Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
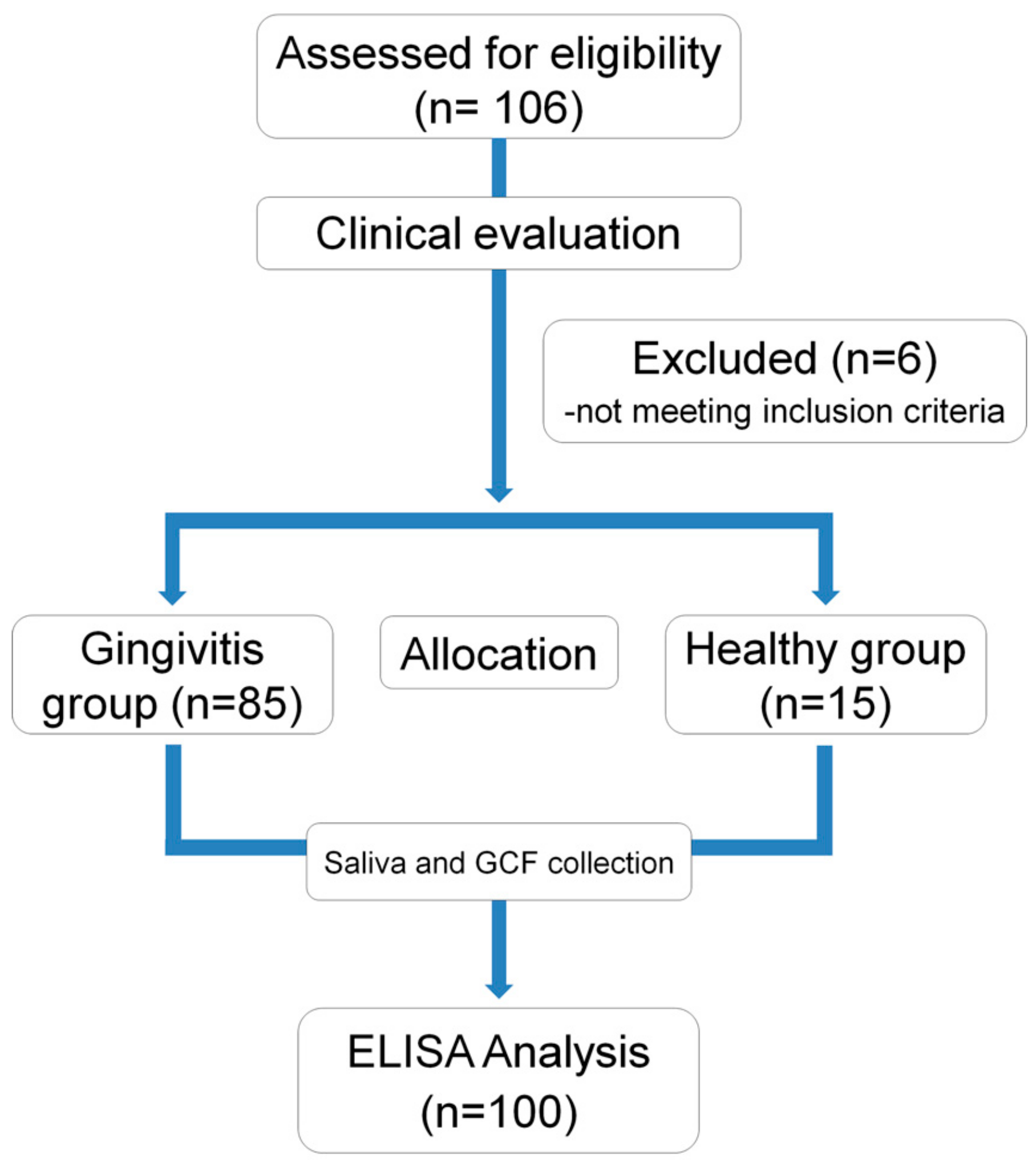
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Evaluation
2.4. GCF and Saliva Collection
2.5. Enzyme-Linked Immunosorbent Assay Analysis of Molecular Biomarkers
2.6. Statistical Analysis
3. Results
3.1. Demographic Analysis
3.2. Correlation Analyses
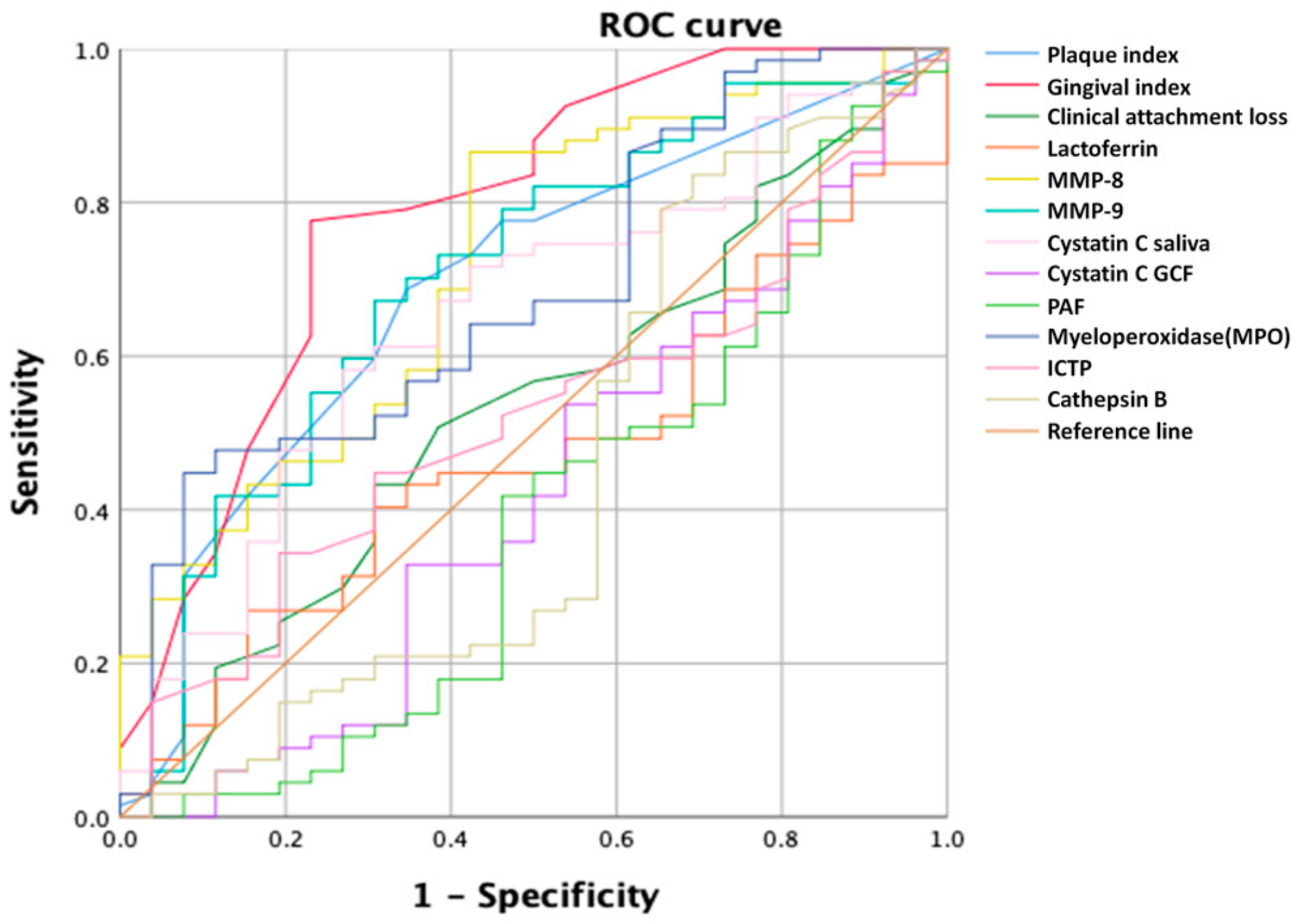
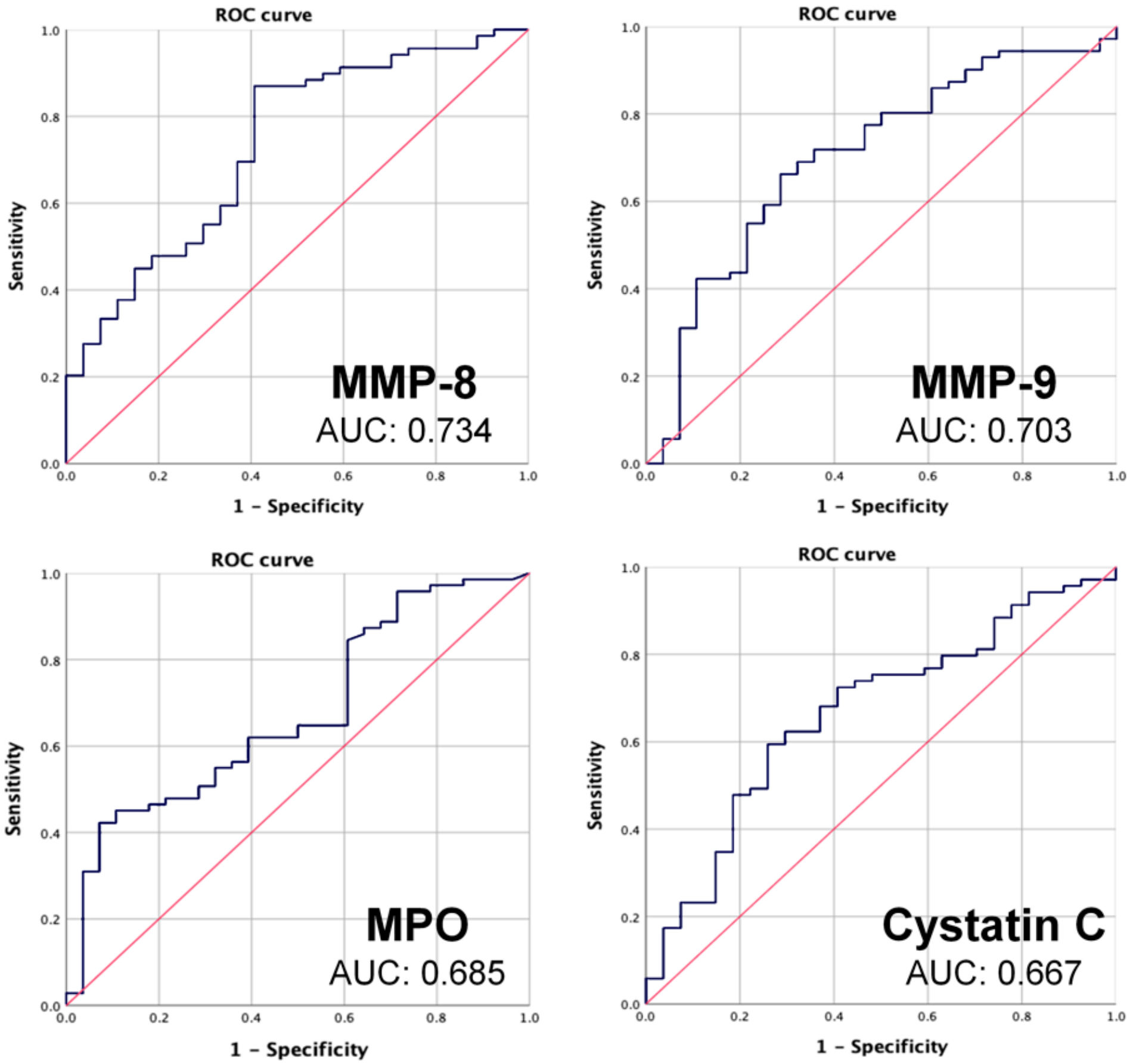
3.3. ROC Curves
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Ogawa, H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control. Periodontology 2000 2012, 60, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Clin. Periodontol. 2018, 45 Suppl. 20, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, U.; de Vries, J.H. Introduction of a new periodontal probe: The pressure probe. J. Clin. Periodontol. 1978, 5, 188–197. [Google Scholar] [CrossRef]
- Listgarten, M.A. Periodontal probing: What does it mean? J. Clin. Periodontol. 1980, 7, 165–176. [Google Scholar] [CrossRef]
- Armitage, G.C. The complete periodontal examination. Periodontology 2000 2004, 34, 22–33. [Google Scholar] [CrossRef]
- Barros, S.P.; Williams, R.; Offenbacher, S.; Morelli, T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontology 2000 2016, 70, 53–64. [Google Scholar] [CrossRef]
- Korte, D.L.; Kinney, J. Personalized medicine: An update of salivary biomarkers for periodontal diseases. Periodontology 2000 2016, 70, 26–37. [Google Scholar] [CrossRef]
- Ghallab, N.A. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: Review of the current evidence. Arch. Oral. Biol. 2018, 87, 115–124. [Google Scholar] [CrossRef]
