Cost-Effectiveness of the Manchester Approach to Identifying Lynch Syndrome in Women with Endometrial Cancer
Abstract
1. Introduction
2. Methods
2.1. Selection of Interventions and Comparators
2.1.1. The Manchester Approach
2.1.2. Comparator Strategies
- No testing for Lynch syndrome in endometrial cancer cases (strategy 0);
- Triage by microsatellite instability testing, followed by NGS (strategy 1);
- Triage by microsatellite instability testing, then MLH1 methylation testing, followed by NGS (strategy 2);
- Direct NGS (strategy 4).
- Using a two-MMR protein IHC panel (including only MSH6 and PMS2 antibodies);
- Treating MSI-L as indicative for further Lynch syndrome testing;
- Using clinical criteria (age or PREMM₅ (PREdiction Model for gene Mutations, 5-gene version) score [17]) to select patients at higher risk of having Lynch syndrome.
2.2. Economic Evaluation Approach
2.2.1. Diagnostic Testing
2.2.2. Extrapolation
2.3. Methods for Estimating Costs
2.3.1. Diagnostic Costs
2.3.2. Long-Term Costs
2.4. Methods for Estimating Health Benefits
2.4.1. Lynch Syndrome Cases Identified
2.4.2. Long-Term QALYs
2.5. Methods for Handling Uncertainty and Heterogeneity
2.6. Further Details of Economic Evaluation
3. Results
3.1. Lynch Syndrome Cases Identified
3.2. Cost per Lynch Syndrome Case Identified
3.3. Impact of Surveillance on Life Expectancy, Colorectal Cancer Outcomes, and QALYs
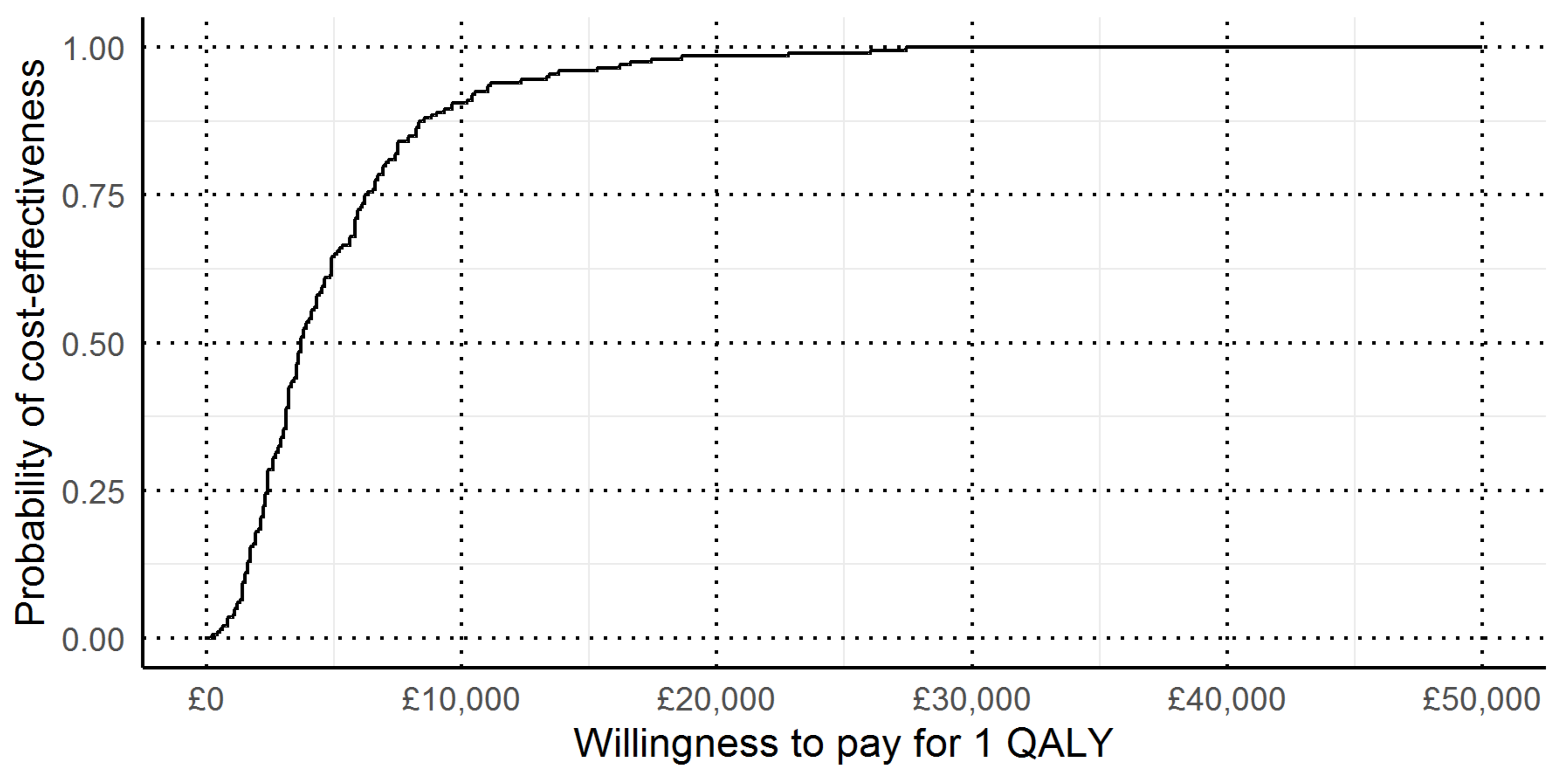
3.4. Cost per QALY
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameter | Base Case Value | Uncertainty (Distribution, 95% CI) ¹ |
|---|---|---|
| Population Characteristics | ||
| Prevalence of Lynch syndrome | 3.20% | Bootstrap, 1.96% to 5.68% |
| Prevalence of path_MLH1 | 0.40% | Bootstrap, 0.03% to 1.41% |
| Prevalence of path_MSH2 | 0.80% | Bootstrap, 0.24% to 2.03% |
| Prevalence of path_MSH6 | 1.60% | Bootstrap, 0.68% to 3.19% |
| Prevalence of path_PMS2 | 0.40% | Bootstrap, 0.02% to 1.33% |
| Age of Lynch syndrome cases (years) | 54 | Bootstrap, 47.0 to 60.9 |
| Age of path_MLH1 cases | 40.5 | Bootstrap, 31.4 to 50.2 |
| Age of path_MSH2 cases | 53.8 | Bootstrap, 44.7 to 62.3 |
| Age of path_MSH6 cases | 59.1 | Bootstrap, 49.5 to 67.8 |
| Age of path_PMS2 cases | 47.5 | Bootstrap, 16.6 to 79.5 |
| Age of sporadic cases | 63.5 | Bootstrap, 62.4 to 64.6 |
| Relatives per proband | 6 | Gamma, 1.80 to 11.96 |
| Probability relative accepts counselling | 77.70% | Beta mixture, 73.8% to 81.1% |
| Probability relative accepts testing after counselling | 71.60% | Ratio, 67.4% to 76.4% |
| Probability relative has Lynch syndrome | 44.00% | Beta, 41.0% to 47.3% |
| Probability relative is female | 52.80% | Beta, 47.6% to 57.0% |
| Diagnostic Effectiveness | ||
