High Blood Pressure Is Associated with Tubulointerstitial Damage along with Glomerular Damage in Glomerulonephritis. A large Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory and Histology
2.3. Statistics
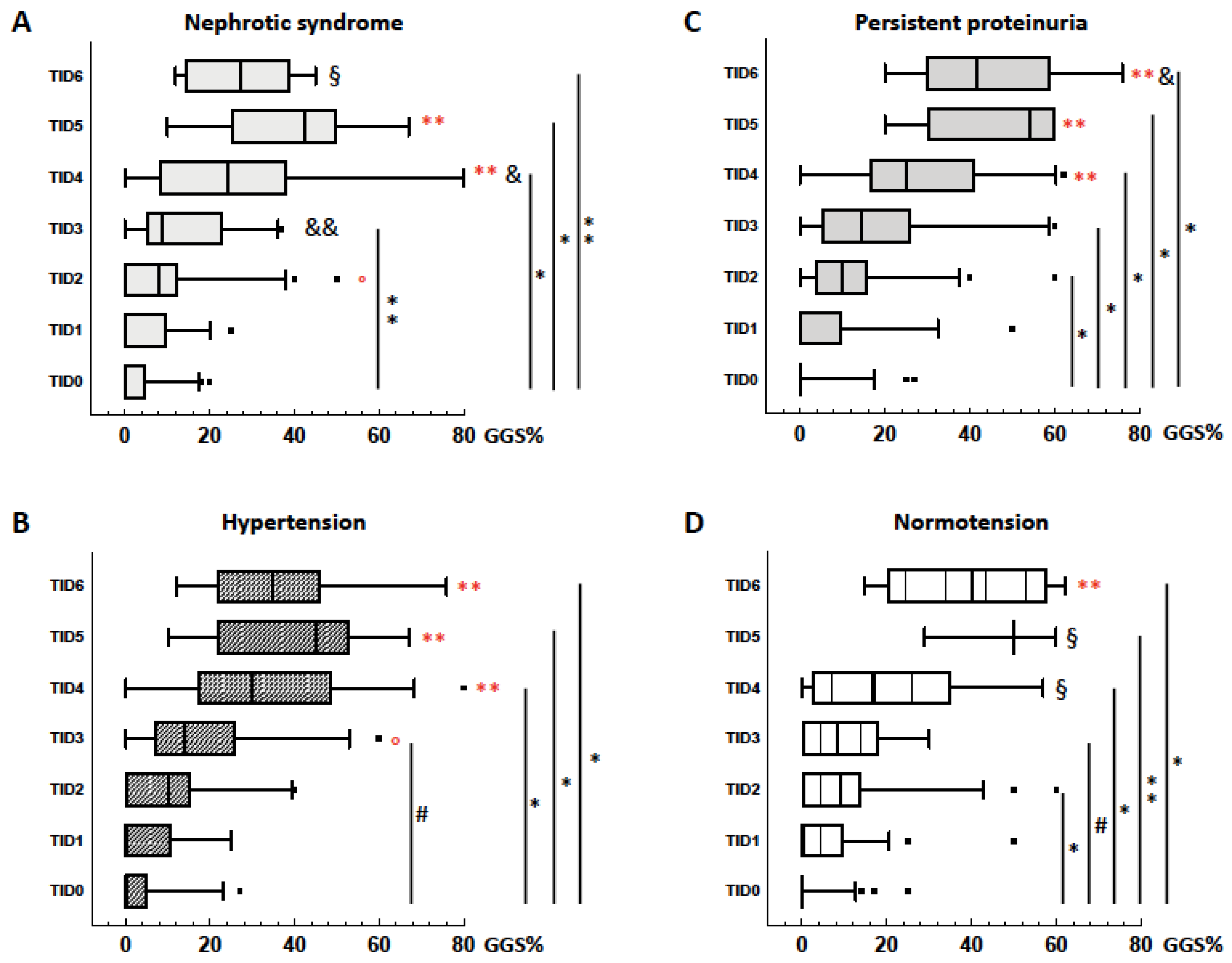
3. Results
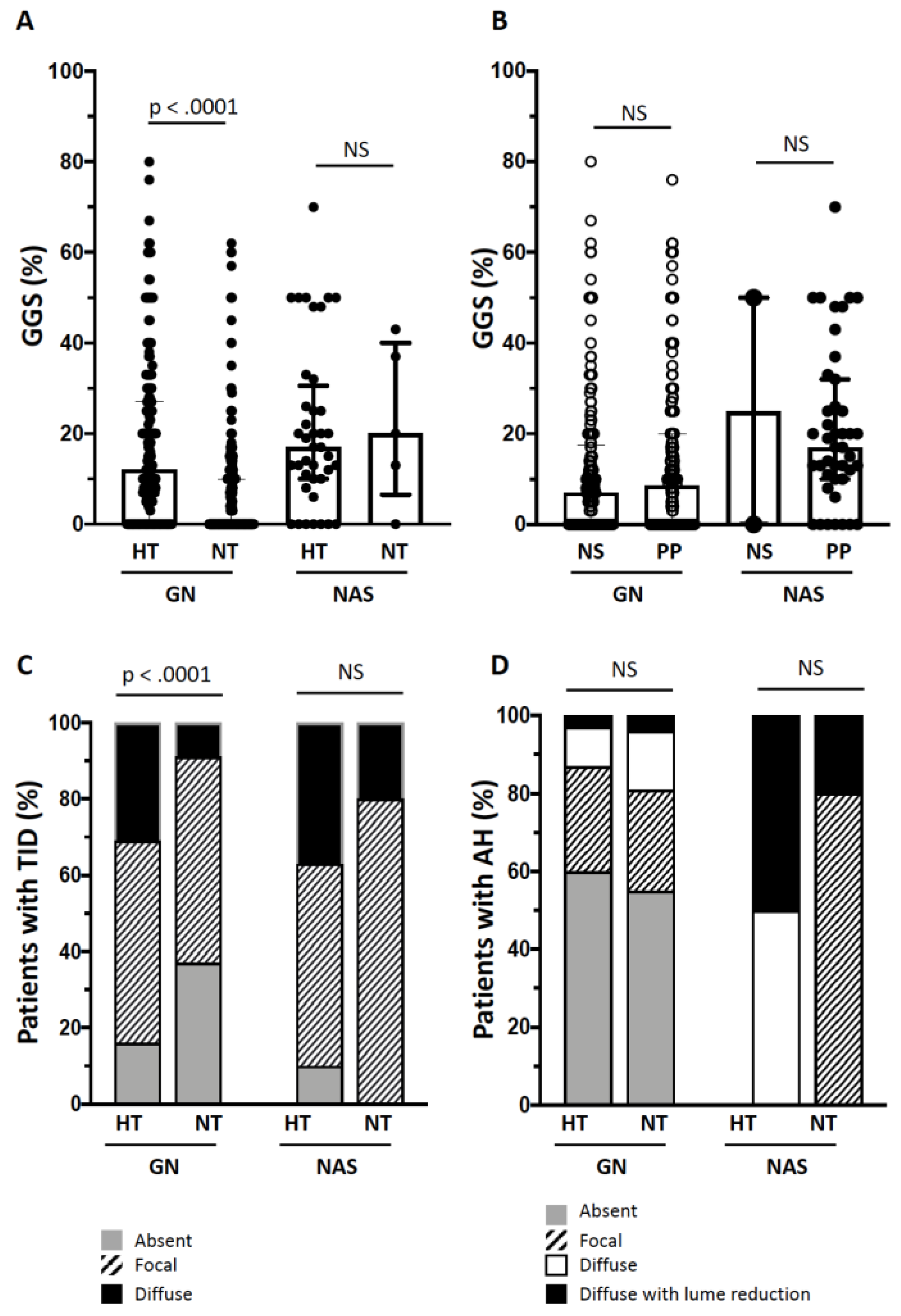
3.1. Kidney Damage in Hypertensive and Normotensives Patients with GN
3.2. Kidney Damage in Hypertensive and Normotensives Patients with NAS
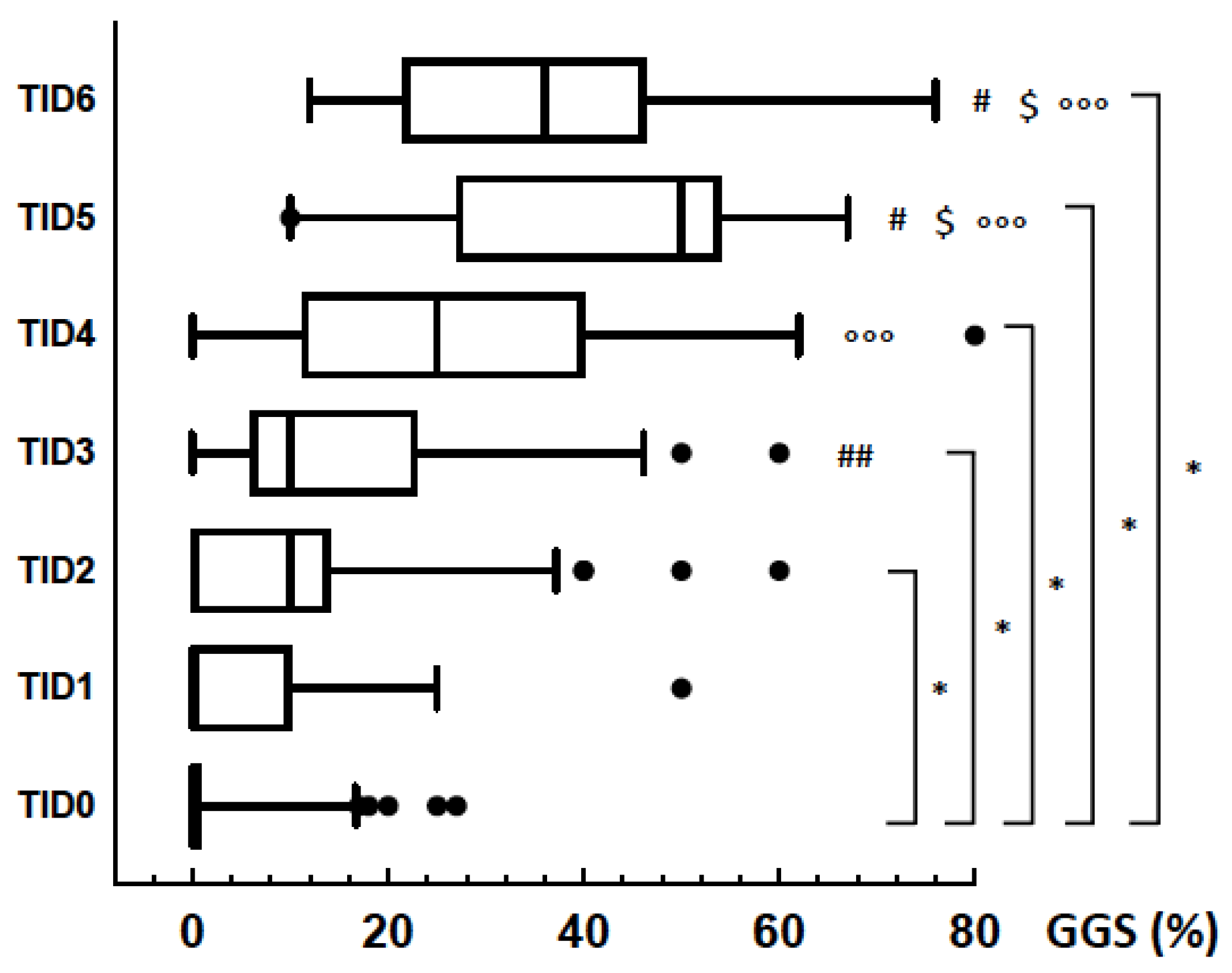
3.3. GGS and TID
3.4. Follow-Up
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Variable | GN and NS | p HT vs. NT | GN and PP | p HT vs. NT | ||
|---|---|---|---|---|---|---|
| HT (n = 122) | NT (n = 82) | HT (n = 79) | NT (n = 120) | |||
| Sex (M, %) | 69 (57) | 36 (44) | 0.08 | 56 (71) | 64 (53) | 0.01 |
| Age (years) | 43 ± 18 | 38 ± 16 | 0.01 | 43 ± 16 | 39 ± 16 | 0.11 |
| eGFR (mL/min/1.73 m2) | 57 ± 30 | 91 ± 26 | 0.01 | 60 ± 24 | 85 ± 25 | 0.01 |
| U-proteins/creatinine (mg/µg) | 4201 | 3277 | 0.31 | 585 | 332 | 0.01 |
| (2760–6224) | (1835–5913) | (214–1176) | (114–848) | |||
| U-IgG/creatinine (mg/µg) | 193 | 102 | 0.01 | 31 | 13 | 0.01 |
| (91–422) | (52–193) | (10–60) | (6–38) | |||
| U-albumin/creatinine (mg/µg) | 3516 | 2978 | 0.11 | 403 | 224 | 0.02 |
| (2157–5155) | (1531–5107) | (108–930) | (54–562) | |||
| U-α1-microglobulin/creatinine (mg/µg) | 43 | 21 | 0.01 | 10 | 5 | 0.01 |
| (24–87) | (10–39) | (4–19) | (3–11) | |||
| U-transferrin/creatinine (mg/µg) | 262 | 232 | 0.34 | 22 | 10 | 0.02 |
| (148–393) | (116–424) | (6–51) | (3–39) | |||
| U-NAG/creatinine (IU/g) | 16.89 | 13.00 | 0.34 | 4.61 | 3.5 | 0.01 |
| (9.3–27.5) | (6.75–21.31) | (3.08–7.88) | (2.2–6.3) | |||
| Variable | cIgAN | MPGN | IMN | IgAN | LN | FSGS | MCD | |
