Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients?
Abstract
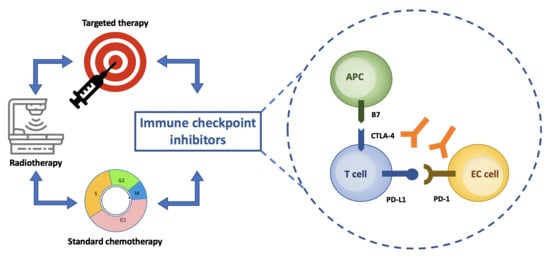
1. Introduction
2. Background
3. Clinical Evidence
3.1. Anti-PD-1: Pembrolizumab
3.2. Anti-PD-1: Nivolumab
3.3. Anti-PD-1: Dostarlimab
3.4. Anti-PD-L1: Atezolizumab
3.5. Anti-PD-L1: Avelumab
3.6. Anti-PD-L1: Durvalumab
4. Main Ongoing Trials
5. Toxicities
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Available online: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html (accessed on 20 February 2020).
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2017, 27, 16–41. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Endometrial cancer treatment Physician Data Query (PDQ). 2019. Available online: http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/healthprofessional (accessed on 20 February 2020).
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 20 February 2020).
- Ventriglia, J.; Paciolla, I.; Pisano, C.; Cecere, C.S.; Di Napoli, M.; Tambaro, R.; Califano, D.; Losito, S.; Scognamiglio, G.; Setola, S.V.; et al. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat. Rev. 2017, 108, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Kratky, W.; Reis e Sousa, C.; Oxenius, A.; Spörri, R. Direct activation of antigen-presenting cells is required for CD8+ T-cell priming and tumor vaccination. Proc. Natl. Acad. Sci. USA 2011, 108, 17414–17419. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Cresswell, P. Antigen Processing and Presentation Mechanisms in Myeloid Cells. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Sharpe, A.H. Mechanisms of costimulation. Immunol. Rev. 2016, 229, 5–11. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Attar, R.; Palaia, I.; Perniola, G.; Marchetti, C.; Di Donato, V.; Farooqui, A.A.; Papadia, A.; Benedetti Panici, P. Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3635–3638. [Google Scholar] [CrossRef]
- Vanderstraeten, A.; Tuyaerts, S.; Amant, F. The immune system in the normal endometrium and implications for endometrial cancer development. J. Reprod. Immunol. 2015, 109, 7–16. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell costimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Di Tucci, C.; Capone, C.; Galati, G.; Iacobelli, V.; Schiavi, M.C.; Di Donato, V.; Muzii, L.; Benedetti Panici, P. Immunotherapy in endometrial cancer: New scenarios on the horizon. J. Gynecol Oncol. 2019, 30, e46. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Lax, S.F. Molecular genetic pathways in various types of endometrial carcinoma: From a phenotypical to a molecular based classification. Virchows. Arch. 2004, 444, 213–223. [Google Scholar] [CrossRef]
- Wilczyński, M.; Danielska, J.; Wilczyński, J. An update of the classical Bokhman’s dualistic model of endometrial cancer. Prz. Menopauzalny. 2016, 15, 63–68. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [PubMed]
- McConechy, M.K.; Talhouk, A.; Leung, S.; Chiu, D.; Yang, W.; Senz, J.; Reha-Krantz, L.J.; Lee, C.-H.; Huntsman, D.G.; Gilks, C.B.; et al. Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin. Cancer Res. 2016, 22, 2865–2873. [Google Scholar] [CrossRef]
- Billingsley, C.C.; Cohn, D.E.; Mutch, D.G.; Hade, E.M.; Goodfellow, P.J. Prognostic significance of POLE exonuclease domain mutations in high-grade endometrioid endometrial cancer on survival and recurrence. Int. J. Gynecol. Cancer 2016, 26, 933–938. [Google Scholar] [CrossRef]
- Meng, B.; Hoang, L.N.; McIntyre, J.B.; Duggan, M.A.; Nelson, G.S.; Lee, C.H.; Köbel, M. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol. Oncol. 2014, 134, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Puzzoni, M.; Silvestris, N.; Leone, F.; Giampieri, R.; Faloppi, L.; Demurtas, L.; Dell’Aquila, E.; Marino, D.; Brunetti, O.; Garattini, S.K.; et al. The Immune Revolution in Gastrointestinal Tumours: Leading the Way or Just Following? Targ. Oncol. 2016, 11, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Aglietta, M.; Genta, S.; Valabrega, G. Checkpoint inhibitors in endometrial cancer: Preclinical rationale and clinical activity. Oncotarget 2017, 8, 90532–90544. [Google Scholar] [CrossRef]