| Strategy 1 sensitivity | 0.563 | Bootstrap, 0.256 to 0.818 |
| Strategy 1 specificity | 0.835 | Bootstrap, 0.804 to 0.868 |
| Strategy 2 sensitivity | 0.563 | Bootstrap, 0.256 to 0.818 |
| Strategy 2 specificity | 0.967 | Bootstrap, 0.948 to 0.981 |
| Strategy 3 sensitivity | 1 | Bootstrap, 1.000 to 1.000 |
| Strategy 3 specificity | 0.967 | Bootstrap, 0.954 to 0.986 |
| Disease Natural History | ||
| Colorectal cancer risk for proband aged 60 years to age 80 years ²,³ | ||
| path_MLH1 | 39.50% | Log-normal model, 33.5% to 47.4% |
| path_MSH2 | 35.70% | Log-normal model, 28.9% to 42.1% |
| path_MSH6 | 19.90% | Log-normal model, 12.1% to 28.1% |
| path_PMS2 | 10.20% | Log-normal model, 1.3% to 30.2% |
| Sporadic | 2.19% | Not varied |
| Colorectal cancer risk for female relative aged 60 years to age 80 years ²,³ | ||
| path_MLH1 | 30.80% | Log-normal model, 24.1% to 40.1% |
| path_MSH2 | 27.00% | Log-normal model, 20.5% to 36.3% |
| path_MSH6 | 12.80% | Log-normal model, 6.9% to 22.7% |
| path_PMS2 | 5.50% | Log-normal model, 0.5% to 24.9% |
| Sporadic | 2.19% | Not varied |
| Colorectal cancer risk for male relative aged 60 years to age 80 years ²,³ | ||
| path_MLH1 | 35.20% | Log-normal model, 28.7% to 43.8% |
| path_MSH2 | 31.40% | Log-normal model, 23.8% to 40.7% |
| path_MSH6 | 16.30% | Log-normal model, 9.5% to 26.2% |
| path_PMS2 | 7.70% | Log-normal model, 0.9% to 30.7% |
| Sporadic | 3.48% | Not varied |
| 5-year endometrial cancer mortality risk for probands ³ | ||
| Lynch syndrome cases | 10.40% | Exponential model, 6.1% to 15.2% |
| Sporadic cases (x = age at diagnosis) | ||
| x < 45 | 12.40% | Exponential model, 10.5% to 14.2% |
| 45 ≤ x < 55 | 13.10% | Exponential model, 11.9% to 14.5% |
| 55 ≤ x < 65 | 14.50% | Exponential model, 13.6% to 15.4% |
| 65 ≤ x < 75 | 21.50% | Exponential model, 20.7% to 22.5% |
| x ≥ 75 | 36.90% | Exponential model, 35.3% to 38.9% |
| 10-year other-cause mortality risk ³ | ||
| Woman aged 60 | 7.60% | Not varied |
| Woman aged 70 | 20.30% | Not varied |
| Man aged 60 | 11.40% | Not varied |
| Man aged 70 | 28.10% | Not varied |
| 5-year colorectal cancer mortality ³ | ||
| Lynch syndrome case, by stage | ||
| Stage I | 4.50% | Exponential model, 2.1% to 8.0% |
| Stage II | 15.80% | Exponential model, 7.7% to 27.0% |
| Stage III | 38.60% | Exponential model, 20.8% to 59.2% |
| Stage IV | 93.40% | Exponential model, 92.9% to 93.9% |
| Sporadic case, by stage | ||
| Stage I | 6.80% | Exponential model, 6.1% to 7.5% |
| Stage II | 22.90% | Exponential model, 22.5% to 23.6% |
| Stage III | 52.30% | Exponential model, 51.7% to 53.0% |
| Stage IV | 93.40% | Exponential model, 92.9% to 93.9% |
| Colonoscopic Surveillance Effectiveness | ||