|---|---|---|---|---|---|---|---|---|
| (n = 37) | (n = 26) | (n = 100) | (n = 127) | (n = 49) | (n = 46) | (n = 18) | ||
| HT/NT (n) | HT | 14/6 | 11/6 | 38/18 | 40/15 | 2/20 | 16/9 | 4/2 |
| NT | 10/7 | 3/6 | 23/21 | 43/29 | 6/21 | 8/13 | 7/5 | |
| Age (years) | HT | 32 ± 12 | 40 ± 15 | 49 ± 18 | 43 ± 17 | 36 ± 16 | 48 ± 18 *** | 50 ± 20 |
| NT | 28 ± 9 | 37 ± 15 | 47± 18 | 42 ± 17 | 31 ± 10 | 29 ± 7 ** | 38 ± 19 | |
| eGFR (mL/min/1.73 m2) | HT | 40.50 *** | 46.00 ** | 58.50 *** | 59.00 *** | 70.50 * | 61.00 *** | 65.00 |
| (17.50–52.25) | (18.50–87.50) | (32.00–78.00) | (45.00–70.00) | (25.25–92.50) | (43.50–94.00) | (48.25–90.25) * | ||
| 94.00 | 96.00 | 90.50 | 79.00 | 91.00 | 102.00 | 98.00 | ||
| (67.50–100.00) | (84.50–117.50) | (75.25–103.50) | (61.00–93.75) | (76.00–102.00) | (88.50–113.50) | (88.00–128.50) | ||
| U-proteins/creatinine (mg/µg) | HT | 1804 ** | 4859 | 3801 ** | 407 | 2820 *** | 5359 | 4277 |
| (965–3829) | (1341–8490) | (2188–6571) | (131–850) * | (1483–4667) | (2796–9052) | (3270–3135) | ||
| NT | 545 | 1761 | 2438 | 204 | 907 | 3000 | 4664 | |
| (228–1395) | (611–5978) | (1297–4287) | (99–532) | (524–1781) | (650–7697) | (2401–6643) | ||
| U-IgG/creatinine (mg/µg) | HT | 113 * | 199 * | 181 *** | 21 ** | 125 * | 195 * | 116 |
| (48–289) | (69–858) | (67–403) | (7–59) | (71–386) | (79–383) | (76–270) | ||
| NT | 32 | 45 | 68 | 10 | 68 | 102 | 94 | |
| (12–76) | (18–197) | (28–131) | (5–28) | (15–149) | (29–186) | (52–148) | ||
| U-albumin/creatinine (mg/g) | HT | 1783 *** | 3973 | 3355 ** | 313 * | 2191 *** | 4656 | 4046 |
| (814–3010) | (1234–5855) | (1788–5103) | (47–704) | (988–3941) | (2070–6556) | (2804–5186) | ||
| NT | 432 | 1735 | 2280 | 126 | 624 | 2621 | 4398 | |
