Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review
Abstract
1. Introduction
2. Histopathological Characteristics of Digestive Net G−3
2.1. Differentiation and Proliferation
2.2. Molecular Biology
2.3. Importance of Ki−67 index
3. Epidemiology and Tumor Presentation of Net G−3
3.1. Incidence and Tumor Site
3.2. Tumor Presentation
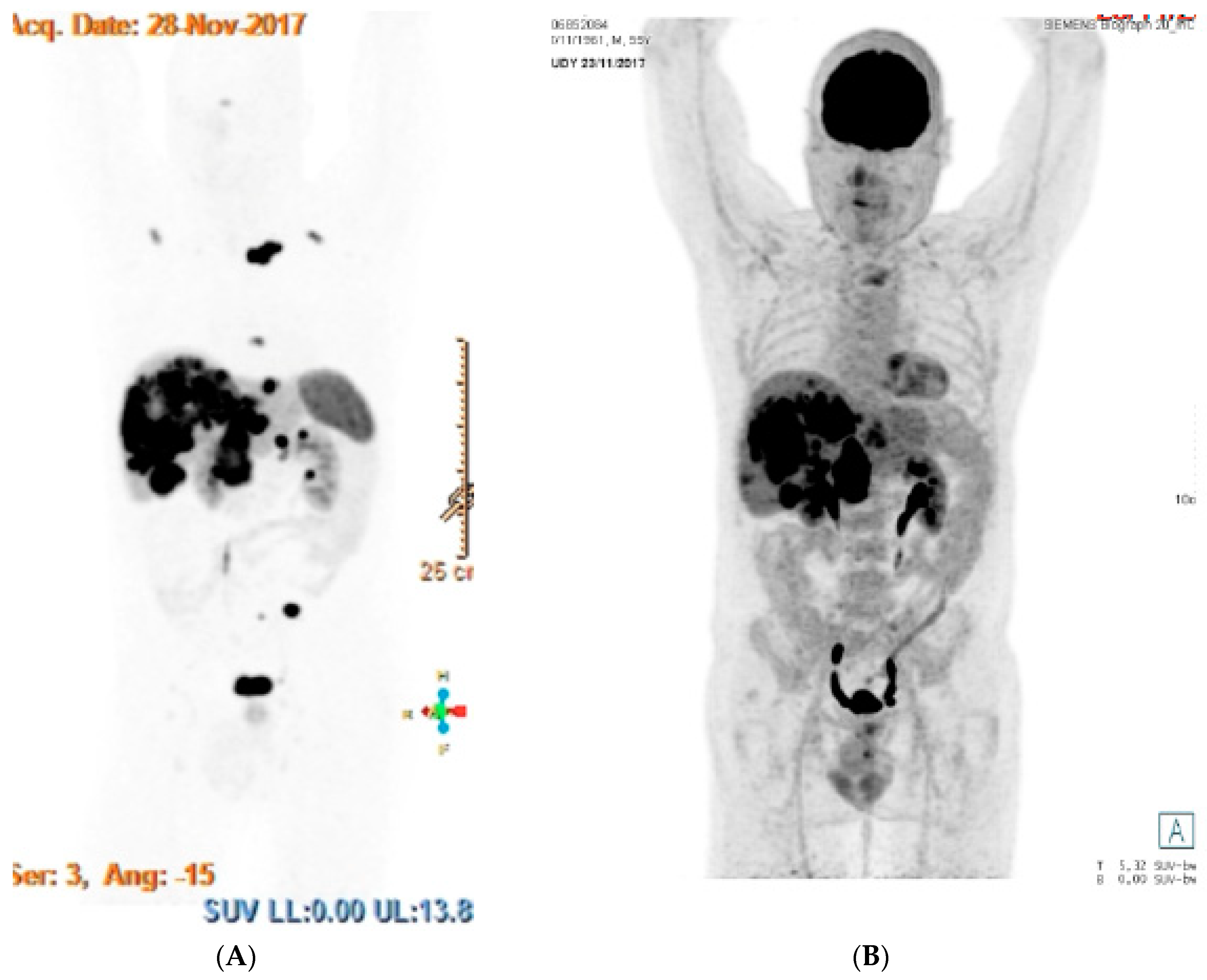
3.3. Functional Imaging
3.4. Prognosis
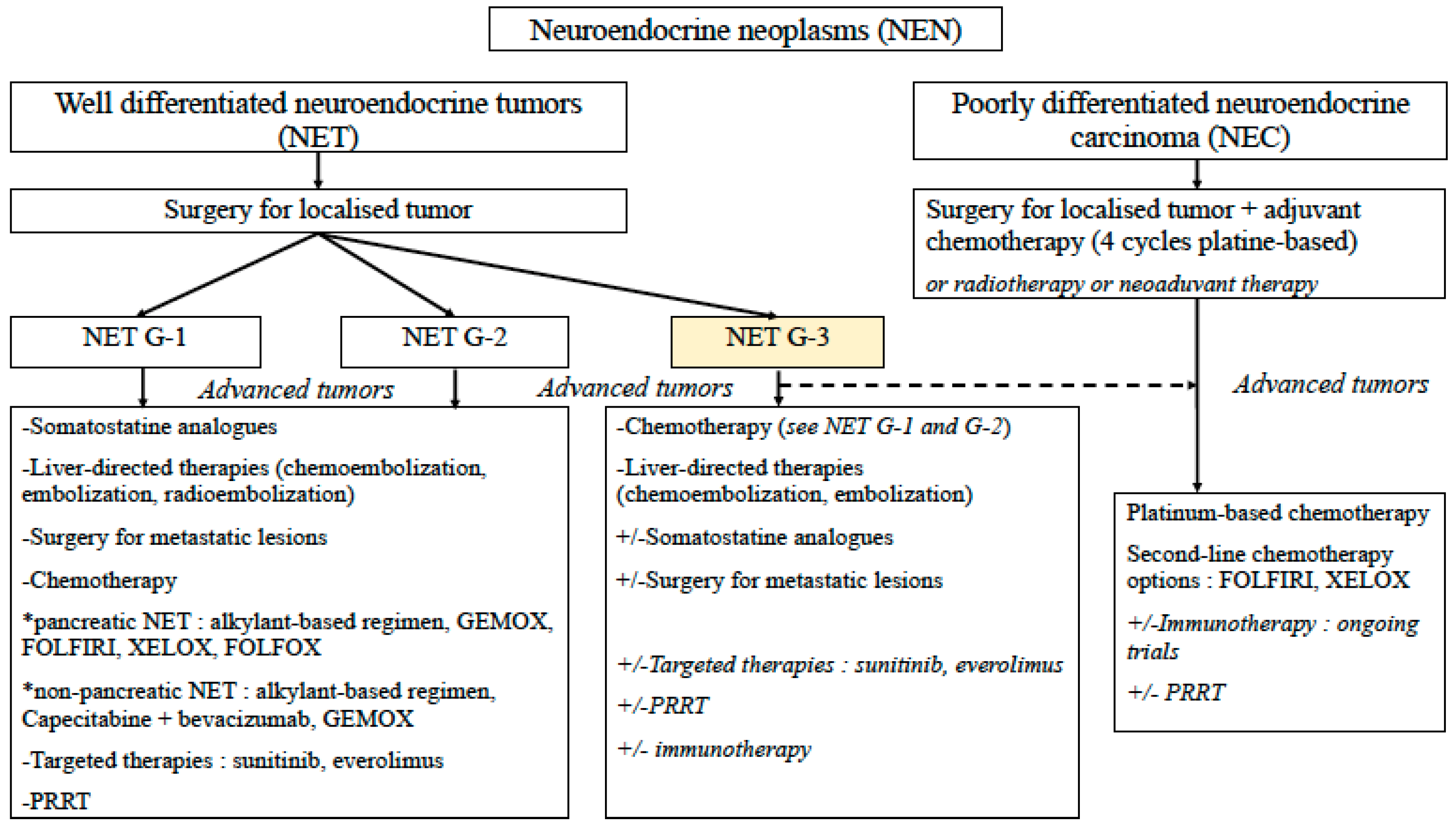
4. Treatment Options
4.1. Surgery and Liver-targeted Therapies
4.2. Somatostatin Analogues (SST)
4.3. Chemotherapy
4.4. Targeted Therapies
4.5. Peptide Receptor Radionuclide Therapy (PRRT)
4.6. Immunotherapy
5. Further Areas of Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Basturk, O.; Yang, Z.; Tang, L.H.; Hruban, R.H.; Adsay, V.; McCall, C.M.; Krasinskas, A.M.; Jang, K.T.; Frankel, W.L.; Balci, S.; et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am. J. Surg. Pathol. 2015, 39, 683–690. [Google Scholar] [CrossRef]
- Velayoudom-Cephise, F.L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T.; et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr.-Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef]
- Fazio, N.; Milione, M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: New insights and treatment implications. Cancer Treat. Rev. 2016, 50, 61–67. [Google Scholar] [CrossRef]
- Perren, A.; Couvelard, A.; Scoazec, J.-Y.; Costa, F.; Borbath, I.; Delle Fave, G.; Gorbounova, V.; Gross, D.; Grossma, A.; Jense, R.T.; et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Pathology-diagnosis and prognostic stratification. Neuroendocrinology 2017. [Google Scholar] [CrossRef]
- Klöppel, G. Neuroendocrine neoplasms: Dichotomy, origin and classifications. Visc. Med. 2017, 33, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Wislez, M.; Svrcek, M.; Hammel, P.; Afchain, P.; Andre, T. Therapeutic management of poorly differentiated neuroendocrine lung tumors and neuroendocrine carcinomas of the digestive system. Bull. Cancer 2016. [Google Scholar] [CrossRef]
- Mitry, E.; Baudin, E.; Ducreux, M.; Sabourin, J.C.; Rufie, P.; Aparicio, T.; Aparicio, T.; Lasser, P.; Elias, D.; Duvillard, P.; et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer 1999, 81, 1351–1355. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Cheema, A.; Weber, J.; Han, G.; Coppola, D.; Kvols, L.K. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 3044–3049. [Google Scholar] [CrossRef]
- Coriat, R.; Walter, T.; Terris, B.; Couvelard, A.; Ruszniewski, P. Gastroenteropancreatic well-differentiated grade 3 neuroendocrine tumors: Review and position statement. Oncologist 2016. [Google Scholar] [CrossRef]
