Caffeine and Gastric Emptying Time in Very Preterm Neonates
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design-Setting
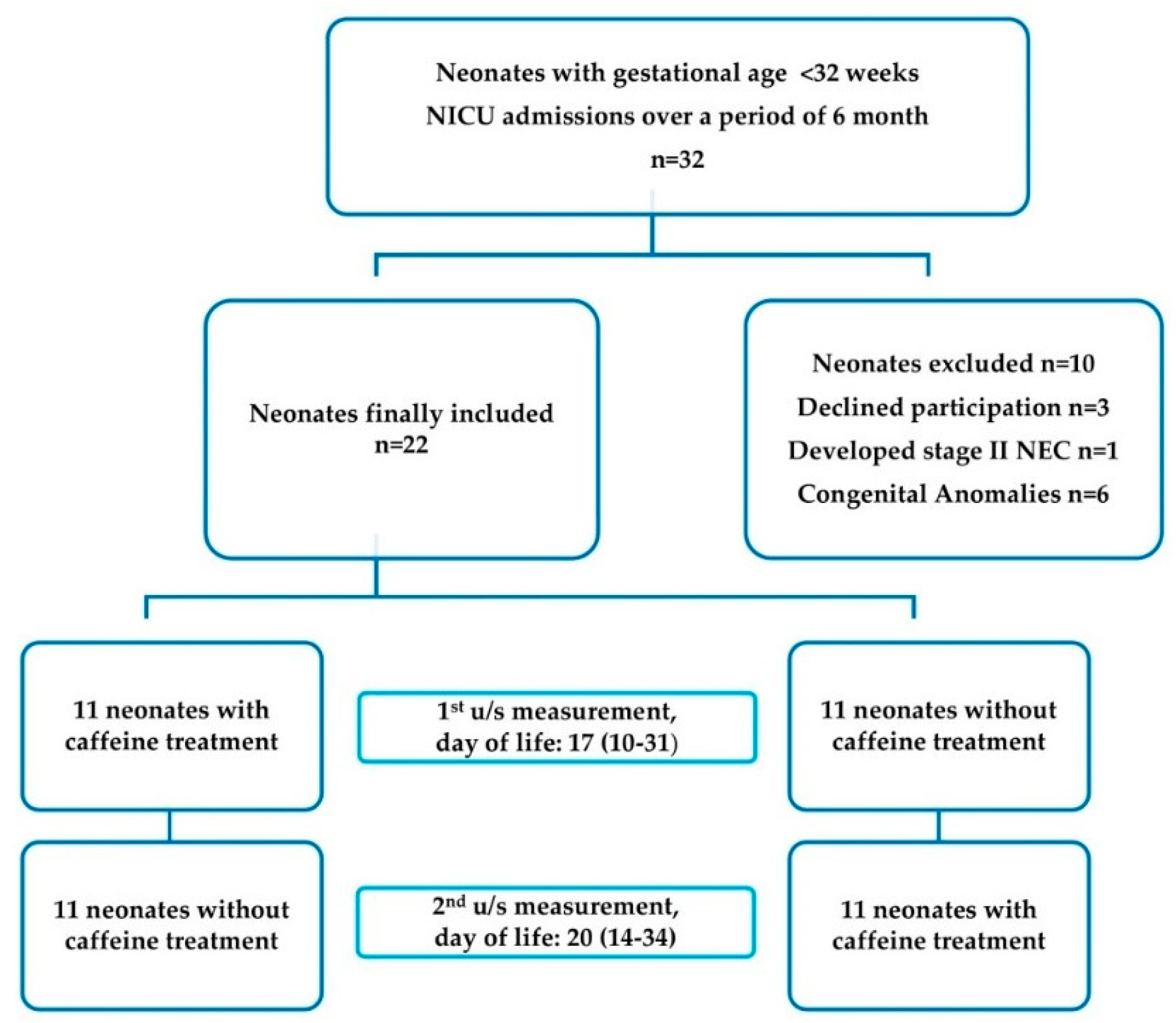
2.2. Participants
2.3. Study Protocol
2.4. Ultrasound Examination
2.5. Outcome Measures
2.6. Statistical Analysis
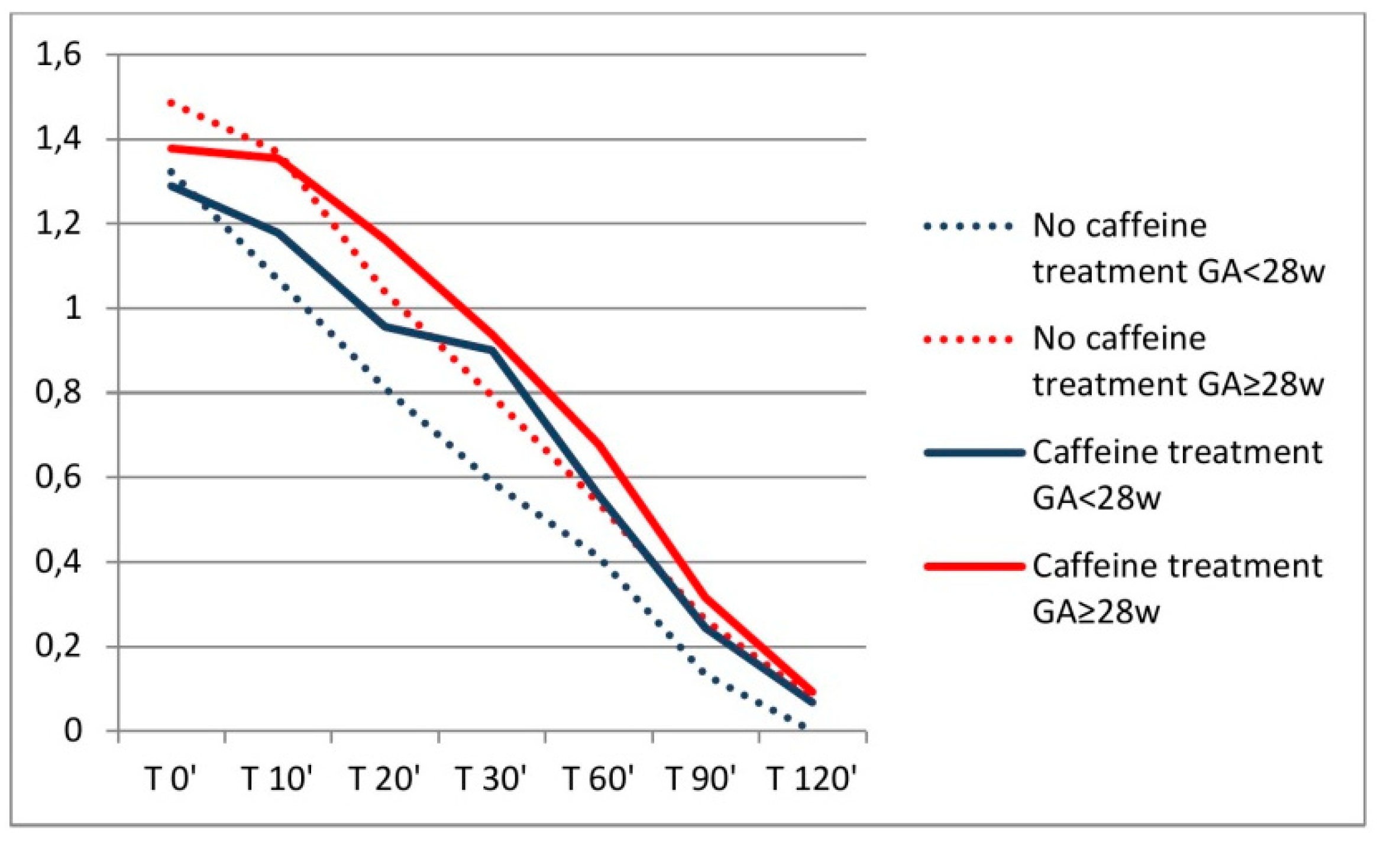
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, R.J.; Abu-Shaweesh, J.M.; Baird, T.M. Apnoea of prematurity. Paediatr. Respir. Rev. 2004, 5 (Suppl. A), S377–S382. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Tin, W. Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Bucher, H.U.; Duc, G. Does caffeine prevent hypoxaemic episodes in premature infants? A randomized controlled trial. Eur. J. Pediatr. 1988, 147, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Henderson-Smart, D.J.; De Paoli, A.G. Methylxanthine treatment for apnoea in preterm infants. Cochrane Database Syst. Rev. 2010, 12, Cd000140. [Google Scholar] [CrossRef]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Tin, W. Long-Term Effects of Caffeine Therapy for Apnea of Prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Anderson, P.J.; Doyle, L.W.; Dewey, D.; Grunau, R.E.; Asztalos, E.V.; Ohlsson, A. Survival Without Disability to Age 5 Years After Neonatal Caffeine Therapy for Apnea of Prematurity. JAMA 2012, 307, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.H.; O’Connor, S.D.; Swiney, B.S.; Salinas-Contreras, P.; Manzella, F.M.; Taylor, G.T.; Noguchi, K.K. Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity. J. Matern. Fetal Neonatal Med. 2017, 30, 2734–2741. [Google Scholar] [CrossRef]
- Fazeli, W.; Zappettini, S.; Marguet, S.L.; Grendel, J.; Esclapez, M.; Bernard, C.; Isbrandt, D. Early-life exposure to caffeine affects the construction and activity of cortical networks in mice. Exp. Neurol. 2017, 295, 88–103. [Google Scholar] [CrossRef]
- Welsh, C.; Pan, J.; Belik, J. Caffeine impairs gastrointestinal function in newborn rats. Pediatr. Res. 2015, 78, 24–28. [Google Scholar] [CrossRef]