| (141–1292) | (497–4461) | (1143–3664) | (32–468) | (266–1259) | (474–6414) | (1941–6225) | ||
| U-α1-microglobulin/creatinine (mg/g) | HT | 32 *** | 78 ** | 42 *** | 10 ** | 26 | 41 * | 30 |
| (16–49) | (16–104) | (25–87) | (4–18) | (13–57) * | (18–62) | (19–42) | ||
| NT | 6 | 5 | 15 | 5 | 11 | 21 | 17 | |
| (2–12) | (3–42) | (7–33) | (2–11) | (5–25) | (7–40) | (15–31) | ||
| U-transferrin/creatinine (mg/g) | HT | 108 ** | 246 | 264 | 13 * | 151 *** | 335 | 319 |
| (38–189) | (55–507) | (117–371) | (3–32) | (92–231) | (155–572) | (234–425) | ||
| NT | 19 | 121 | 189 | 6 | 43 | 211 | 332 | |
| (8–65) | (25–437) | (66–317) | (2–23) | (19–87) | (36–536) | (208–464) | ||
| U-NAG/creatinine (IU/g) | HT | 10.32 ** | 18.75 | 15.81 ** | 4.05 * | 12.35 | 14.89 | 7.61 |
| (6.62–16.63) | (4.67–36.21) | (9.90–26.14) | (2.82–6.36) | (6.47–31.02) | (9.02–26.72) | (5.61–15.51) | ||
| NT | 4.68 | 5.55 | 9.86 | 2.94 | 7.66 | 10.00 | 12.01 | |
| (3.19–6.53) | (2.66–38.41) | (5.44–18.01) | (2.08–5.33) | (5.77–13.36) | (5.58–25.81) | (7.04–17.3) |
References
- Risdon, R.A.; Sloper, J.C.; De Wardener, H.E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 1968, 2, 363–366. [Google Scholar] [CrossRef]
- Srivastava, A.; Palsson, R.; Kaze, A.D.; Chen, M.E.; Palacios, P.; Sabbisetti, V.; Betensky, R.A.; Steinman, T.I.; Thadhani, R.I.; McMahon, G.M.; et al. The Prognostic Value of Histopathologic Lesions in Native Kidney Biopsy Specimens: Results from the Boston Kidney Biopsy Cohort Study. J. Am. Soc. Nephrol. 2018, 29, 2213–2224. [Google Scholar] [CrossRef] [PubMed]