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Bellone, S.; Centritto, F.; Black, J.; Schwab, C.; English, D.; Cocco, E.; Lopez, S.; Bonazzoli, E.; Predolini, F.; Ferrari, F.; et al. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol. Oncol. 2015, 138, 11–17. [Google Scholar] [CrossRef]
- Di Donato, V.; Iacobelli, V.; Schiavi, M.C.; Colagiovanni, V.; Pecorella, I.; Palaia, I.; Perniola, G.; Marchetti, C.; Musella, A.; Tomao, F.; et al. Impact of Hormone Receptor Status and Ki-67 Expression on Disease-Free Survival in Patients Affected by High-risk Endometrial Cancer. Int. J. Gynecol Cancer 2018, 28, 505–513. [Google Scholar] [CrossRef]
- Gatalica, Z.; Vranic, S.; Xiu, J.; Swensen, J.; Reddy, S. High microsatellite instability (MSI-H) colorectal carcinoma: A brief review of predictive biomarkers in the era of personalized medicine. Fam. Cancer 2016, 15, 405–412. [Google Scholar] [CrossRef]
- Inaguma, S.; Lasota, J.; Wang, Z.; Felisiak-Golabek, A.; Ikeda, H.; Miettinen, M. Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod. Pathol. 2017, 30, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.H.; McAlpine, J.N. The more tumors change, the more they stay tame: Do T cells keep POLE ultramutated endometrial carcinomas in check? Gynecol. Oncol. 2015, 138, 1–2. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Nakayama, K.; Ishikawa, M.; Nakamura, K.; Ishibashi, T.; Sanuki, K.; Ono, R.; Sasamori, H.; Minamoto, T.; Iida, K.; et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2018, 9, 5652–5664. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Panda, A.; Zhong, H.; Hirshfield, K.; Damare, S.; Lane, K.; Sokol, L.; Stein, M.N.; Rodriguez-Rodriquez, L.; Kaufman, H.L. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J. Clin. Investig. 2016, 126, 2334–2340. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1-Positive Endometrial Cancer: Results From the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 2535–2541. [Google Scholar] [CrossRef]
- Makker, V.; Rasco, D.; Vogelzang, N.J.; Brose, M.S.; Cohn, A.L.; Mier, J.; Di Simone, C.; Hyman, D.M.; Stepan, D.E.; Dutcus, C.E.; et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 711–718. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Buza, N.; Choi, J.; Schwartz, P.E.; Schlessinger, J.; Lifton, R.P. Regression of Chemotherapy-Resistant Polymerase ε (POLE) Ultra-Mutated and MSH6 Hyper-Mutated Endometrial Tumors with Nivolumab. Clin. Cancer Res. 2016, 22, 5682–5687. [Google Scholar] [CrossRef]
- Oaknin ADuska, L.R.; Sullivan, R.J.; Pothuri, B.; Ellard, S.L.; Leath, C.A., III; Moreno, V.; Kristeleit, R.S.; Guo, W.; Danaee, H. Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol. Oncol. 2019, 154, 17. [Google Scholar] [CrossRef]
- Fleming, G.F.; Emens, L.A.; Eder, J.P.; Hamilton, E.P.; Liu, J.F.; Liu, B.; Molinero, L.; Fasso, M.; O’Hear, C.; Braiteh, F.S. Clinical activity, sefety and biomarker results from a phase Ia study of atezolizumab (atezo)in advanced/recurrent endometrial cancer(rEC). J. Clin. Oncol. 2017, 35, 5585. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Luo, W.; Liu, J.F.; Gulhan, D.C.; Krasner, C.; Ishizuka, J.J.; Gockley, A.A.; Buss, M.; Growdon, W.B.; Crowe, H. Phase II Study of Avelumab in Patients with Mismatch Repair Deficient and Mismatch Repair Proficient Recurrent/Persistent Endometrial Cancer. J. Clin. Oncol. 2019, 37, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Antill, Y.C.; Kok, P.S.; Robledo, K.; Barnes, E.; Friedlander, M.; Baron-Hay, S.E.; Shannon, C.M.; Coward, J.; Beale, P.J.; Goss, G.; et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). Presented at ASCO 2019. J. Clin. Oncol. 2019, 37, 5501. [Google Scholar] [CrossRef]
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03572478 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02982486 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03241745 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02549209 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03276013 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03517449 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03914612 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03884101 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03503786 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04269200 (accessed on 20 February 2020).