| Hazard ratio for incidence of colorectal cancer from colonoscopic surveillance | 0.387 | Log-normal model, 0.164 to 0.753 |
| Colorectal cancer stage distribution | ||
| Lynch syndrome case, under surveillance | ||
| Stage I | 68.60% | Dirichlet distribution, 52.9% to 81.8% |
| Stage II | 10.50% | Dirichlet distribution, 3.3% to 22.3% |
| Stage III | 12.80% | Dirichlet distribution, 5.0% to 23.4% |
| Stage IV | 8.10% | Dirichlet distribution, 1.8% to 18.8% |
| Lynch syndrome case, not under surveillance | ||
| Stage I | 18.80% | Dirichlet distribution, 9.2% to 34.2% |
| Stage II | 48.80% | Dirichlet distribution, 31.7% to 63.9% |
| Stage III | 21.20% | Dirichlet distribution, 10.7% to 32.8% |
| Stage IV | 11.20% | Dirichlet distribution, 4.1% to 22.8% |
| Sporadic case | ||
| Stage I | 17.60% | Dirichlet distribution, 17.2% to 18.0% |
| Stage II | 27.00% | Dirichlet distribution, 26.5% to 27.5% |
| Stage III | 29.50% | Dirichlet distribution, 29.1% to 30.0% |
| Stage IV | 25.90% | Dirichlet distribution, 25.4% to 26.4% |
| Utility Values | ||
| Baseline utility by gender and age (years) | ||
| Woman, 40 | 0.887 | Regression model, 0.826 to 0.949 |
| Woman, 50 | 0.855 | Regression model, 0.766 to 0.944 |
| Woman, 60 | 0.816 | Regression model, 0.696 to 0.937 |
| Woman, 70 | 0.77 | Regression model, 0.611 to 0.931 |
| Woman, 80 | 0.718 | Regression model, 0.513 to 0.924 |
| Woman, 90 | 0.659 | Regression model, 0.403 to 0.917 |
| Man, 40 | 0.909 | Regression model, 0.845 to 0.974 |
| Man, 50 | 0.876 | Regression model, 0.787 to 0.968 |
| Man, 60 | 0.837 | Regression model, 0.717 to 0.963 |
| Man, 70 | 0.791 | Regression model, 0.630 to 0.954 |
| Man, 80 | 0.739 | Regression model, 0.534 to 0.945 |
| Man, 90 | 0.68 | Regression model, 0.424 to 0.938 |
| Utility modifiers (multipliers) | ||
| Stage IV colorectal cancer | 0.789 | Beta, 0.721 to 0.844 |
| Costs | ||
| Diagnostic costs | ||
| IHC | £30.36 | Gamma, £21.10 to £44.95 |
| MSI | £36.63 | Gamma, £23.90 to £55.35 |
| MLH1 methylation post-IHC | £32.65 | Gamma, £20.96 to £44.72 |
| MLH1 methylation post-MSI | £22.84 | Gamma, £14.53 to £35.86 |
| NGS | £236.35 | Gamma, £152.85 to £326.52 |
| Post-test counselling (proband) | £133.15 | Gamma, £83.00 to £185.05 |
| Consent to test (proband) | £13.64 | Gamma, £12.60 to £14.79 |
| GP referral (relative) | £36.40 | Gamma, £24.16 to £48.46 |
| Pre-test counselling (relative) | £171.73 | Gamma, £110.07 to £252.03 |
| Predictive mutation testing (relative) | £166.32 | Gamma, £116.27 to £245.42 |
| Post-test counselling (relative) | £133.15 | Gamma, £84.75 to £183.80 |
| PREMM5 scoring | £3.58 | Gamma, £2.35 to £5.29 |
| Surveillance costs | ||
| Colonoscopy | £583.34 | Gamma, £383.84 to £809.88 |
| Interval between colonoscopies | 2.1 years | Log-normal, 1.54 to 2.89 years |