- Mitic, B.; Lazarevic, G.; Vlahovic, P.; Rajic, M.; Stefanovic, V. Diagnostic value of the aminopeptidase N, N-acetyl-beta-D-glucosaminidase and dipeptidylpeptidase IV in evaluating tubular dysfunction in patients with glomerulopathies. Ren. Fail. 2008, 30, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Zhu, X.; Feng, Q.; Li, X.; Feng, X. Urinary protein markers predict the severity of renal histological lesions in children with mesangial proliferative glomerulonephritis. BMC Nephrol. 2012, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.; van den Brand, J.A.; Waanders, F.; Meijer, E.; Goor van, H.; Peters, H.P.; Hofstra, J.M.; Wetzels, J.F. Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as prognostic markers in idiopathic membranous nephropathy. Ann. Clin. Biochem. 2016, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pagni, F.; Galimberti, S.; Galbiati, E.; Rebora, P.; Pietropaolo, V.; Pieruzzi, F.; Smith, A.J.; Ferrario, F. Tubulointerstitial lesions in lupus nephritis: International multicentre study in a large cohort of patients with repeat biopsy. Nephrology (Carlton) 2016, 21, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hagiyama, M.; Nakatani, Y.; Takashima, Y.; Kato, T.; Inoue, T.; Kimura, R.; Otani, T.; Sato, Y.; Mori, H.; Arima, S.; et al. Urinary Cell Adhesion Molecule 1 Is a Novel Biomarker That Links Tubulointerstitial Damage to Glomerular Filtration Rates in Chronic Kidney Disease. Front. Cell Dev. Biol. 2019, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Meyrier, A. Nephrosclerosis: Update on a centenarian. Nephrol. Dial. Transplant. 2015, 30, 1833–1841. [Google Scholar] [CrossRef]
- Seccia, T.M.; Caroccia, B.; Calò, L.A. Hypertensive nephropathy. Moving from classic to emerging pathogenetic mechanisms. J. Hypertens. 2017, 35, 205–212. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. D.I.A.F.M. