEC | (n/%) | 8 | 26.7 | 7 | 29.2 |
| Liver metastases at intended CRS-HIPEC | (n/%) | 3 | 10 | 2 | 8.3 |
| Lymph node metastases at intended CRS-HIPEC | (n/%) | 7 | 24.1 | 6 | 25 |
| PCI | Mean/SD | 10.1 | 8.4 | 9.3 | 7.8 |
| Median/range | 7 | 0–31 | 7 | 0–26 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beagan, J.J.; Sluiter, N.R.; Bach, S.; Eijk, P.P.; Vlek, S.L.; Heideman, D.A.M.; Kusters, M.; Pegtel, D.M.; Kazemier, G.; van Grieken, N.C.T.; et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. J. Clin. Med. 2020, 9, 1738. https://doi.org/10.3390/jcm9061738
Beagan JJ, Sluiter NR, Bach S, Eijk PP, Vlek SL, Heideman DAM, Kusters M, Pegtel DM, Kazemier G, van Grieken NCT, et al. Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. Journal of Clinical Medicine. 2020; 9(6):1738. https://doi.org/10.3390/jcm9061738
Chicago/Turabian StyleBeagan, Jamie J., Nina R. Sluiter, Sander Bach, Paul P. Eijk, Stijn L. Vlek, Daniëlle A. M. Heideman, Miranda Kusters, D. Michiel Pegtel, Geert Kazemier, Nicole C. T. van Grieken, and et al. 2020. "Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study" Journal of Clinical Medicine 9, no. 6: 1738. https://doi.org/10.3390/jcm9061738
APA StyleBeagan, J. J., Sluiter, N. R., Bach, S., Eijk, P. P., Vlek, S. L., Heideman, D. A. M., Kusters, M., Pegtel, D. M., Kazemier, G., van Grieken, N. C. T., Ylstra, B., & Tuynman, J. B. (2020). Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study. Journal of Clinical Medicine, 9(6), 1738. https://doi.org/10.3390/jcm9061738






