Long-Term Changes in Sleep Disordered Breathing in Renal Transplant Patients: Relevance of the BMI
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design
2.2. Patients
2.3. Study Procedures
2.4. Assessment of Sleep Disordered Breathing
2.5. Statistical Analysis
3. Results
3.1. Description of the Study Cohort
3.2. Baseline Polygraphic Sleep Data
3.3. Longitudinal Polygraphic Sleep Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Mucsi, I.; Novak, M.; Szabo, Z.; Freire, A.X.; Huch, K.M.; Arah, O.A.; Ma, J.Z.; Lu, J.L.; Sim, J.J.; et al. Association of incident obstructive sleep apnoea with outcomes in a large cohort of US veterans. Thorax 2015, 70, 888–895. [Google Scholar] [CrossRef]
- Nicholl, D.D.M.; Ahmed, S.B.; Loewen, A.H.S.; Hemmelgarn, B.R.; Sola, D.Y.; Beecroft, J.M.; Turin, T.C.; Hanly, P.J. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest 2012, 141, 1422–1430. [Google Scholar] [CrossRef]
- Xu, J.; Yoon, I.Y.; Chin, H.J. The effect of sleep apnea on all-cause mortality in nondialyzed chronic kidney disease patients. Sleep Med. 2016, 27, 32–38. [Google Scholar] [CrossRef]
- Roumelioti, M.-E.; Buysse, D.J.; Sanders, M.H.; Strollo, P.; Newman, A.B.; Unruh, M.L. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 986–994. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Tripepi, G. Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J. Am. Soc. Nephrol. 2002, 13, 729–733. [Google Scholar]
- Tang, S.C.W.; Lam, B.; Yao, T.J.; Leung, W.S.; Chu, C.M.; Ho, Y.W.; Ip, M.S.M.; Lai, K.N. Sleep apnea is a novel risk predictor of cardiovascular morbidity and death in patients receiving peritoneal dialysis. Kidney Int. 2010, 77, 1031–1038. [Google Scholar]
- Elias, R.M.; Chan, C.T.; Paul, N.; Motwani, S.S.; Kasai, T.; Gabriel, J.M.; Spiller, N.; Bradley, T.D. Relationship of pharyngeal water content and jugular volume with severity of obstructive sleep apnea in renal failure. Nephrol. Dial. Transplant. 2013, 28, 937–944. [Google Scholar] [PubMed]
- Ogna, A.; Forni Ogna, V.; Mihalache, A.; Pruijm, M.; Halabi, G.; Phan, O.; Cornette, F.; Bassi, I.; Rubio, J.H.; Burnier, M.; et al. Obstructive Sleep Apnea Severity and Overnight Body Fluid Shift before and after Hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1002–1010. [Google Scholar]