- Clinicaltrial.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03694262 (accessed on 20 February 2020).
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- De Felice, F.; Marchetti, C.; Palaia, I.; Musio, D.; Muzii, L.; Tombolini, V.; Benedetti Panici, P. Immunotherapy of ovarian cancer: The role of checkpoint inhibitors. J. Immunol. Res. 2015, 2015, 191832. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 378, 158–168. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellman, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: Hereditary or Sporadic Origin Matters. Clin. Cancer Res. 2017, 23, 4473–4481. [CrossRef] [PubMed]
- Ramchander, N.C.; Ryan, N.A.J.; Walker, T.D.J.; Harries, L.; Bolton, J.; Bosse, T.; Evans, D.G.; Crosbie, E.J. Distinct Immunological Landscapes Characterize Inherited and Sporadic Mismatch Repair Deficient Endometrial Cancer. Front. Immunol. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Ryan, N.A.; Arends, M.J.; Bosse, T.; Burn, J.; Cornes, J.M.; Crawford, R.; Eccles, D.; Frayling, I.M.; Ghaem-Maghami, S.; et al. The Manchester International Consensus Group recommendations for the management of gynecological cancers in Lynch syndrome. Genet. Med. 2019, 21, 2390–2400. [Google Scholar] [CrossRef]
- Jiao, S.; Xia, W.; Yamaguchi, H.; Wei, Y.; Chen, M.K.; Hsu, J.M.; Hsu, J.L.; Yu, W.H.; Du, Y.; Lee, H.H.; et al. PARP Inhibitor Upregulates PD-L1 Expression and Enhances Cancer-Associated Immunosuppression. Clin. Cancer Res. 2017, 23, 3711–3720. [Google Scholar] [CrossRef]
| Authors, Years | Phase | Patient Population | Treatment | Findings |
|---|---|---|---|---|
| Le et al., 2015 | II | 41 pts with metastatic cancer with or without MMRd, including EC | Pembrolizumab 10 mg/kg IV every 14 days | MMRd CRC ORR: 40% PFS12: 78% MMRp CRC ORR: 0% PFS12: 11% MMRd non-CRC ORR: 71% PFS12: 67% |
| Ott et al., 2017 | Ib | 24 pts with PD-L1 positive locally advanced or metastatic EC | Pembrolizumab 10 mg/kg IV every 2 weeks up to 24 months | ORR: 13% PFS: 1.8 months |
| Fleming et al., 2017 | Ia | 15 pts with advanced or recurrent EC | Atezolizumab 1200 mg or 15 mg/kg IV every 3 weeks | ORR: 13% PFS: 1.7 months OS: 9.6 months |
| Makker et al., 2019 | II | 108 pts with advanced or recurrent EC | Pembrolizumab 200 mg IV every 3 weeks plus Lenvatinib 20 mg orally daily | ORR (24 weeks): 38.0% DOR: 21.2 months PFS: 7.4 months OS: 16.7 months |
| Kostantinopoulos, 2019 | II | 33 pts with MMRd/MMRp recurrent or persistent EC | Avelumab 10 mg/kg IV every 2 weeks | MMRd cohort ORR: 26.7% PFS6: 40% *MMRp cohort was closed at the first stage because of futility |
| Marabelle et al., 2020 (Keynote-158) | II | 233 pts with MSI/MMRd advanced non-CRC | Pembrolizumab 200 mg IV every 3 weeks | ORR: 34.3% PFS: 4.1 months OS: 23.5 months |
| Antill, 2017 (PHAEDRA study) | II | 71 pts with advanced or unresectable MMRd/MMRp EC | Durvalumab 1500 mg IV every 28 days until disease progression or prohibitive toxicity | MMRd OTR: 40% MMRp OTR: 3% |