- Miller, C.S.; King, C.P., Jr.; Langub, M.C.; Kryscio, R.J.; Thomas, M.V. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J. Am. Dent. Assoc. 2006, 137, 322–329. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Kononen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Beikler, T.; Kinney, J.S.; Ramseier, C.A.; Morelli, T.; Wong, D.T. Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontology 2000 2009, 50, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Leppilahti, J.M.; Hernandez-Rios, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernandez, M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Chen, H.; Shi, S.; Jepsen, S.; Eberhard, J. Periodontal parameters and platelet-activating factor levels in serum and gingival crevicular fluid in a Chinese population. J. Clin. Periodontol. 2006, 33, 797–802. [Google Scholar] [CrossRef]
- De Morais, E.F.; Pinheiro, J.C.; Leite, R.B.; Santos, P.P.A.; Barboza, C.A.G.; Freitas, R.A. Matrix metalloproteinase-8 levels in periodontal disease patients: A systematic review. J. Periodontal. Res. 2018, 53, 156–163. [Google Scholar] [CrossRef]
- Pepe, M.S. An interpretation for the ROC curve and inference using GLM procedures. Biometrics 2000, 56, 352–359. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of victamine C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef]
- Kraft-Neumarker, M.; Lorenz, K.; Koch, R.; Hoffmann, T.; Mantyla, P.; Sorsa, T.; Netuschil, L. Full-mouth profile of active MMP-8 in periodontitis patients. J. Periodontal. Res. 2012, 47, 121–128. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Nagarajan, R.; Akers, D.; Miller, C.S. Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front. Cell. Infect. Microbiol 2015, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Blicher, B.; Joshipura, K.; Eke, P. Validation of self-reported periodontal disease: A systematic review. J. Dent. Res. 2005, 84, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.S.; Ramseier, C.A.; Giannobile, W.V. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann. N. Y. Acad. Sci. 2007, 1098, 230–251. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Gupta, N.; Gupta, N.D.; Gupta, A.; Khan, S.; Bansal, N. Role of salivary matrix metalloproteinase-8 (MMP-8) in chronic periodontitis diagnosis. Front. Med. 2015, 9, 72–76. [Google Scholar] [CrossRef]
- Kinney, J.S.; Morelli, T.; Braun, T.; Ramseier, C.A.; Herr, A.E.; Sugai, J.V.; Shelburne, C.E.; Rayburn, L.A.; Singh, A.K.; Giannobile, W.V. Saliva/pathogen biomarker signatures and periodontal disease progression. J. Dent. Res. 2011, 90, 752–758. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Hernandez, M.; Dutzan, N.; Garcia-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; Gonzalez, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.R.; Boon, N.; Bernaerts, K.; Slomka, V.; Verspecht, T.; Quirynen, M.; Teughels, W. Clinical concentrations of peroxidases cause dysbiosis in in vitro oral biofilms. J. Periodontal Res. 2018, 53, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Korostoff, J.M. Revisiting the Page & Schroeder model: The good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol 2000 2017, 75, 116–151. [Google Scholar] [CrossRef] [PubMed]
- Lalkhen, A.G.; McCluskey, A. Clinical tests: Sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 221–223. [Google Scholar] [CrossRef]