- Jensen, R.T.; Bodei, L.; Capdevila, J.; Couvelard, A.; Falconi, M.; Glasberg, S.; Kloppel, G.; Lamberts, S.; Peeters, M.; Rindi, G.; et al. Unmet needs in functional and nonfunctional pancreatic neuroendocrine neoplasms. Neuroendocrinology 2019, 108, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr.-Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Hijioka, S.; Hosoda, W.; Matsuo, K.; Ueno, M.; Furukawa, M.; Yoshitomi, H.; Kobayashi, N.; Ikeda, M.; Ito, T.; Nakamori, S.; et al. Rb loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: A Japanese multicenter pancreatic NEN-G3 study. Clin. Cancer Res. 2017, 23, 4625–4632. [Google Scholar] [CrossRef]
- van Velthuysen, M.-L.F.; Groen, E.J.; van der Noort, V.; van de Pol, A.; Tesselaar, M.E.T.; Korse, C.M. Grading of neuroendocrine neoplasms: Mitoses and Ki-67 are both essential. Neuroendocrinology 2014, 100, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Sigel, C.S.; Krauss Silva, V.W.; Reid, M.D.; Chhieng, D.; Basturk, O.; Sigel, K.M.; Daniel, T.D.; Klimstra, D.S.; Tang, L.H. Well differentiated grade 3 pancreatic neuroendocrine tumors compared with related neoplasms: A morphologic study. Cancer Cytopathol. 2018, 126, 326–335. [Google Scholar] [CrossRef]
- Yachida, S.; Vakiani, E.; White, C.M.; Zhong, Y.; Saunders, T.; Morgan, R.; de Wilde, R.F.; Maitra, A.; Hicks, J.; Demarzo, A.M.; et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am. J. Surg. Pathol. 2012, 36, 173–184. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.-M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef]
- Wong, H.-L.; Yang, K.C.; Shen, Y.; Zhao, E.Y.; Loree, J.M.; Kennecke, H.F.; Kalloger, S.E.; Karasinska, J.M.; Lim, H.J.; Mungall, A.J.; et al. Molecular characterization of metastatic pancreatic neuroendocrine tumors (PNETs) using whole-genome and transcriptome sequencing. Cold Spring Harb. Mol. Case Stud. 2018, 4. [Google Scholar] [CrossRef]
- Williamson, L.M.; Steel, M.; Grewal, J.K.; Thibodeau, M.L.; Zhao, E.Y.; Loree, J.M.; Yang, K.C.; Gorski, S.M.; Mungall, A.J.; Mungall, K.L.; et al. Genomic characterization of a well-differentiated grade 3 pancreatic neuroendocrine tumor. Cold Spring Harb. Mol. Case Stud. 2019, 5. [Google Scholar] [CrossRef]
- Weynand, B.; Borbath, I.; Bernard, V.; Sempoux, C.; Gigot, J.-F.; Hubert, C.; Lannoy, V.; Deprez, P.H.; Jouret-Mourin, A. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: High reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology 2014, 25, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Gonen, M.; Hedvat, C.; Modlin, I.M.; Klimstra, D.S. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: A comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012, 36, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.D.; Bagci, P.; Ohike, N.; Saka, B.; Erbarut Seven, I.; Dursun, N.; Balci, S.; Gucer, H.; Jang, K.-T.; Tajiri, T.; et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: A comparative analysis of four counting methodologies. Mod. Pathol. 2015, 28, 686–694. [Google Scholar] [CrossRef] [PubMed]