| Oaknin, 2019 (GARNET study) | II | 110 pts with recurrent or advanced MSI/MSS EC | Dostarlimab 500 mg IV every 3 weeks | MSI cohort ORR: 50% MSS cohort ORR: 19.1 |
| DRUGS | Description/Condition | Setting | Primary Endpoint | Phase | Status | Trial Identifier |
|---|---|---|---|---|---|---|
| Nivolumab | A Phase Ib/IIa Study of Rucaparib (PARP Inhibitor) Combined with Nivolumab in Metastatic Castrate—Resistant Prostate Cancer and Advanced/Recurrent Endometrial Cancer | Advanced/Recurrent EC | DLT | I/II | Recruiting | NCT03572478 |
| Nivolumab | A Phase II Trial of IDO-Inhibitor, BMS-986205, and PD-1 Inhibitor, Nivolumab, in Patients with Recurrent or Persistent Endometrial Cancer or Endometrial Carcinosarcomas (CA017-056) | Recurrent/Persistent EC | ORR | II | Recruiting | NCT04106414 |
| Nivolumab + Ipilimumab | A Phase II Single Arm Study Assessing Efficacy and Safety of Nivolumab Plus Ipilimumab in Nonresectable/Metastatic Sarcoma and Endometrial Carcinoma Patients with Somatic Deficient MMR as a Selection Tool | EC with Somatic MMRd | ORR | II | Not yet Recruiting | NCT02982486 |
| Nivolumab | Phase II Trial of Single-Agent Nivolumab in Patients with Microsatellite Unstable/Mismatch Repair Deficient/Hypermutated Uterine Cancer | Metastatic/Recurrent EC | PFS | II | Recruiting | NCT03241745 |
| Nivolumab +/− Ipilimumab | Phase Ib Clinical Investigation of Intraperitoneal Ipilimumab and Nivolumab in Patients with Peritoneal Carcinomatosis Due to Gynecologic Cancers | Recurrent EC | MTD | I | Recruiting | NCT03508570 |
| Nivolumab | A Phase 1a/1b Study of COM701 as Monotherapy and in Combination with an Anti-PD-1 Antibody in Subjects with Advanced Solid Tumors | Advanced or Metastatic Solid Tumor included EC | MTD, DLT, AE | I | Recruiting | NCT03667716 |
| Nivolumab | Targeted Therapy Directed by Genetic Testing in Treating Patients with Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma (The MATCH Screening Trial) | Advanced or Metastatic Solid Tumor included EC | ORR | II | Recruiting | NCT02465060 |
| Pembrolizumab | A Phase 2, Two-Stage Study of Mirvetuximab Soravtansine (IMGN853) in Combination with Pembrolizumab in Patients with Microsatellite Stable (MSS) Recurrent or Persistent Endometrial Cancer (EC) | Advanced/Recurrent EC | ORR, PFS | II | Recruiting | NCT03835819 |
| Pembrolizumab | A Phase II Evaluation of Pembrolizumab, a Humanized Antibody Against PD-1, in the Treatment of Persistent or Recurrent Hypermutated/Ultramutated Endometrial Cancer Identified by Next Generation Sequencing (NGS) and Comprehensive Genomic Profiling (CGP) | Persistent/Recurrent EC | ORR, AE | II | Recruiting | NCT02899793 |
| Pembrolizumab | A Phase Ib Trial of Vaginal Cuff Brachytherapy + Pembrolizumab (MK3475) Followed by 3 Cycles of Dose Dense Paclitaxel/q 21 Day Carboplatin + Pembrolizumab (MK3475) in High Intermediate Risk Endometrial Cancer | High/Intermediate Risk EC | Proportion of patients completing three cycles | I | Not yet Recruiting | NCT03932409 |
| Pembrolizumab | Pembrolizumab with Axitinib in Recurrent Endometrial Cancer with Deficient Mismatch Repair System Post PD1 Exposure: Phase II Trial | Recurrent EC with MMRd | ORR | II | Not yet recruiting | NCT04197219 |