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication—worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Canetta, P.A.; Troost, J.P.; Mahoney, S.; Kogon, A.J.; Carlozzi, N.; Bartosh, S.M.; Cai, Y.; Davis, T.K.; Fernandez, H.; Fornoni, A.; et al. Health-related quality of life in glomerular disease. Kidney Int. 2019, 95, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.K.-C.; Li, P.K.-T. Chronic kidney disease epidemic: How do we deal with it? Nephrology (Carlton) 2018, 23 (Suppl. 4), 116–120. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- de la Sierra, A.; Segura, J.; Banegas, J.R.; Gorostidi, M.; de la Cruz, J.J.; Armario, P.; Oliveras, A.; Ruilope, L.M. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension (Dallas, Tex. 1979) 2011, 57, 898–902. [Google Scholar] [CrossRef]
- Gesualdo, L.; Di Palma, A.M.; Morrone, L.F.; Strippoli, G.F.; Schena, F.P. Italian Immunopathology Group, Italian Society of Nephrology The Italian experience of the national registry of renal biopsies. Kidney Int. 2004, 66, 890–894. [Google Scholar] [CrossRef]
- Zink, C.M.; Ernst, S.; Riehl, J.; Helmchen, U.; Gröne, H.-J.; Floege, J.; Schlieper, G. Trends of renal diseases in Germany: Review of a regional renal biopsy database from 1990 to 2013. Clin. Kidney J. 2019, 12, 795–800. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.M.; Hogan, S.L.; Thompson, B.D.; Coppo, R.; Fogo, A.B.; Jennette, J.C. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol. Dial. Transplant. 2018, 33, 661–669. [Google Scholar] [CrossRef]
- Sim, J.J.; Batech, M.; Hever, A.; Harrison, T.N.; Avelar, T.; Kanter, M.H.; Jacobsen, S.J. Distribution of Biopsy-Proven Presumed Primary Glomerulonephropathies in 2000-2011 Among a Racially and Ethnically Diverse US Population. Am. J. Kidney Dis. 2016, 68, 533–544. [Google Scholar] [CrossRef]