| Colorectal cancer costs (lifetime discounted costs, by stage and age) | ||
| Stage I, <50 | £8754 | Gamma, £5907 to £11,966 |
| Stage I, 50–59 | £5712 | Gamma, £3793 to £7993 |
| Stage I, 60–69 | £4623 | Gamma, £2990 to £6602 |
| Stage I, 70–79 | £3178 | Gamma, £2125 to £4701 |
| Stage I, ≥80 | £1380 | Gamma, £917 to £1868 |
| Stage II, <50 | £8741 | Gamma, £5529 to £12,172 |
| Stage II, 50–59 | £7016 | Gamma, £4367 to £10,348 |
| Stage II, 60–69 | £5352 | Gamma, £3447 to £7751 |
| Stage II, 70–79 | £3455 | Gamma, £2166 to £5124 |
| Stage II, ≥80 | £1546 | Gamma, £923 to £2216 |
| Stage III, <50 | £14,490 | Gamma, £8742 to £21,370 |
| Stage III, 50–59 | £9692 | Gamma, £6269 to £13,227 |
| Stage III, 60–69 | £7259 | Gamma, £4500 to £10,369 |
| Stage III, 70–79 | £4485 | Gamma, £2965 to £6123 |
| Stage III, ≥80 | £1561 | Gamma, £1044 to £2424 |
| Stage IV, <50 | £11,705 | Gamma, £7719 to £17,169 |
| Stage IV, 50–59 | £8444 | Gamma, £5762 to £11,688 |
| Stage IV, 60–69 | £6509 | Gamma, £4461 to £9038 |
| Stage IV, 70–79 | £4365 | Gamma, £2636 to £5946 |
| Stage IV, ≥80 | £807 | Gamma, £514 to £1097 |
References
- Vasen, H.; Blanco, I.; Aktan-Collan, K.; Gopie, J.P.; Alonso, A.; Aretz, S.; Bernstein, I.T.; Bertario, L.; Burn, J.; Capellá, G.; et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): Recommendations by a group of European experts. Gut 2013, 62, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Frankel, W.; Panescu, J.; Lockman, J.; Sotamaa, K.; Fix, D.; Comeras, I.; La Jeunesse, J.; Nakagawa, H.; Westman, J.A.; et al. Screening for Lynch Syndrome (Hereditary Nonpolyposis Colorectal Cancer) among Endometrial Cancer Patients. Cancer Res. 2006, 66, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Frankel, W.L.; Martin, E.; Arnold, M.; Khanduja, K.; Kuebler, P.; Clendenning, M.; Sotamaa, K.; Prior, T.; Westman, J.A.; et al. Feasibility of Screening for Lynch Syndrome Among Patients With Colorectal Cancer. J. Clin. Oncol. 2008, 26, 5783–5788. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D. When is Genomic Testing Cost-Effective? Testing for Lynch Syndrome in Patients with Newly-Diagnosed Colorectal Cancer and Their Relatives. Healthcare 2015, 3, 860–878. [Google Scholar] [CrossRef] [PubMed]
- Ladabaum, U.; Wang, G.; Terdiman, J.; Blanco, A.; Kuppermann, M.; Boland, C.R.; Ford, J.; Elkin, E.; Phillips, K.A. Strategies to Identify the Lynch Syndrome Among Patients with Colorectal Cancer. Ann. Intern. Med. 2011, 155, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mvundura, M.; Grosse, S.D.; Hampel, H.; Palomaki, G.E. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet. Med. 2009, 12, 93–104. [Google Scholar] [CrossRef]