| Pembrolizumab | Phase II Study of Pembrolizumab in Combination with Carboplatin and Paclitaxel for Advanced or Recurrent Endometrial Adenocarcinoma | Advanced or Recurrent EC | ORR | II | Recruiting | NCT02549209 |
| Pembrolizumab | Study of Pembrolizumab Combined with Ataluren in Patients with Metastatic pMMR and dMMR Colorectal Adenocarcinomas or Metastatic dMMR Endometrial Carcinoma: the ATAPEMBRO Study | Metastatic EC MMRd | AE | I/II | Recruiting | NCT04014530 |
| Pembrolizumab | Immunotherapy with MK-3475 in Surgically Resectable Endometrial Carcinoma | Advanced EC | AE | I | Active, not recruiting | NCT02630823 |
| Pembrolizumab | Phase II Trial of Pembrolizumab in Combination with Doxorubicin in Advanced, Recurrent or Metastatic Endometrial Cancer (TOPIC) | Advanced or Metastatic EC | PFS | II | Active, not recruiting | NCT03276013 |
| Pembrolizumab | A Multicenter, Open-label, Randomized, Phase 3 Trial to Compare the Efficacy and Safety of Lenvatinib in Combination with Pembrolizumab Versus Treatment of Physician’s Choice in Participants with Advanced Endometrial Cancer | Advanced EC | PFS, OS | III | Active, not recruiting | NCT03517449 |
| Pembrolizumab | A Phase III Randomized, Placebo-Controlled Study of Pembrolizumab (MK-3475, NSC #776864) in Addition to Paclitaxel and Carboplatin for Measurable Stage III or IVA, Stage IVB, or Recurrent Endometrial Cancer | Advanced or Recurrent EC | PFS | III | Recruiting | NCT03914612 |
| Pembrolizumab | A Phase III Randomized Trial of Radiation +/− MK-3475 (Pembrolizumab) for Newly Diagnosed, High Intermediate Risk Mismatch Repair Deficient (dMMR) Endometrioid Endometrial Cancer | Early stage EC | 3 years RFS | III | Not yet recruiting | NCT04214067 |
| Pembrolizumab | A Phase 3 Randomized, Open-Label, Study of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for First-Line Treatment of Advanced or Recurrent Endometrial Carcinoma (LEAP-001) | Advanced or Recurrent EC | PFS, OS | III | Recruiting | NCT03884101 |
| Pembrolizumab | A Phase II Investigation of Pembrolizumab (Keytruda) in Combination with Radiation and an Immune Modulatory Cocktail in Patients with Cervical and Uterine Cancer (PRIMMO Trial) | Advanced or Refractory EC | ORR | II | Recruiting | NCT03192059 |
| Pembrolizumab | A Pilot Study Investigating the Effect of Pembrolizumab on the Tumoral Immunoprofile of Gynecologic Cancers of Mullerian Origin | EC any stages | Change in tumor Immune Infiltrates | Early phase I | Active, not recruiting | NCT02728830 |
| Pembrolizumab | A Phase 1a/1b Study of FPA150, an Anti-B7-H4 Antibody, in Patients with Advanced Solid Tumors | Advanced EC | MTD | Early phase I | Active, not recruiting | NCT03514121 |
| Pembrolizumab | A Platform Study Exploring the Safety, Tolerability, Effect on the Tumor Microenvironment, and Efficacy of Pembrolizumab + INCB Combinations in Advanced Solid Tumors | Advanced or Metastatic EC | AE | I | Active, not recruiting | NCT02646748 |