- van Velthuysen, M.-L.F.; Groen, E.J.; Sanders, J.; Prins, F.A.; van der Noort, V.; Korse, C.M. Reliability of proliferation assessment by Ki-67 expression in neuroendocrine neoplasms: Eyeballing or image analysis? Neuroendocrinology 2014, 100, 288–292. [Google Scholar] [CrossRef]
- Korse, C.M.; Taal, B.G.; van Velthuysen, M.L.; Visser, O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: Experience of two decades of cancer registry. Eur. J. Cancer 2013, 49, 1975–1983. [Google Scholar] [CrossRef]
- Walter, T.; Tougeron, D.; Baudin, E.; Le Malicot, K.; Lecomte, T.; Malka, D.; Hentic, O.; Manfredi, S.; Bonnet, I.; Guimbaud, R.; et al. Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur. J. Cancer 2017, 79, 158–165. [Google Scholar] [CrossRef]
- Dasari, A.; Mehta, K.; Byers, L.A.; Sorbye, H.; Yao, J.C. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 2017. [Google Scholar] [CrossRef]
- Lepage, C.; Rachet, B.; Coleman, M.P. Survival from malignant digestive endocrine tumors in England and Wales: A population-based study. Gastroenterology 2007, 132, 899–904. [Google Scholar] [CrossRef]
- Walter, T.; Scoazec, J.-Y.; Lepage, C. Epidemiology of digestive neuroendocrine tumors with focus on French data. Hepato-Gastro 2013, 20, 160–166. [Google Scholar]
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic neuroendocrine tumours: The current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: An analysis based on prospectively collected parameters. Endocr.-Relat. Cancer 2010, 17, 909–918. [Google Scholar] [CrossRef]
- Scoazec, J.-Y.; Couvelard, A.; Monges, G.; Leteurtre, E.; Belleannee, G.; Guyetant, S.; Duvillard, P.; Danjoux, M.; Parot, X.; Lepage, C. Well-differentiated grade 3 digestive neuroendocrine tumors: Myth or reality? The PRONET Study Group. JCO 2012, 30, 4129. [Google Scholar] [CrossRef]
- Scoazec, J.-Y.; Couvelard, A.; Monges, G.; Guyétant, S.; Bisot-Locard, S.; Parot, X.; Lepage, C.; PRONET Study Group. Professional practices and diagnostic issues in neuroendocrine tumour pathology: Results of a prospective one-year survey among French pathologists (the PRONET study). Neuroendocrinology 2017, 105, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. ESMO 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Mortensen, J.; Pfeifer, A.; Federspiel, B.; Hansen, C.P.; Hojgaard, L.; Kjaer, A. Functional imaging of neuroendocrine tumors: A head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2010, 51, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Cros, J.; Neuzillet, C.; Hentic, O.; Egal, A.; Muller, N.; Bouché, O.; Cadiot, G.; Ruszniewski, P.; Couvelard, A.; et al. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms. Neuroendocrinology 2017, 105, 412–425. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Coppola, D.; Klimstra, D.S.; Phan, A.T.; Kulke, M.H.; Wiseman, G.A.; Kvols, L.K.; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas 2010, 39, 799–800. [Google Scholar] [CrossRef]
- Boudreaux, J.P.; Klimstra, D.S.; Hassan, M.M.; Woltering, E.A.; Jensen, R.T.; Goldsmith, S.J.; Nutting, C.; Bushnell, D.L.; Caplin, M.E.; Yao, J.C.; et al. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: Well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010, 39, 753–766. [Google Scholar] [CrossRef]
- Falconi, M.; Bartsch, D.K.; Eriksson, B.; Klöppel, G.; Lopes, J.M.; O’Connor, J.M.; Salazar, R.; Taal, B.G.; Vullierme, M.P.; O’Toole, D.; et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: Well-differentiated pancreatic non-functioning tumors. Neuroendocrinology 2012, 95, 120–134. [Google Scholar] [CrossRef]
- Kulke, M.H.; Anthony, L.B.; Bushnell, D.L.; de Herder, W.W.; Goldsmith, S.J.; Klimstra, D.S.; Marx, S.J.; Pasieka, J.L.; Pommier, R.F.; Yao, J.C.; et al. NANETS treatment guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010, 39, 735–752. [Google Scholar] [CrossRef]
- Pape, U.-F.; Perren, A.; Niederle, B.; Gross, D.; Gress, T.; Costa, F.; Arnold, R.; Denecke, T.; Plöckinger, U.; Salazar, R.; et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 2012, 95, 135–156. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Kianmanesh, R.; Caplin, M. ENETS 2016 Consensus Guidelines for the management of patients with digestive neuroendocrine tumors: An update. Neuroendocrinology 2016, 103, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, H.; Ali, S.; Salhab, M.; Olszewski, A.J. Survival of patients with neuroendocrine carcinoma of the colon and rectum: A population-based analysis. Dis. Colon Rectum 2015, 58, 294–303. [Google Scholar] [CrossRef]
- Smith, J.D.; Reidy, D.L.; Goodman, K.A.; Shia, J.; Nash, G.M. A retrospective review of 126 high-grade neuroendocrine carcinomas of the colon and rectum. Ann. Surg. Oncol. 2014, 21, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Haugvik, S.-P.; Janson, E.T.; Österlund, P.; Langer, S.W.; Falk, R.S.; Labori, K.J.; Vestermark, L.W.; Grønbæk, H.; Gladhaug, I.P.; Sorbye, H. surgical treatment as a principle for patients with high-grade pancreatic neuroendocrine carcinoma: A nordic multicenter comparative study. Ann. Surg. Oncol. 2016, 23, 1721–1728. [Google Scholar] [CrossRef]