- Hanly, P.J.; Pierratos, A. Improvement of Sleep Apnea in Patients with Chronic Renal Failure Who Undergo Nocturnal Hemodialysis. N. Engl. J. Med. 2001, 344, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Abuyassin, B.; Sharma, K.; Ayas, N.T.; Laher, I. Obstructive Sleep Apnea and Kidney Disease: A Potential Bidirectional Relationship? J. Clin. Sleep. Med. 2015, 11, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Leonardis, D.; Tripepi, R.; Parlongo, G.; Catalano, C.; Tripepi, G.; Castronovo, V.; Ferini Strambi, L.; Zoccali, C. Sleep disordered breathing in renal transplant patients. Am. J. Transplant. 2009, 9, 1373–1381. [Google Scholar]
- Tandukar, S.; Hou, S.; Yabes, J.; Ran, X.; Fletcher, M.; Strollo, P.; Patel, S.R.; Unruh, M.; Jhamb, M. Does Kidney Transplantation Affect Sleep and Fatigue in Patients With Kidney Disease? Transplant. Direct 2019, 5, e461. [Google Scholar] [CrossRef] [PubMed]
- Auckley, D.H.; Schmidt-Nowara, W.; Brown, L.K. Reversal of sleep apnea hypopnea syndrome in end-stage renal disease after kidney transplantation. Am. J. Kidney Dis. 1999, 34, 739–744. [Google Scholar] [CrossRef]
- Langevin, B.; Fouque, D.; Léger, P.; Robert, D. Sleep apnea syndrome and end-stage renal disease: Cure after renal transplantation. Chest 1993, 103, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Gamez, B.; Martin-Malo, A.; Rodriguez-Benot, A.; Muñoz-Cabrera, L.; Cosano Povedano, A.; Aljama, P. Kidney Transplantation Improves Sleep-Related Breathing in Hemodialysis Patients. Blood Purif. 2008, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Gupta, K.; Sinha, S.; Malhotra, A.; Mahajan, S. Effect of kidney transplantation on sleep-disordered breathing in patients with End Stage Renal Disease: A polysomnographic study. Sleep Med. 2018, 45, 140–145. [Google Scholar] [CrossRef]
- Forni Ogna, V.; Ogna, A.; Haba-Rubio, J.; Nowak, G.; Venetz, J.-P.; Golshayan, D.; Matter, M.; Burnier, M.; Pascual, M.; Heinzer, R. Impact of kidney transplantation on sleep apnea severity: A prospective polysomnographic study. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Hayer, M.K.; Radhakrishnan, A.; Price, A.M.; Baig, S.; Liu, B.; Ferro, C.J.; Captur, G.; Townend, J.N.; Moon, J.C.; Edwards, J.C. Early effects of kidney transplantation on the heart–A cardiac magnetic resonance multi-parametric study. Int. J. Cardiol. 2019, 293, 272–277. [Google Scholar] [CrossRef]