| Pembrolizumab | A Phase 1/1b Multicenter Study to Evaluate the Humanized Anti-cd73 Antibody, cpi-006, as a Single Agent or in Combination with Ciforadenant, with Pembrolizumab, and with Ciforadenant plus Pembrolizumab in Adult Subjects with Advanced Cancers | Advanced EC | DLT, AE, MDL | I | Recruiting | NCT03454451 |
| Pembrolizumab | A Phase 1/2 Study Exploring the Safety, Tolerability, and Efficacy of MK-3475 in Combination with INCB024360 in Subjects with Selected Cancers (ECHO-202/KEYNOTE-037) | Advanced or Metastatic EC | AE, ORR | I/II | Active, not recruiting | NCT02178722 |
| Pembrolizumab | A Clinical Trial of Pembrolizumab (MK-3475) Evaluating Predictive Biomarkers in Subjects with Advanced Solid Tumors (KEYNOTE 158) | Advanced EC | ORR | II | Recruiting | NCT02628067 |
| Pembrolizumab | A Phase 1/2 Safety and Efficacy Study of INCAGN01876 in Combination with Immune Therapies in Subjects with Advanced or Metastatic Malignancies | Advanced or Metastatic EC | AE, ORR, CRR | I/II | Active, not recruiting | NCT03277352 |
| Pembrolizumab | An Open-Label Phase 1b Trial of Lenvatinib Plus Pembrolizumab in Subjects with Selected Solid Tumors | Refractory EC | AE, DLT | I | Active, not recruiting | NCT03006887 |
| Pembrolizumab | A Phase 1A/B Study to Evaluate the Safety and Tolerability of ETC-1922159 in Advanced Solid Tumours | Advanced, Metastatic or Refractory EC | MTD, AE | I | Active, not recruiting | NCT02521844 |
| Avelumab | A Phase 2, Two-Group, Two-Stage, Open-Label Study of Avelumab (MSB0010718C) in Patients with MSS, MSI-H, and POLE-mutated Recurrent or Persistent Endometrial Cancer and of Avelumab (MSB0010718C)/Talazoparib (MDV3800, BMN 673) in Patients with MSS Recurrent or Persistent Endometrial Cancer | Recurrent or Metastatic EC | PFR6 | II | Recruiting | NCT02912572 |
| Avelumab | MITO END-3: A Randomized Phase II Trial of Carboplatin + Paclitaxel Compared to Carboplatin + Paclitaxel + Avelumab in Advanced (Stages III–IV) or Recurrent Endometrial Cancer | Advanced or Recurrent EC | PFS | II | Not yet recruiting | NCT03503786 |
| Durvalumab | Durvalumab and Olaparib in Metastatic or Recurrent Endometrial Cancer | Advanced or Metastatic EC | PFS | II | Recruiting | NCT03951415 |
| Durvalumab | A Phase 2 Trial of Durvalumab (MEDI4736) (Anti-PD-L1 Antibody) with or without Tremelimumab (Anti-CTLA-4 Antibody) in Patients with Persistent or Recurrent Endometrial Carcinoma and Endometrial Carcinosarcoma | Recurrent or Persistent EC | ORR | II | Recruiting | NCT03015129 |
| Durvalumab | A Randomised, Multicentre, Double-blind, Placebo-controlled, Phase III Study of First-Line Carboplatin and Paclitaxel in Combination with Durvalumab, Followed by Maintenance Durvalumab with or without Olaparib in Patients with Newly Diagnosed Advanced or Recurrent Endometrial Cancer (DUO-E) | Advanced or Recurrent EC | PFS | III | Not yet recruiting | NCT04269200 |
| Durvalumab | A Phase 1 Study of Durvalumab, Tremelimumab, and Radiotherapy in Recurrent Gynecologic Cancer | Metastatic or Unresectable EC | MTD | I | Recruiting | NCT03277482 |