- Pellat, A.; Walter, T.; Augustin, J.; Hautefeuille, V.; Hentic, O.; Do Cao, C.; Lièvre, A.; Coriat, R.; Hammel, P.; Dubreuil, O.; et al. Chemotherapy in resected neuroendocrine carcinomas of the digestive tract: A national study from the French group of endocrine tumours (GTE). Neuroendocrinology 2019. [Google Scholar] [CrossRef]
- Worth, P.J.; Leal, J.; Ding, Q.; Trickey, A.; Dua, M.M.; Chatzizacharias, N.; Soonawalla, Z.; Athanasopoulos, P.; Toumpanakis, C.; Hansen, P.; et al. Pancreatic grade 3 neuroendocrine tumors behave similarly to neuroendocrine carcinomas following resection: A multi-center, international appraisal of the WHO 2010 and WHO 2017 staging schema for pancreatic neuroendocrine lesions. HPB (Oxf.) 2020. [Google Scholar] [CrossRef]
- Zappa, M.; Abdel-Rehim, M.; Hentic, O.; Vullierme, M.-P.; Ruszniewski, P.; Vilgrain, V. Liver-directed therapies in liver metastases from neuroendocrine tumors of the gastrointestinal tract. Target. Oncol. 2012, 7, 107–116. [Google Scholar] [CrossRef]
- de Baere, T.; Deschamps, F.; Tselikas, L.; Ducreux, M.; Planchard, D.; Pearson, E.; Berdelou, A.; Leboulleux, S.; Elias, D.; Baudin, E. GEP-NETS update: Interventional radiology: Role in the treatment of liver metastases from GEP-NETs. Eur. J. Endocrinol. 2015, 172, R151–R166. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ruszniewski, P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, M.; Lombard-Bohas, C.; Cadiot, G.; Matysiak-Budnik, T.; Rebours, V.; Vullierme, M.-P.; Couvelard, A.; Hentic, O.; Ruszniewski, P. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. Eur. J. Gastroenterol. Hepatol. 2013, 25, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.; Klaassen, D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Rimassa, L.; Carnaghi, C.; Seregni, E.; Ferrari, L.; Di Bartolomeo, M.; Regalia, E.; Cassata, A.; Procopio, G.; Mariani, L. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer 1998, 83, 372–378. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.-T.; Helm, J.; Kvols, L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). JCO 2018, 36, 4004. [Google Scholar] [CrossRef]
- Ducreux, M.; Dahan, L.; Smith, D.; O’Toole, D.; Lepère, C.; Dromain, C.; Vilgrain, V.; Baudin, E.; Lombard-Bohas, C.; Scoazec, J.-Y.; et al. Bevacizumab combined with 5-FU/streptozocin in patients with progressive metastatic well-differentiated pancreatic endocrine tumours (BETTER trial)—A phase II non-randomised trial. Eur. J. Cancer 2014, 50, 3098–3106. [Google Scholar] [CrossRef]