- Snowsill, T.; Coelho, H.; Huxley, N.; Jones-Hughes, T.; Briscoe, S.; Frayling, I.M.; Hyde, C. Molecular testing for Lynch syndrome in people with colorectal cancer: Systematic reviews and economic evaluation. Health Technol. Assess. 2017, 21, 1–238. [Google Scholar] [CrossRef]
- Snowsill, T.; Huxley, N.; Hoyle, M.; Jones-Hughes, T.; Coelho, H.; Cooper, C.; Frayling, I.M.; Hyde, C. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol. Assess. 2014, 18, 1–406. [Google Scholar] [CrossRef]
- Bruegl, A.S.; Djordjevic, B.; Batte, B.; Daniels, M.; Fellman, B.; Urbauer, D.; Luthra, R.; Sun, C.; Lu, K.H.; Broaddus, R.R. Evaluation of clinical criteria for the identification of Lynch syndrome among unselected patients with endometrial cancer. Cancer Prev. Res. 2014, 7, 686–697. [Google Scholar] [CrossRef]
- Goverde, A.; Spaander, M.C.; Van Doorn, H.C.; Dubbink, H.J.; Ouweland, A.M.V.D.; Tops, C.M.; Kooi, S.G.; De Waard, J.; Hoedemaeker, R.F.; Bruno, M.; et al. Cost-effectiveness of routine screening for Lynch syndrome in endometrial cancer patients up to 70 years of age. Gynecol. Oncol. 2016, 143, 453–459. [Google Scholar] [CrossRef]
- Kwon, J.S.; Scott, J.L.; Gilks, C.B.; Daniels, M.; Sun, C.C.; Lu, K.H. Testing Women with Endometrial Cancer to Detect Lynch Syndrome. J. Clin. Oncol. 2011, 29, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Resnick, K.; Straughn, J.M., Jr.; Backes, F.; Hampel, H.; Matthews, K.S.; Cohn, D.E. Lynch syndrome screening strategies among newly diagnosed endometrial cancer patients. Obstet. Gynecol. 2009, 114, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Snowsill, T.M.; Ryan, N.A.J.; Crosbie, E.J.; Frayling, I.M.; Evans, D.G.; Hyde, C.J. Cost-effectiveness analysis of reflex testing for Lynch syndrome in women with endometrial cancer in the UK setting. PLoS ONE 2019, 14, e0221419. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Nuovo, G.J.; Zervos, E.E.; Martin, E.W.; Salovaara, R.; Aaltonen, L.A.; de la Chapelle, A. Age-related Hypermethylation of the 5′ Region of MLH1 in Normal Colonic Mucosa Is Associated with Microsatellite-unstable Colorectal Cancer Development. Cancer Res. 2001, 61, 6991. [Google Scholar]
- Wu, Y.; Berends, M.J.W.; Mensink, R.G.J.; Kempinga, C.; Sijmons, R.; Van Der Zee, A.G.J.; Hollema, H.; Kleibeuker, J.H.; Buys, C.H.C.M.; Hofstra, R. Association of Hereditary Nonpolyposis Colorectal Cancer–Related Tumors Displaying Low Microsatellite Instability with MSH6 Germline Mutations. Am. J. Hum. Genet. 1999, 65, 1291–1298. [Google Scholar] [CrossRef]
- Ryan, N.; McMahon, R.; Tobi, S.; Snowsill, T.; Esquibel, S.; Wallace, A.; Bunstone, S.; Bowers, N.; Mosneag, I.; Kitson, S.; et al. The Proportion of Endometrial Tumors Associated with Lynch Syndrome (PETALS study). PLOS Med. (Under review).