- Gounaris, A.; Kokori, P.; Varchalama, L.; Konstandinidi, K.; Skouroliakou, M.; Alexiou, N.; Costalos, C. Theophylline and gastric emptying in very low birthweight neonates: A randomised controlled trial. Arch. Dis. Child Fetal. Neonatal Ed. 2004, 89, F297–F299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, J.L.; Wang, R.Q.; Chen, D.M. Comparison of caffeine citrate and aminophylline for treating primary apnea in premature infants. Zhongguo Dang Dai Er Ke Za Zhi 2014, 16, 1129–1132. [Google Scholar] [PubMed]
- Belén Rivas, A.; Arruza, L.; Pacheco, E.; Portoles, A.; Diz, J.; Vargas, E. Adverse drug reactions in neonates: A prospective study. Arch. Dis. Child. 2016, 101, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, G. Clinical Pharmacology of Caffeine Citrate in Preterm Infants. Med. Express 2014, 1, 243–250. [Google Scholar] [CrossRef]
- Moschino, L.; Zivanovic, S.; Hartley, C.; Trevisanuto, D.; Baraldi, E.; Roehr, C.C. Caffeine in preterm infants: Where are we in 2020? ERJ Open Res. 2020, 6, 00330–2019. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Jawa, G. Caffeine citrate—Is it a silver bullet in neonatology? Pediatrics Neonatol. 2017, 58, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Botica, M.L.; Thomas, R.; Aranda, J.V. Therapeutic Drug Monitoring for Caffeine in Preterm Neonates: An Unnecessary Exercise? Pediatrics 2007, 119, 936. [Google Scholar] [CrossRef]
- Panagiotounakou, P.; Sokou, R.; Gounari, E.; Konstantinidi, A.; Antonogeorgos, G.; Grivea, I.N.; Gounaris, A. Very preterm neonates receiving “aggressive” nutrition and early nCPAP had similar long-term respiratory outcomes as term neonates. Pediatric Res. 2019, 86, 742–748. [Google Scholar] [CrossRef]
- Gounaris, A.; Sokou, R.; Panagiotounakou, P.; Grivea, I.N. It is Time for a Universal Nutrition Policy in Very Preterm Neonates during the Neonatal Period? Comment on: “Applying Methods for Postnatal Growth Assessment in the Clinical Setting: Evaluation in a Longitudinal Cohort of Very Preterm Infants”. Nutrients 2020, 12, 980. [Google Scholar] [CrossRef]