- Brixi-Benmansour, H.; Jouve, J.-L.; Mitry, E.; Bonnetain, F.; Landi, B.; Hentic, O.; Bedenne, L.; Cadiot, G. Phase II study of first-line FOLFIRI for progressive metastatic well-differentiated pancreatic endocrine carcinoma. Dig. Liver Dis. 2011, 43, 912–916. [Google Scholar] [CrossRef]
- Bajetta, E.; Catena, L.; Procopio, G.; De Dosso, S.; Bichisao, E.; Ferrari, L.; Martinetti, A.; Platania, M.; Verzoni, E.; Formisano, B.; et al. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother. Pharmacol. 2007, 59, 637–642. [Google Scholar] [CrossRef]
- Dussol, A.-S.; Joly, M.-O.; Vercherat, C.; Forestier, J.; Hervieu, V.; Scoazec, J.-Y.; Lombard-Bohas, C.; Walter, T. Gemcitabine and oxaliplatin or alkylating agents for neuroendocrine tumors: Comparison of efficacy and search for predictive factors guiding treatment choice. Cancer 2015, 121, 3428–3434. [Google Scholar] [CrossRef]
- Pavel, M.E.; Baum, U.; Hahn, E.G.; Schuppan, D.; Lohmann, T. Efficacy and tolerability of pegylated IFN-alpha in patients with neuroendocrine gastroenteropancreatic carcinomas. J. Interferon Cytokine Res. 2006, 26, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dahan, L.; Bonnetain, F.; Rougier, P.; Raoul, J.-L.; Gamelin, E.; Etienne, P.-L.; Cadiot, G.; Mitry, E.; Smith, D.; Cvitkovic, F.; et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon alpha for advanced carcinoid tumors: FNCLCC-FFCD 9710. Endocr.-Relat. Cancer 2009, 16, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Altimari, A.F.; Badrinath, K.; Reisel, H.J.; Prinz, R.A. DTIC therapy in patients with malignant intra-abdominal neuroendocrine tumors. Surgery 1987, 102, 1009–1017. [Google Scholar] [PubMed]
- Bajetta, E.; Ferrari, L.; Procopio, G.; Catena, L.; Ferrario, E.; Martinetti, A.; Di Bartolomeo, M.; Buzzoni, R.; Celio, L.; Vitali, M.; et al. Efficacy of a chemotherapy combination for the treatment of metastatic neuroendocrine tumours. Ann. Oncol. 2002, 13, 614–621. [Google Scholar] [CrossRef]
- Ekeblad, S.; Sundin, A.; Janson, E.T.; Welin, S.; Granberg, D.; Kindmark, H.; Dunder, K.; Kozlovacki, G.; Orlefors, H.; Sigurd, M.; et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin. Cancer Res. 2007, 13, 2986–2991. [Google Scholar] [CrossRef]
- Yang, Q.-C.; Wang, Y.-H.; Lin, Y.; Xue, L.; Chen, Y.-J.; Chen, M.-H.; Chen, J. Expression of O(6)-methylguanine DNA methyltransferase (MGMT) and its clinical significance in gastroenteropancreatic neuroendocrine neoplasm. Int. J. Clin. Exp. Pathol. 2014, 7, 4204–4212. [Google Scholar]
- Cassier, P.A.; Walter, T.; Eymard, B.; Ardisson, P.; Perol, M.; Paillet, C.; Chayvialle, J.-A.; Scoazec, J.-Y.; Hervieu, V.; Bohas, C.L. Gemcitabine and oxaliplatin combination chemotherapy for metastatic well-differentiated neuroendocrine carcinomas: A single-center experience. Cancer 2009, 115, 3392–3399. [Google Scholar] [CrossRef]