- Krieger, J.; Sforza, E.; Delanoe, C.; Petiau, C. Decrease in haematocrit with continuous positive airway pressure treatment in obstructive sleep apnoea patients. Eur. Respir. J. 1992, 2, 228–233. [Google Scholar]
- Neale, J.; Smith, A.C. Cardiovascular risk factors following renal transplant. World J. Transplant. 2015, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Workeneh, B.; Moore, L.W.; Nolte Fong, J.V.; Shypailo, R.; Gaber, A.O.; Mitch, W.E. Successful Kidney Transplantation Is Associated With Weight Gain From Truncal Obesity and Insulin Resistance. J. Ren. Nutr. 2019, 29, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Bosch, J.A.; Jones, D.; McTernan, P.G.; Inston, N.; Moore, S.; Kaur, O.; Phillips, A.C.; Borrows, R. Hypervolemia and Blood Pressure in Prevalent Kidney Transplant Recipients. Transplantation 2014, 98, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.D.; Logan, A.G.; Kimoff, R.J.; Sériès, F.; Morrison, D.; Ferguson, K.; Belenki, I.; Pfifer, M.; Fleetham, J.; Hanly, P. Continuous positive airway pressure for central sleep apnea and heart failure. N. Engl. J. Med. 2005, 353, 2025–2033. [Google Scholar]
- Bia, M.; Adey, D.B.; Bloom, R.D.; Chan, L.; Kulkarni, S.; Tomlanovich, S. KDOQI US Commentary on the 2009 KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Kidney Dis. 2010, 56, 189–218. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D.; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999, 22, 667–689. [Google Scholar]
- Hanley, J.A.; Negassa, A.; Edwardes, M.D.; Forrester, J.E. Statistical Analysis of Correlated Data Using Generalized Estimating Equations: An Orientation. Am. J. Epidemiol. 2003, 157, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, M.C.K. An Index, Which Distinguishes between Some Important Exponential Families. In Statistics: Applications and New Directions—Proceedings of the Indian Statistical Institute Golden Jubilee International Conference; Ghosh, J.K., Roy, J., Eds.; Indian Statistical Institute: Calcutta, India, 1984; p. 579. [Google Scholar]