- Bonner, J.J.; Vajjah, P.; Abduljalil, K.; Jamei, M.; Rostami-Hodjegan, A.; Tucker, G.T.; Johnson, T.N. Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults. Biopharm. Drug Dispos. 2015, 36, 245–257. [Google Scholar] [CrossRef]
- Newell, S.J.; Chapman, S.; Booth, I.W. Ultrasonic assessment of gastric emptying in the preterm infant. Arch. Dis. Child. 1993, 69, 32–36. [Google Scholar] [CrossRef]
- Taha, D.; Kirkby, S.; Nawab, U.; Dysart, K.C.; Genen, L.; Greenspan, J.S.; Aghai, Z.H. Early caffeine therapy for prevention of bronchopulmonary dysplasia in preterm infants. J. Matern. Fetal Neonatal Med. 2014, 27, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.; Hashem, N.G.; Tebbs, J.; Bookstaver, P.B.; Iskersky, V. Evaluation of caffeine and the development of necrotizing enterocolitis. J. Neonatal Perinat. Med. 2015, 8, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, V.; Paterlini, G.; DeLorenzo, P.; Meroni, V.; Gazzolo, D.; Van Bel, F.; Tagliabue, P.E. Feeding tolerance of preterm infants appropriate for gestational age (AGA) as compared to those small for gestational age (SGA). J. Matern Fetal Neonatal. Med. 2013, 26, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Q.; Peng, W. Clinical characteristics and risk factors for feeding intolerance in preterm infants. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2018, 43, 797–804. [Google Scholar]
- Vesoulis, Z.A.; McPherson, C.; Neil, J.J.; Mathur, A.M.; Inder, T.E. Early High-Dose Caffeine Increases Seizure Burden in Extremely Preterm Neonates: A Preliminary Study. J. Caffeine Res. 2016, 6, 101–107. [Google Scholar] [CrossRef]
- Philip, R.K.; Ismail, A.; Murphy, B.; Mirza, A.; Quinn, C.; Dunworth, M. Caffeine Treatment for Apnea of Prematurity and the Influence on Dose-Dependent Postnatal Weight Gain Observed Over 15 Years. J. Caffeine Adenosine Res. 2018, 8, 99–106. [Google Scholar] [CrossRef]