- Mitry, E.; Walter, T.; Baudin, E.; Kurtz, J.-E.; Ruszniewski, P.; Dominguez-Tinajero, S.; Bengrine-Lefevre, L.; Cadiot, G.; Dromain, C.; Farace, F.; et al. Bevacizumab plus capecitabine in patients with progressive advanced well-differentiated neuroendocrine tumors of the gastro-intestinal (GI-NETs) tract (BETTER trial)—A phase II non-randomised trial. Eur. J. Cancer 2014, 50, 3107–3115. [Google Scholar] [CrossRef]
- Moertel, C.G.; Kvols, L.K.; O’Connell, M.J.; Rubin, J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer 1991, 68, 227–232. [Google Scholar] [CrossRef]
- Nakano, K.; Takahashi, S.; Yuasa, T.; Nishimura, N.; Mishima, Y.; Sakajiri, S.; Yokoyama, M.; Tsuyama, N.; Ishikawa, Y.; Hatake, K. Feasibility and efficacy of combined cisplatin and irinotecan chemotherapy for poorly differentiated neuroendocrine carcinomas. Jpn. J. Clin. Oncol. 2012, 42, 697–703. [Google Scholar] [CrossRef]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Levy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr.-Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Malka, D.; Planchard, D.; Scoazec, J.Y.; Caramella, C.; Guigay, J.; Boige, V.; Leboulleux, S.; Burtin, P.; Berdelou, A.; et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr.-Relat. Cancer 2015, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Oberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Öberg, K.; Steinmüller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R. Barcelona Consensus Conference participants ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.-L.; Bang, Y.-J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Pellat, A.; Dreyer, C.; Couffignal, C.; Walter, T.; Lombard-Bohas, C.; Niccoli, P.; Seitz, J.F.; Hentic, O.; André, T.; Coriat, R.; et al. Clinical and biomarker evaluations of sunitinib in patients with grade 3 digestive neuroendocrine neoplasms. Neuroendocrinology 2018, 107, 24–31. [Google Scholar] [CrossRef]
- Panzuto, F.; Rinzivillo, M.; Spada, F.; Antonuzzo, L.; Ibrahim, T.; Campana, D.; Fazio, N.; Delle Fave, G. Everolimus in pancreatic neuroendocrine carcinomas G3. Pancreas 2017, 46, 302–305. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-term efficacy, survival, and safety of [177Lu-DOTA0,Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O’Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gönen, M.; Lohrmann, C.; et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist 177Lu-Satoreotide tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Kong, G.; Grozinsky-Glasberg, S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr.-Relat. Cancer 2020, 27, R67–R77. [Google Scholar] [CrossRef]