- Dawson, D.R., III; Branch-Mays, G.; Gonzalez, O.A.; Ebersole, J.L.J.P. Dietary modulation of the inflammatory cascade. Periodontology 2000 2014, 64, 161–197. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lee, J.S.; Choi, S.H.; Shin, H.S.; Park, J.C.; Shin, S.I.; Chung, J.H. A randomized, double-blind, placebo-controlled multicenter study for evaluating the effects of fixed-dose combinations of vitamin C, vitamin E, lysozyme, and carbazochrome on gingival inflammation in chronic periodontitis patients. BMC Oral Health 2019, 19, 40. [Google Scholar] [CrossRef]
- Singh, N.; Chander Narula, S.; Kumar Sharma, R.; Tewari, S.; Kumar Sehgal, P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: A randomized clinical trial. J. Periodontol. 2014, 85, 242–249. [Google Scholar] [CrossRef]
- Wang, S.F.; Leknes, K.N.; Zimmerman, G.J.; Sigurdsson, T.J.; Wikesjo, U.M.; Selvig, K.A. Intra- and inter-examiner reproducibility in constant force probing. J. Clin. Periodontol. 1995, 22, 918–922. [Google Scholar] [CrossRef]
- Grossi, S.G.; Dunford, R.G.; Ho, A.; Koch, G.; Machtei, E.E.; Genco, R.J. Sources of error for periodontal probing measurements. J. Periodontal. Res. 1996, 31, 330–336. [Google Scholar] [CrossRef]
| Healthy Group (n = 15) | Gingivitis Group (n = 85) | p-Value | |
|---|---|---|---|
| Age (years) | 34.93 ± 15.79 | 32.65 ± 12.21 | 0.85 |
| Female/Male (n) | 7/8 | 47/38 | 0.54 |
| BOP site (%) | 5.56 ± 0.89 | 26.96 ± 4.70 * | <0.001 |
| CAL (mm) | 2.55 ± 0.30 | 2.60 ± 0.27 | 0.71 |
| PI | 0.13 ± 0.22 | 0.53 ± 0.39 * | <0.001 |
| GI | 0.39 ± 0.35 | 0.96 ± 0.37 * | <0.001 |
| Coefficient of Correlation | p-Value | |