- Parikh, N.I.; Hwang, S.-J.; Larson, M.G.; Meigs, J.B.; Levy, D.; Fox, C.S. Cardiovascular Disease Risk Factors in Chronic Kidney Disease. Arch. Intern. Med. 2006, 166, 1884. [Google Scholar] [CrossRef]
- Alencar de Pinho, N.; Levin, A.; Fukagawa, M.; Hoy, W.E.; Pecoits-Filho, R.; Reichel, H.; Robinson, B.; Kitiyakara, C.; Wang, J.; Eckardt, K.-U.; et al. Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019, 96, 983–994. [Google Scholar] [CrossRef]
- Yu, Z.; Rebholz, C.M.; Wong, E.; Chen, Y.; Matsushita, K.; Coresh, J.; Grams, M.E. Association Between Hypertension and Kidney Function Decline: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2019, 74, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Anderson, A.; Charleston, J.; Chen, Z.; Ford, V.; Makos, G.; O’Connor, A.; Perumal, K.; Rahman, M.; Steigerwalt, S.; et al. Hypertension awareness, treatment, and control in adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2010, 55, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. Caring for individuals with hypertension in CKD, especially those with low education. Kidney Int. 2019, 96, 820–822. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A. Tubulointerstitial Changes as a Major Determinant in the Progression of Renal Damage. Am. J. Kidney Dis. 1992, 20, 1–17. [Google Scholar] [CrossRef]
- Malhotra, R.; Craven, T.; Ambrosius, W.T.; Killeen, A.A.; Haley, W.E.; Cheung, A.K.; Chonchol, M.; Sarnak, M.; Parikh, C.R.; Shlipak, M.G.; et al. Effects of Intensive Blood Pressure Lowering on Kidney Tubule Injury in CKD: A Longitudinal Subgroup Analysis in SPRINT. Am. J. Kidney Dis. 2019, 73, 21–30. [Google Scholar] [CrossRef]