- Basu, S.; Adnan, A. Well-differentiated grade 3 neuroendocrine tumours and poorly differentiated grade 3 neuroendocrine carcinomas: Will dual tracer PET-computed tomography (68Ga-DOTATATE and FDG) play a pivotal role in differentiation and guiding management strategies? Nucl. Med. Commun. 2019, 40, 1086–1087. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C. Immune checkpoint inhibitors in the treatment of patients with neuroendocrine neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Belousova, E.; Karmazanovsky, G.; Kriger, A.; Kalinin, D.; Mannelli, L.; Glotov, A.; Karelskaya, N.; Paklina, O.; Kaldarov, A. Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumours: Correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin. Radiol. 2017, 72, 150–158. [Google Scholar] [CrossRef]
- Waseem, N.; Aparici, C.M.; Kunz, P.L. Evaluating the role of theranostics in grade 3 neuroendocrine neoplasms. J. Nucl. Med. 2019, 60, 882–891. [Google Scholar] [CrossRef]
| Well Differentiated NEN | Ki−67 Index (%) | Mitotic Index (HPF/10HPF) |
|---|---|---|
| Neuroendocrine tumor (NET) G1 | <3 | <2/10 |
| Neuroendocrine tumor (NET) G2 | 3–20 | 2–20/10 |
| Neuroendocrine tumor (NET) G3 | >20 | >20/10 |
| Poorly differentiated NEN | ||
| Neuroendocrine carcinoma (NEC) G3 Small-cell type Large-cell type | >20 | >20/10 |
| Mixed Neuroendocrine-nonneuroendocrine neoplasm (MiNEN) | ||
| Study, Year | Number of Patients | Median of Age (Years) | Tumor Sites | Metastatic State (%) | Median Ki−67 (%) | Median Survival (Months) |
|---|---|---|---|---|---|---|
| Velayoudom-Céphise et al. [3], 2013 | 12 | 56 | Pancreas, non-digestive sites | 100 | 21 | 41 |
| Heetfeld et al. [12], 2015 | 37 | 52 | Pancreas, rectum, stomach, small bowel | 62 | 30 | 99 |
| Basturk et al. [2], 2015 | 19 | 54 | Pancreas | 67 | 40 | 54 |
| Scoazec et al. [32], 2017 | 21 | NA | Pancreas, colorectal, stomach, small bowel | NA | 35 | NA |
| Hijioka et al. [13], 2017 | 21 | 63 | Pancreas | 71 | 29 | 42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellat, A.; Coriat, R. Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review. J. Clin. Med. 2020, 9, 1677. https://doi.org/10.3390/jcm9061677
Pellat A, Coriat R. Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review. Journal of Clinical Medicine. 2020; 9(6):1677. https://doi.org/10.3390/jcm9061677
Chicago/Turabian StylePellat, Anna, and Romain Coriat. 2020. "Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review" Journal of Clinical Medicine 9, no. 6: 1677. https://doi.org/10.3390/jcm9061677
APA StylePellat, A., & Coriat, R. (2020). Well Differentiated Grade 3 Neuroendocrine Tumors of the Digestive Tract: A Narrative Review. Journal of Clinical Medicine, 9(6), 1677. https://doi.org/10.3390/jcm9061677