- Young, T.; Peppard, P.E.; Taheri, S. Physiology and Pathophysiology of Sleep Apnea Excess weight and sleep-disordered breathing Cross-Sectional Studies. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Baum, C.L.; Thielke, K.; Westin, E.; Kogan, E.; Cicalese, L.; Benedetti, E. Predictors of weight gain and cardiovascular risk in a cohort of racially diverse kidney transplant recipients. In Nutrition; 2002; Volume 18, pp. 139–146. [Google Scholar]
- Armstrong, K.A.; Campbell, S.B.; Hawley, C.M.; Johnson, D.W.; Isbel, N.M. Impact of obesity on renal transplant outcomes. Nephrology 2005, 10, 405–413. [Google Scholar] [CrossRef]
- Lafranca, J.A.; IJermans, J.N.; Betjes, M.G.; Dor, F.J. Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis. BMC Med. 2015, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular risk in renal transplant recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Stone, K.L.; Blackwell, T.; Ancoli Israel, S.; Dam, T.-T.L.; Stefanick, M.L.; Redline, S. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic fractures in men sleep study. J. Am. Geriatr. Soc. 2007, 55, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Wall, S.; Auguste, E.; Myers, A.K.; Stefanick, G.L.; McFarlane, S.I. Obstructive Sleep Apnea and Obesity: Implications for Public Health. Sleep Med. Disord. Intern. J. 2017, 1, 00019. [Google Scholar]
- Molnar, M.Z.; Lazar, A.S.; Lindner, A.; Fornadi, K.; Czira, M.E.; Dunai, A.; Zoller, R.; Szentikiraly, A.; Rosivall, L.; Shapiro, C.M.; et al. Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 125–132. [Google Scholar] [CrossRef]
- Beecroft, J.M.; Zaltzman, J.; Prasad, R.; Meliton, G.; Hanly, P.J. Impact of kidney transplantation on sleep apnoea in patients with end-stage renal disease. Nephrol. Dial. Transplant. 2007, 22, 3028–3033. [Google Scholar] [CrossRef]
- Rodrigues, C.J.O.; Marson, O.; Togeiro, S.M.G.P.; Tufik, S.; Ribeiro, A.B.; Tavares, A. Sleep-disordered breathing changes after kidney transplantation:a polysomnographic study. Nephrol. Dial. Transplant. 2010, 25, 2011–2015. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Sackett, D.L.; Sinclair, J.C.; Hayward, R.; Cook, D.J.; Cook, R.J. Users’ Guides to the Medical Literature. JAMA 1995, 274, 1800. [Google Scholar] [CrossRef]