- Kastrinos, F.; Uno, H.; Ukaegbu, C.; Alvero, C.; McFarland, A.; Yurgelun, M.B.; Kulke, M.H.; Schrag, D.; Meyerhardt, J.A.; Fuchs, C.S.; et al. Development and Validation of the PREMM5 Model for Comprehensive Risk Assessment of Lynch Syndrome. J. Clin. Oncol. 2017, 35, 2165–2172. [Google Scholar] [CrossRef]
- Anonymous. The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- Filipovic-Pierucci, A.; Zarca, K.; Durand-Zaleski, I. Markov Models for Health Economic Evaluations: The R Package heemod. Value Health 2017, 19, 369. [Google Scholar] [CrossRef]
- Järvinen, H.J.; Aarnio, M.; Mustonen, H.; Aktan-Collan, K.; Aaltonen, L.; Peltomaki, P.; De La Chapelle§, A.; Mecklin, J. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000, 118, 829–834. [Google Scholar] [CrossRef]
- Mecklin, J.-P.; Aarnio, M.; Läärä, E.; Kairaluoma, M.V.; Pylvänäinen, K.; Peltomaki, P.; Aaltonen, L.; Järvinen, H.J. Development of Colorectal Tumors in Colonoscopic Surveillance in Lynch Syndrome. Gastroenterology 2007, 133, 1093–1098. [Google Scholar] [CrossRef]
- Curtis, L.; Burns, A. Unit Costs of Health and Social Care 2018; Personal Social Services Research Unit (PSSRU); University of Kent: Caterbury, Kent, UK, 2018. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Davison, N.; Payne, K.; Cole, A.; Evans, D.G.; Crosbie, E.J. A Micro-Costing Study of Screening for Lynch Syndrome-Associated Pathogenic Variants in an Unselected Endometrial Cancer Population: Cheap as NGS Chips? Front. Oncol. 2019, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Slade, I.; Hanson, H.; George, A.; Kohut, K.; Strydom, A.; Wordsworth, S.; Rahman, N.; Programme, M. A cost analysis of a cancer genetic service model in the UK. J. Community Genet. 2016, 7, 185–194. [Google Scholar] [CrossRef]
- Rahman, N. Mainstreaming genetic testing of cancer predisposition genes. Clin. Med. 2014, 14, 436–439. [Google Scholar] [CrossRef] [PubMed]
- NHS Improvement. National Schedule of Reference Costs 2016–2017. Available online: https://improvement.nhs.uk/resources/reference-costs/ (accessed on 3 April 2020).
- Department of Health. NHS Reference Costs 2015 to 2016. Available online: https://www.gov.uk/government/publications/nhs-reference-costs-2015-to-2016 (accessed on 6 December 2017).
- Whyte, S.; Harnan, S.; Scope, A.; Simpson, E.; Tappenden, P.; Duffy, S.; Rachet, B.; Sculpher, M.; Hinde, S.; McKenna, C.; et al. Early Awareness Interventions for Cancer: Colorectal Cancer; Economic Evaluation of Health and Social Care Interventions Policy Research Unit: University of Sheffield; University of York: Sheffield, UK; York, UK, 2012. [Google Scholar]
- Ara, R.; Brazier, J. Populating an Economic Model with Health State Utility Values: Moving toward Better Practice. Value Health 2010, 13, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Djalalov, S.; Rabeneck, L.; Tomlinson, G.; Bremner, K.E.; Hilsden, R.; Hoch, J.S. A Review and Meta-analysis of Colorectal Cancer Utilities. Med. Decis. Mak. 2014, 34, 809–818. [Google Scholar] [CrossRef]