| Durvalumab | Pilot Study of Durvalumab (MEDI4736) in Combination with Vigil in Advanced Women’s Cancers | Advanced EC | AE | II | Not yet recruiting | NCT02725489 |
| Durvalumab | Phase 1B, Open-Label, Dose Escalation, and Cohort Expansions Trial of Naptumomab Estafenatox (Nap, ABR-217620) in Combination with Durvalumab (MEDI4736) in Subjects with Selected Advanced or Metastatic Solid Tumors | Advanced EC | AE, MTD, RP2D | II | Recruiting | NCT03983954 |
| Atezolizumab | Phase III Double-blind Randomized Placebo Controlled Trial of Atezolizumab in Combination with Paclitaxel and Carboplatin in Women with Advanced/Recurrent Endometrial Cancer | Advanced or Recurrent EC | OS, PFS | III | Recruiting | NCT03603184 |
| Atezolizumab | A Phase II, Single Arm Study of Atezolizumab + Bevacizumab in Women with Advanced, Recurrent, or Persistent Endometrial Cancer | Recurrent EC | OTR | II | Recruiting | NCT03526432 |
| Atezolizumab | An Open Label, Non-Randomized Multisite Phase II Trial Combining Bevacizumab, Atezolizumab, and Rucaparib for the Treatment of Previously Treated Recurrent and Progressive Endometrial Carcinoma | Recurrent or Progressive EC | ORR | II | Recruiting | NCT03694262 |
| Atezolizumab | A Phase 1b to Assess the Safety and Tolerability of Carboplatin-Cyclophosphamide Combined with Atezolizumab, an Antibody that Targets Programmed Death Ligand 1 (PD-L1), in Patients with Advanced Breast Cancer and Gynaecologic Cancer | Advanced EC | Toxicity | I | Active, not recruiting | NCT02914470 |
| Atezolizumab | A Phase 1b Dose-Escalation Study of Cabozantinib (XL184) Administered Alone or in Combination with Atezolizumab to Subjects with Locally Advanced or Metastatic Solid Tumors | Advanced or Metastatic EC | MTD, ORR | I/II | Recruiting | NCT03170960 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musacchio, L.; Boccia, S.M.; Caruso, G.; Santangelo, G.; Fischetti, M.; Tomao, F.; Perniola, G.; Palaia, I.; Muzii, L.; Pignata, S.; et al. Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients? J. Clin. Med. 2020, 9, 1721. https://doi.org/10.3390/jcm9061721
Musacchio L, Boccia SM, Caruso G, Santangelo G, Fischetti M, Tomao F, Perniola G, Palaia I, Muzii L, Pignata S, et al. Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients? Journal of Clinical Medicine. 2020; 9(6):1721. https://doi.org/10.3390/jcm9061721
Chicago/Turabian StyleMusacchio, Lucia, Serena Maria Boccia, Giuseppe Caruso, Giusi Santangelo, Margherita Fischetti, Federica Tomao, Giorgia Perniola, Innocenza Palaia, Ludovico Muzii, Sandro Pignata, and et al. 2020. "Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients?" Journal of Clinical Medicine 9, no. 6: 1721. https://doi.org/10.3390/jcm9061721
APA StyleMusacchio, L., Boccia, S. M., Caruso, G., Santangelo, G., Fischetti, M., Tomao, F., Perniola, G., Palaia, I., Muzii, L., Pignata, S., Benedetti Panici, P., & Di Donato, V. (2020). Immune Checkpoint Inhibitors: A Promising Choice for Endometrial Cancer Patients? Journal of Clinical Medicine, 9(6), 1721. https://doi.org/10.3390/jcm9061721