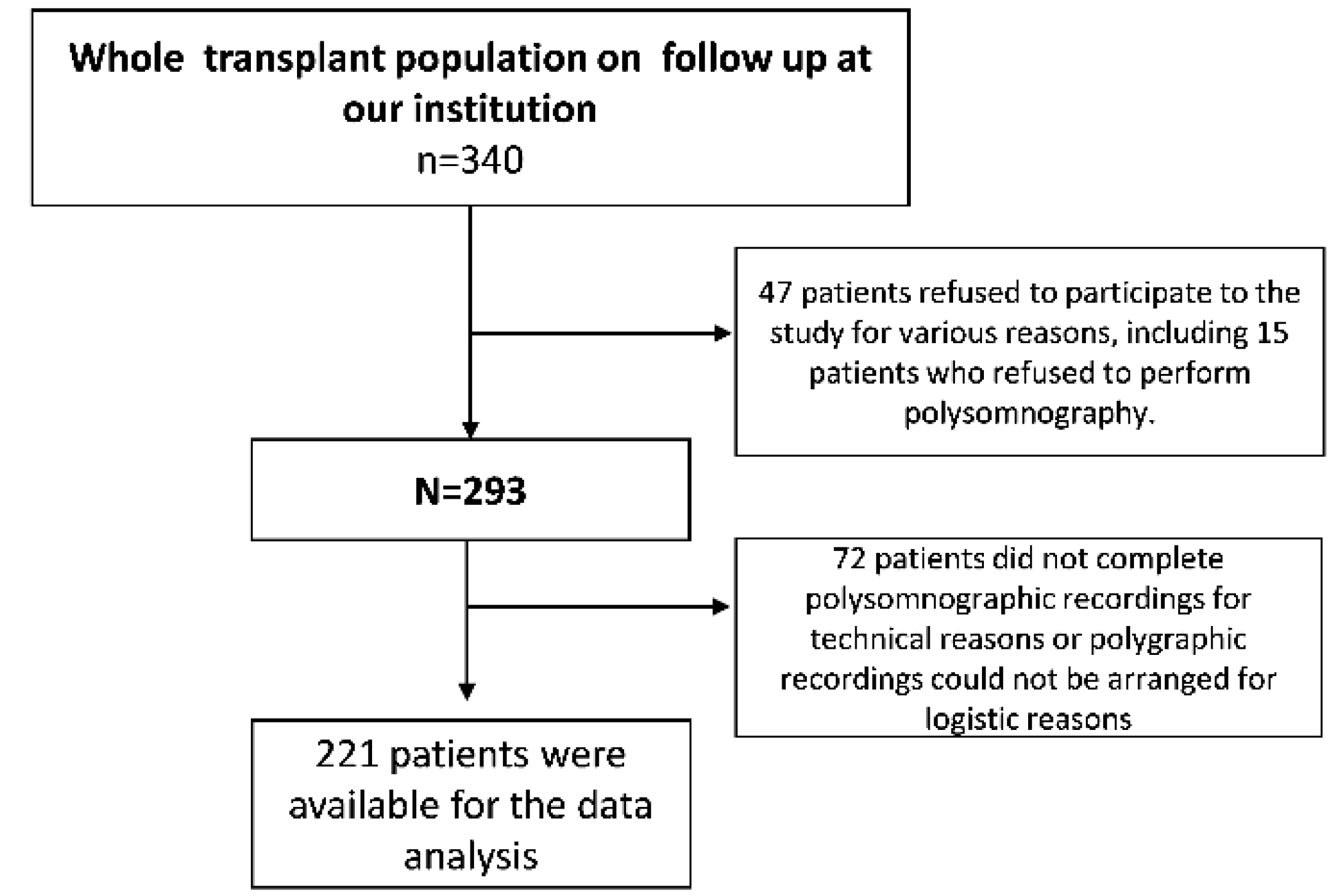
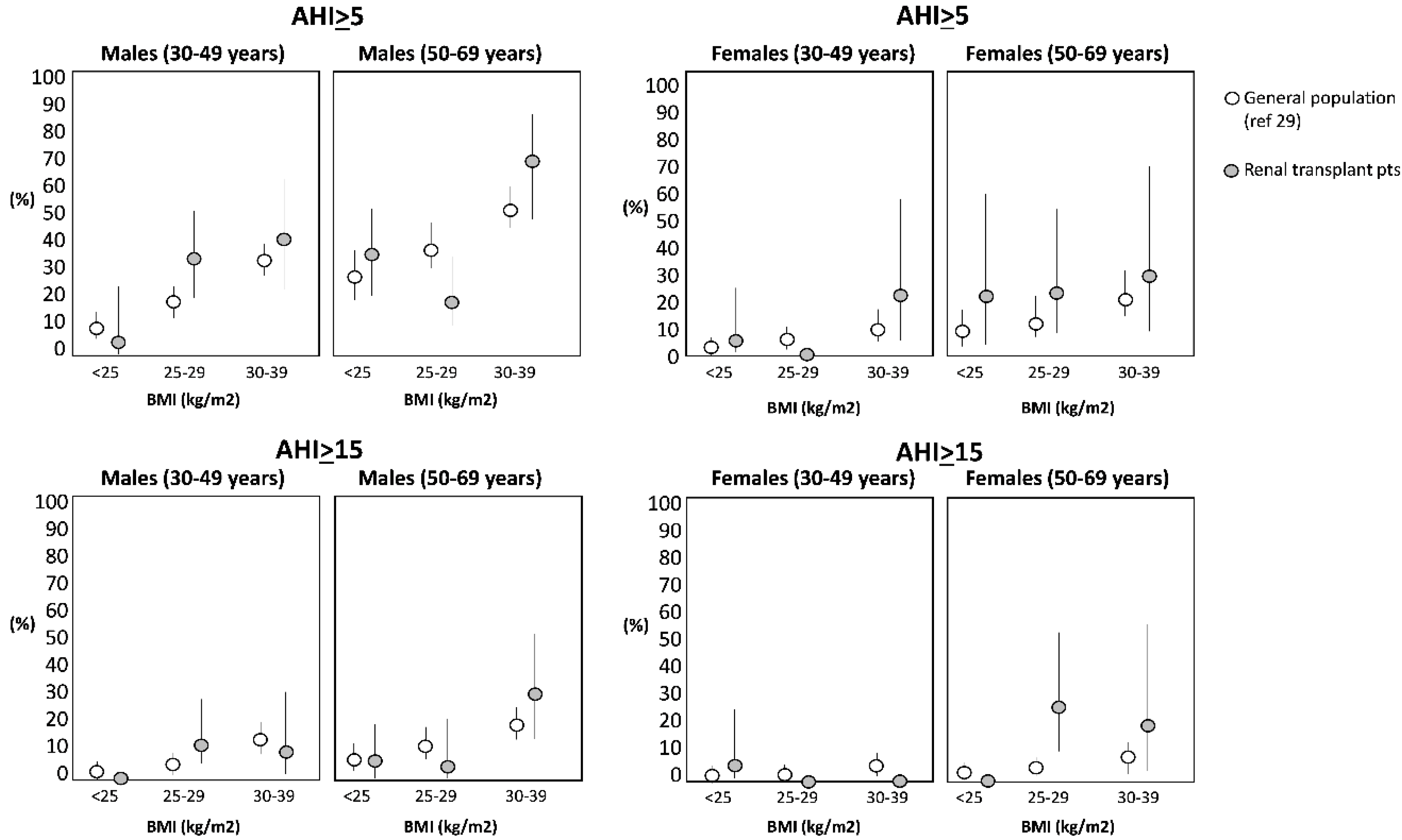

| Baseline Values | Baseline Apnea-Hypopnea Index (Episodes/h) | ||||
|---|---|---|---|---|---|
| Whole Group (n = 221) | <5.0 (n = 166) | from ≥5 to <15 (n = 37) | ≥15 (n = 18) | p (See also Statistical Methods) | |
| Age (years) | 47 ± 12 | 45 ± 12 | 51 ± 10 | 53 ± 9 | 0.001 * |
| Organ from living donors (%) | 12% | 11% | 19% | 6% | 0.99 ^ |
| Male sex (%) | 70% | 66% | 89% | 72% | 0.065 ^ |
| Active smokers (%) | 10% | 10% | 10% | 14% | 0.70 ^ |
| Past smokers (%) | 41% | 38% | 48% | 50% | 0.23 ^ |
| Diabetes (%) | 9.0% | 8% | 8% | 22.% | 0.097 ^ |
| Background CV complications (%) | 11% | 10% | 11% | 22% | 0.16 ^ |
| Systolic BP (mmHg) | 132 ± 16 | 132 ± 15 | 134 ± 15 | 136 ± 23 | 0.24 * |
| Diastolic BP (mmHg) | 78 ± 10 | 78 ± 10 | 78 ± 10 | 79 ± 9 | 0.69 * |
| Sodium (mEq/L) | 140.0 ± 4.7 | 139.9 ± 4.9 | 140.2 ± 4.9 | 140.7 ± 2.5 | 0.51 * |
| Potassium (mEq/L) | 4.2 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.5 | 4.4 ± 0.5 | 0.33 * |
| Cholesterol (mg/dL) | 180 ± 36 | 180 ± 37 | 177 ± 33 | 182 ± 37 | 0.96 * |
| HDL cholesterol (mg/dL) | 55 ± 16 | 55 ± 15 | 55 ± 20 | 51 ± 11 | 0.34 * |
| LDL cholesterol (mg/dL) | 100 ± 34 | 102 ± 36 | 92 ± 27 | 104 ± 33 | 0.64 * |
| BMI (kg/m2) | 25.9 ± 3.6 | 25.4 ± 3.3 | 27.2 ± 4.0 | 28.1 ± 4.0 | <0.001 * |
| Hemoglobin (g/dL) | 13.0 ± 1.6 | 12.9 ± 1.7 | 13.3 ± 1.4 | 12.6 ± 2.0 | 0.998 * |
| Albumin (g/dl) | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.3 | 0.18 * |
| Phosphate (mg/dl) | 3.3 ± 0.8 | 3.3 ± 0.8 | 3.2 ± 0.7 | 3.7 ± 0.8 | 0.16 * |
| PTH (pg/mL) | 67 (43–106) | 67 (41–100) | 64 (45–191) | 70 (49–115) | 0.72 # |
| hs-CRP (mg/L) | 1.5 (0.6–3.1) | 1.4 (0.6–2.9) | 1.2 (0.5–3.4) | 2.4 (1.3–7.2) | 0.58 # |
| eGFR-MDRD186 (mL/min/1.73 m2) | 56.1 ± 20 | 56 ± 21 | 56 ± 18 | 58 ± 21 | 0.10 * |
| Polygraphic Sleep Data | |||||
| Apnea-hypopnea index (episodes/h) | 1.8 (0.6–4.9) | 1.1 (0.5–2.2) | 7.5 (5.9–9.9) | 28.5 (19.5–51.2) | <0.001 # |
| ODI (number of O2 desaturation episodes/h) | 1.30 (0.30–4.45) | 0.70 (0.18–2.23) | 5.6 (2.5–8.6) | 18.6(11.0–43.7) | <0.001 # |