|---|---|---|
| Average plaque index (PI) | 0.411 ** | <0.001 |
| Average gingival index (GI) | 0.766 ** | <0.001 |
| Clinical attachment loss (CAL) | 0.096 | 0.346 |
| MPO | 0.399 ** | <0.001 |
| MMP-8 | 0.217 * | 0.034 |
| MMP-9 | 0.032 | 0.750 |
| Cystatin C | 0.055 | 0.595 |
| Lactoferrine | 0.137 | 0.183 |
| PAF | 0.034 | 0.742 |
| Cathepsin B | -0.093 | 0.366 |
| ICTP | -0.004 | 0.966 |
| AUC | Cut-Off Value (ng/mL) | Sensitivity | Specificity | |
|---|---|---|---|---|
| MMP-8 | 0.734 | 6.464 | 0.87 | 0.60 |
| MMP-9 | 0.703 | 38.075 | 0.739 | 0.63 |
| MPO | 0.685 | 12.75 | 0.87 | 0.42 |
| Cystatin C | 0.667 | 59.28 | 0.725 | 0.59 |
| AUC | |
|---|---|
| Gingival index | 0.788 |
| Plaque index | 0.692 |
| Clinical attachment loss | 0.532 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, I.; Pae, H.-C.; Song, Y.W.; Cha, J.-K.; Lee, J.-S.; Paik, J.-W.; Choi, S.-H. Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 1720. https://doi.org/10.3390/jcm9061720
Hong I, Pae H-C, Song YW, Cha J-K, Lee J-S, Paik J-W, Choi S-H. Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(6):1720. https://doi.org/10.3390/jcm9061720
Chicago/Turabian StyleHong, Inpyo, Hyung-Chul Pae, Young Woo Song, Jae-Kook Cha, Jung-Seok Lee, Jeong-Won Paik, and Seong-Ho Choi. 2020. "Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 6: 1720. https://doi.org/10.3390/jcm9061720
APA StyleHong, I., Pae, H.-C., Song, Y. W., Cha, J.-K., Lee, J.-S., Paik, J.-W., & Choi, S.-H. (2020). Oral Fluid Biomarkers for Diagnosing Gingivitis in Human: A Cross-Sectional Study. Journal of Clinical Medicine, 9(6), 1720. https://doi.org/10.3390/jcm9061720