- Jadcherla, S.R.; Berseth, C.L. Effect of erythromycin on gastroduodenal contractile activity in developing neonates. J. Pediatric Gastroenterol. Nutr. 2002, 34, 16–22. [Google Scholar] [CrossRef]
- Aly, H.; Abdel-Hady, H.; Khashaba, M.; El-Badry, N. Erythromycin and feeding intolerance in premature infants: A randomized trial. J. Perinatol. 2007, 27, 39–43. [Google Scholar] [CrossRef]
- Perrella, S.L.; Hepworth, A.R.; Simmer, K.N.; Geddes, D.T. Influences of breast milk composition on gastric emptying in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 264–271. [Google Scholar] [CrossRef]
- Ferreira, C.H.F.; Shifrin, Y.; Pan, J.; Ivanovska, J.; McNamara, P.J.; Belik, J. The newborn rat gastric emptying rate is volume and not developmentally dependent. Neurogastroenterol. Motil. 2018, 30, e13233. [Google Scholar] [CrossRef]
- Ramirez, A.; Wong, W.W.; Shulman, R.J. Factors regulating gastric emptying in preterm infants. J. Pediatr. 2006, 149, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Hepworth, A.R.; Gridneva, Z.; Simmer, K.N.; Hartmann, P.E.; Geddes, D.T. Gastric emptying of different meal volumes of identical composition in preterm infants: A time series analysis. Pediatr. Res. 2018, 83, 778–783. [Google Scholar] [CrossRef] [PubMed]

| Variables | |
|---|---|
| Number of neonates | 22 |
| Birth weight (g) (mean ± SD) | 1077 (229) |
| Gestational age (weeks) (mean ± SD) | 28.6 (2.1) |
| Male (n,%) | 11 (50) |
| Antenatal corticosteroids (n,%) | 12 (54.5) |
| AGA (n,%) | 22 (100) |
| Apgar score (median, range) | |
| 1st min | 6 (2–9) |
| 5th min | 8 (7–10) |
| Age of measurements (days) | |
| (median, range) | |
| 1st | 17 (10–31) |
| 2nd | 20 (14–34) |
| Nasal CPAP during measurements (n,%) | 7 (31.8) |
| Milk volume during measurements (mL/2 h) | |
| (mean ± SD) | 19.3 (4.8) |
| (median, range) | 18 (12–26) |
| Mother’s milk with fortifier (n,%) | 9 (40.9) |
| BW Mean (Range) | GA Mean (Range) | No Caffeine * | Caffeine * | p-Value | |
|---|---|---|---|---|---|