| Minimum O2 saturation, min SaO2 (%) | 89 (86–92) | 90 (88–93) | 86.3 (80–88) | 80.0 (70–88) | <0.001 # |
| Average O2 saturation, mean SaO2 (%) | 96 (94–96.) | 96 0(94–97) | 95 (93–96) | 94 (90–96) | <0.001 # |
| Apnea Hypopnea Index | Minimal O2 Saturation | |
|---|---|---|
| Variables (units of increase) | Regression coefficients (95% CI) | Regression coefficients (95% CI) |
| Age (5 years) | 1.105 (1.017–1.201), p = 0.019 | 0.996 (0.992–0.999), p = 0.02 |
| Male/females | 1.725 (1.014–2.935), p = 0.044 | 0.980 (0.963–0.997), p = 0.02 |
| Diabetes (yes/no) | 1.217 (0.483–3.069), p = 0.68 | 0.960 (0.904–1.025), p = 0.25 |
| * BMI (1 kg/m2) | 1.098 (1.037–1.164), p = 0.001 | 0.990 (0.980–0.996), p < 0.001 |
| * eGFR (1 mL/min/1.73 m2) | 1.001 (0.991–1.011), p = 0.90 | 1.000 (0.999–1.000), p = 0.92 |
| Visit (0,1,2) | 1.409 (1.170–1.697), p < 0.001 | 0.985 (0.972–0.998), p = 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamaci, F.; Tripepi, R.; D’Arrigo, G.; Porto, G.; Versace, M.C.; Marino, C.; Sanguedolce, M.C.; Tripepi, G.; Zoccali, C. Long-Term Changes in Sleep Disordered Breathing in Renal Transplant Patients: Relevance of the BMI. J. Clin. Med. 2020, 9, 1739. https://doi.org/10.3390/jcm9061739
Mallamaci F, Tripepi R, D’Arrigo G, Porto G, Versace MC, Marino C, Sanguedolce MC, Tripepi G, Zoccali C. Long-Term Changes in Sleep Disordered Breathing in Renal Transplant Patients: Relevance of the BMI. Journal of Clinical Medicine. 2020; 9(6):1739. https://doi.org/10.3390/jcm9061739
Chicago/Turabian StyleMallamaci, Francesca, Rocco Tripepi, Graziella D’Arrigo, Gaetana Porto, Maria Carmela Versace, Carmela Marino, Maria Cristina Sanguedolce, Giovanni Tripepi, and Carmine Zoccali. 2020. "Long-Term Changes in Sleep Disordered Breathing in Renal Transplant Patients: Relevance of the BMI" Journal of Clinical Medicine 9, no. 6: 1739. https://doi.org/10.3390/jcm9061739
APA StyleMallamaci, F., Tripepi, R., D’Arrigo, G., Porto, G., Versace, M. C., Marino, C., Sanguedolce, M. C., Tripepi, G., & Zoccali, C. (2020). Long-Term Changes in Sleep Disordered Breathing in Renal Transplant Patients: Relevance of the BMI. Journal of Clinical Medicine, 9(6), 1739. https://doi.org/10.3390/jcm9061739