- Arrigoni, A.; Sprujevnik, T.; Alvisi, V.; Rossi, A.; Ricci, G.; Pennazio, M.; Spandre, M.; Cavallero, M.; Bertone, A.; Foco, A.; et al. Clinical identification and long-term surveillance of 22 hereditary non-polyposis colon cancer Italian families. Eur. J. Gastroenterol. Hepatol. 2005, 17, 213–219. [Google Scholar] [CrossRef]
- Ladabaum, U.; Ford, J.M.; Martel, M.; Barkun, A.N. American Gastroenterological Association Technical Review on the Diagnosis and Management of Lynch Syndrome. Gastroenterology 2015, 149, 783–813. [Google Scholar] [CrossRef]
- Møller, P.; Seppälä, T.; Bernstein, I.T.; Holinski-Feder, E.; Sala, P.; Evans, D.G.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2017, 67, 1306–1316. [Google Scholar] [CrossRef]
- Ryan, N.A.J.; Morris, J.; Green, K.; Lalloo, F.; Woodward, E.R.; Hill, J.; Crosbie, E.J.; Evans, D.G. Association of Mismatch Repair Mutation with Age at Cancer Onset in Lynch Syndrome. JAMA Oncol. 2017, 3, 1702–1706. [Google Scholar] [CrossRef]
- Crim, A.; Perkins, V.; Husain, S.; Ding, K.; Holman, L. Feasibility of two-antibody vs four-antibody mismatch repair protein immunohistochemistry as initial screening for Lynch syndrome in patients with endometrial adenocarcinoma. Gynecol. Oncol. 2017, 145, 44. [Google Scholar] [CrossRef]
- Kausmeyer, D.T.; Lengerich, E.J.; Kluhsman, B.C.; Morrone, D.; Harper, G.R.; Baker, M.J. A Survey of Patients’ Experiences with the Cancer Genetic Counseling Process: Recommendations for Cancer Genetics Programs. J. Genet. Couns. 2006, 15, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Gerdes, A.-M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.M.; Evans, D.G.R.; Maher, E.R.; Bertario, L.; et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet 2011, 378, 2081–2087. [Google Scholar] [CrossRef]
- Sculpher, M.J.; Pang, F.S.; Manca, A.; Drummond, M.F.; Golder, S.; Urdahl, H.; Davies, L.; Eastwood, A. Generalisability in economic evaluation studies in healthcare: A review and case studies. Health Technol. Assess. 2004, 8, 8. [Google Scholar] [CrossRef] [PubMed]
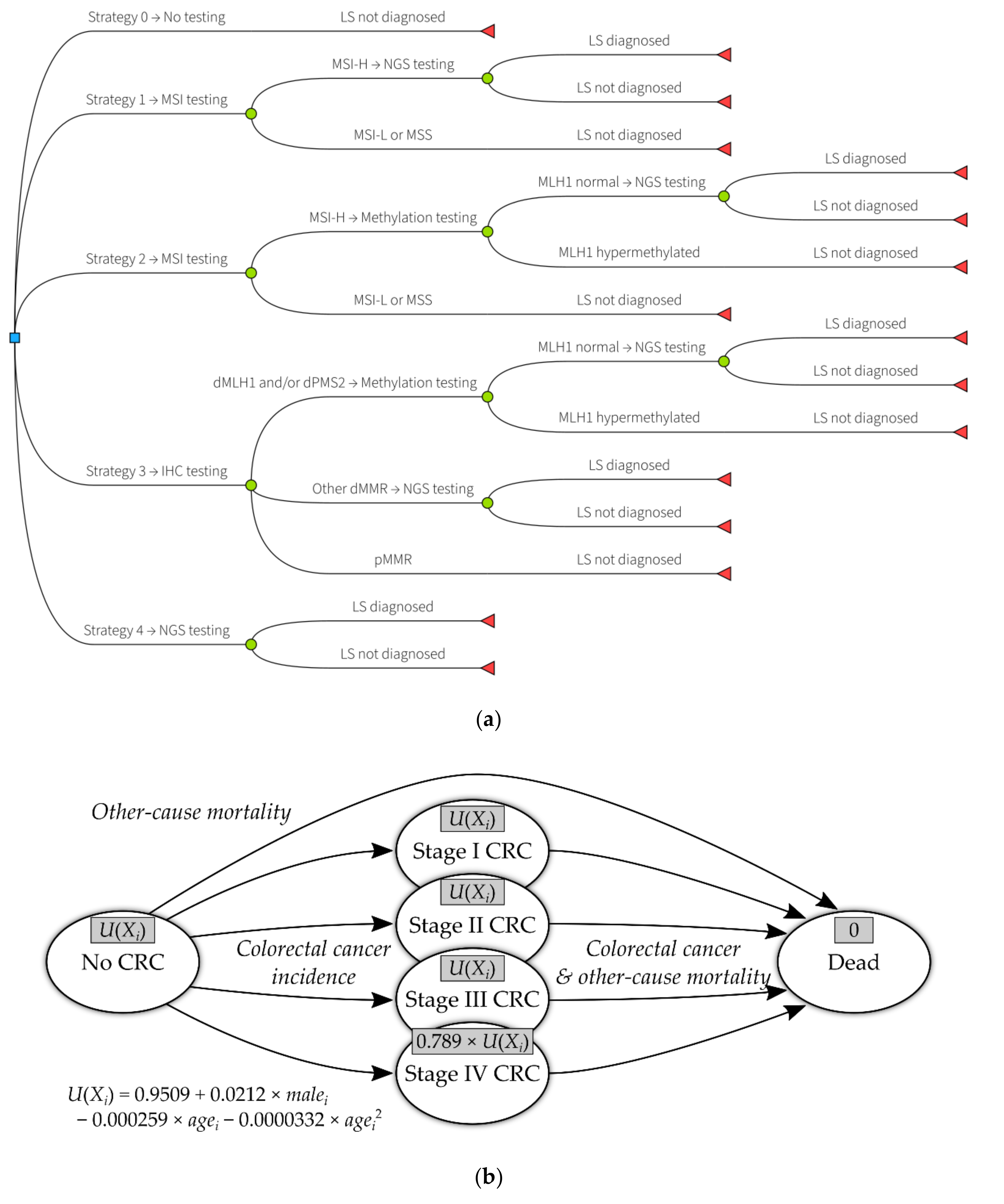
| Decision Problem | What is the Relative Cost-Effectiveness of Strategies to Identify Lynch Syndrome in Women with Endometrial Cancer |
|---|---|
| Interventions and comparators | Strategy 0: No testing Strategy 1: MSI triage followed by NGS Strategy 2: MSI and MLH1 methylation testing triage followed by NGS Strategy 3: IHC and MLH1 methylation testing triage followed by NGS (the Manchester approach) Strategy 4: Direct NGS |
| Type of economic evaluation, costs, and health outcomes | Cost-effectiveness analysis: Diagnostic costs and Lynch syndrome cases identified (no cost-effectiveness threshold identified) Cost-utility analysis: Lifetime costs and QALYs for women with endometrial cancer and their relatives (cost-effectiveness threshold £20,000 per QALY) |
| Model type | Decision tree and Markov model implemented in R |
| Key data source | PETALS study (diagnostic accuracy study conducted in Manchester) |
| Perspective | NHS and PSS, costs in pounds sterling (£; GBP) in 2016/17 prices |
| Time horizon | Lifetime |
| Discounting | 3.5% for costs and QALYs |