| Total (n = 22) | 26 (24–30) | 36.5 (25.2–64.2) | 0.040 | ||
| BW < 1000 g (n = 10) ** | 864 (770–970)g | 26.7 (26–29)w | 24.5 (20.7–33) | 24.5 (20–47) | 0.879 |
| BW = 1000–1500 g (n = 12) | 1212 (1020–1440)g | 29.3 (28–31)w | 27 (24.2–30) | 44.5 (36–68.2) | 0.002 |
| GA < 28 w (n = 9) | 865 (770–970)g | 26.4 (26–27)w | 24 (20.5–38) | 23 (20–54) | 0.964 |
| GA = 28–32 w (n = 13) ** | 1185 (860–1440)g | 29.3 (28–31)w | 27 (24.5–30) | 40 (34.5–66.5) | 0.001 |
| GI Complication | |||
|---|---|---|---|
| No Caffeine | Caffeine | p-Value | |
| Total (n = 22) | 1 (4.6%) | 6 (27.7%) | 0.039 |
| BW = 1000–1500 g (n = 12) | 1 (8.3%) | 4 (33.3%) | 0.028 |
| BW < 1000 g (n = 10) | 0 | 2 (20%) | 0.531 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gounaris, A.K.; Grivea, I.N.; Baltogianni, M.; Gounari, E.; Antonogeorgos, G.; Kokori, F.; Panagiotounakou, P.; Theodoraki, M.; Konstantinidi, A.; Sokou, R. Caffeine and Gastric Emptying Time in Very Preterm Neonates. J. Clin. Med. 2020, 9, 1676. https://doi.org/10.3390/jcm9061676
Gounaris AK, Grivea IN, Baltogianni M, Gounari E, Antonogeorgos G, Kokori F, Panagiotounakou P, Theodoraki M, Konstantinidi A, Sokou R. Caffeine and Gastric Emptying Time in Very Preterm Neonates. Journal of Clinical Medicine. 2020; 9(6):1676. https://doi.org/10.3390/jcm9061676
Chicago/Turabian StyleGounaris, Antonios K., Ioanna N. Grivea, Maria Baltogianni, Eleni Gounari, George Antonogeorgos, Fedra Kokori, Polytimi Panagiotounakou, Martha Theodoraki, Aikaterini Konstantinidi, and Rozeta Sokou. 2020. "Caffeine and Gastric Emptying Time in Very Preterm Neonates" Journal of Clinical Medicine 9, no. 6: 1676. https://doi.org/10.3390/jcm9061676
APA StyleGounaris, A. K., Grivea, I. N., Baltogianni, M., Gounari, E., Antonogeorgos, G., Kokori, F., Panagiotounakou, P., Theodoraki, M., Konstantinidi, A., & Sokou, R. (2020). Caffeine and Gastric Emptying Time in Very Preterm Neonates. Journal of Clinical Medicine, 9(6), 1676. https://doi.org/10.3390/jcm9061676