| Analysis of uncertainty | Non-parametric bootstrap resampling of participants in a clinical study and parametric sampling of model parameters (probabilistic sensitivity analysis) |
| Item | Unit Cost (£, GBP) | Source |
|---|---|---|
| Calculate PREMM₅ score | 3.58 | PSSRU 2017 [22] |
| MMR IHC (4 protein panel) | 30.36 ¹ | Ryan et al. 2019 [23] |
| MMR IHC (2 protein panel) | 15.18 | Assumed half cost of 4 protein panel |
| MSI testing | 36.63 ¹ | Ryan et al. 2019 [23] |
| MLH1 methylation testing (strategy 2) | 22.84 ¹ | Ryan et al. 2019 [23] |
| MLH1 methylation testing (strategy 3) | 32.65 ¹ | Ryan et al. 2019 [23] |
| Obtain consent for NGS | 13.64 ¹ | Ryan et al. 2019 [23] |
| NGS | 236.35 ¹ | Ryan et al. 2019 [23] |
| Post-test genetic counselling (probands) | 133.15 | Slade et al. 2016 [24] |
| Pre-test genetic counselling (relatives) | 171.73 | Slade et al. 2016 [24] |
| Predictive genetic testing (relatives) | 166.32 | Slade et al. 2016 [24] |
| Post-test genetic counselling (relatives) | 133.15 | Slade et al. 2016 [24] |
| Strategy | Costs (£) | Effectiveness Outcome | ICER |
|---|---|---|---|
| Short-term | Diagnostic pathway costs ¹ | Lynch syndrome cases identified ¹ | Additional diagnostic pathway cost per Lynch syndrome case identified |
| Strategy 0 No testing | 0 | 0 | — |
| Strategy 1 MSI | 41,512 | 9 | Dominated |
| Strategy 2 MSI and MLH1 methylation | 27,523 | 9 | Dominated |
| Strategy 3 IHC and MLH1 methylation | 27,183 | 16 | 1699 |
| Strategy 4 Direct NGS | 127,125 | 16 | Dominated |
| Lifetime | Lifetime costs (proband/proband and relatives) | Lifetime QALYs (proband/proband and relatives) | Additional cost per QALY gained (proband only/proband and relatives) |
| Strategy 0 No testing | 120 642 | 7.64 104.61 | — — |
| Strategy 1 MSI | 223 842 | 7.65 104.65 | Dominated Dominated |
| Strategy 2 MSI and MLH1 methylation | 195 815 | 7.65 104.65 | Extendedly dominated 3738 |
| Strategy 3 IHC and MLH1 methylation | 220 928 | 7.66 104.67 | 5003 5459 |
| Strategy 4 Direct NGS | 419 1128 | 7.66 104.67 | Dominated Dominated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snowsill, T.M.; Ryan, N.A.J.; Crosbie, E.J. Cost-Effectiveness of the Manchester Approach to Identifying Lynch Syndrome in Women with Endometrial Cancer. J. Clin. Med. 2020, 9, 1664. https://doi.org/10.3390/jcm9061664
Snowsill TM, Ryan NAJ, Crosbie EJ. Cost-Effectiveness of the Manchester Approach to Identifying Lynch Syndrome in Women with Endometrial Cancer. Journal of Clinical Medicine. 2020; 9(6):1664. https://doi.org/10.3390/jcm9061664
Chicago/Turabian StyleSnowsill, Tristan M., Neil A. J. Ryan, and Emma J. Crosbie. 2020. "Cost-Effectiveness of the Manchester Approach to Identifying Lynch Syndrome in Women with Endometrial Cancer" Journal of Clinical Medicine 9, no. 6: 1664. https://doi.org/10.3390/jcm9061664
APA StyleSnowsill, T. M., Ryan, N. A. J., & Crosbie, E. J. (2020). Cost-Effectiveness of the Manchester Approach to Identifying Lynch Syndrome in Women with Endometrial Cancer. Journal of Clinical Medicine, 9(6), 1664. https://doi.org/10.3390/jcm9061664