- Bullen, A.L.; Katz, R.; Lee, A.K.; Anderson, C.A.M.; Cheung, A.K.; Garimella, P.S.; Jotwani, V.; Haley, W.E.; Ishani, A.; Lash, J.P.; et al. The SPRINT trial suggests that markers of tubule cell function in the urine associate with risk of subsequent acute kidney injury while injury markers elevate after the injury. Kidney Int. 2019, 96, 470–479. [Google Scholar] [CrossRef]
- Garimella, P.S.; Lee, A.K.; Ambrosius, W.T.; Bhatt, U.; Cheung, A.K.; Chonchol, M.; Craven, T.; Hawfield, A.T.; Jotwani, V.; Killeen, A.; et al. Markers of kidney tubule function and risk of cardiovascular disease events and mortality in the SPRINT trial. Eur. Heart J. 2019, 40, 3486–3493. [Google Scholar] [CrossRef]
- Ikee, R.; Kobayashi, S.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Hemmi, N.; Imakiire, T.; Kikuchi, Y.; Suzuki, S.; Miura, S. Impact of Hypertension and Hypertension-Related Vascular Lesions in IgA Nephropathy. Hypertens Res. 2006, 29, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Haruhara, K.; Tsuboi, N.; Koike, K.; Kanzaki, G.; Okabayashi, Y.; Miyazaki, Y.; Kawamura, T.; Ogura, M.; Yokoo, T. Ambulatory blood pressure and tubulointerstitial injury in patients with IgA nephropathy. Clin. Kidney J. 2015, 8, 716–721. [Google Scholar] [CrossRef]
- Ku, E.; Lee, B.J.; Wei, J.; Weir, M.R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 74, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Mashima, Y.; Konta, T.; Kudo, K.; Takasaki, S.; Ichikawa, K.; Suzuki, K.; Shibata, Y.; Watanabe, T.; Kato, T.; Kawata, S.; et al. Increases in Urinary Albumin and beta2-microglobulin are Independently Associated With Blood Pressure in the Japanese General Population: The Takahata Study. Hypertens. Res. 2011, 34, 831–835. [Google Scholar] [CrossRef] [PubMed]
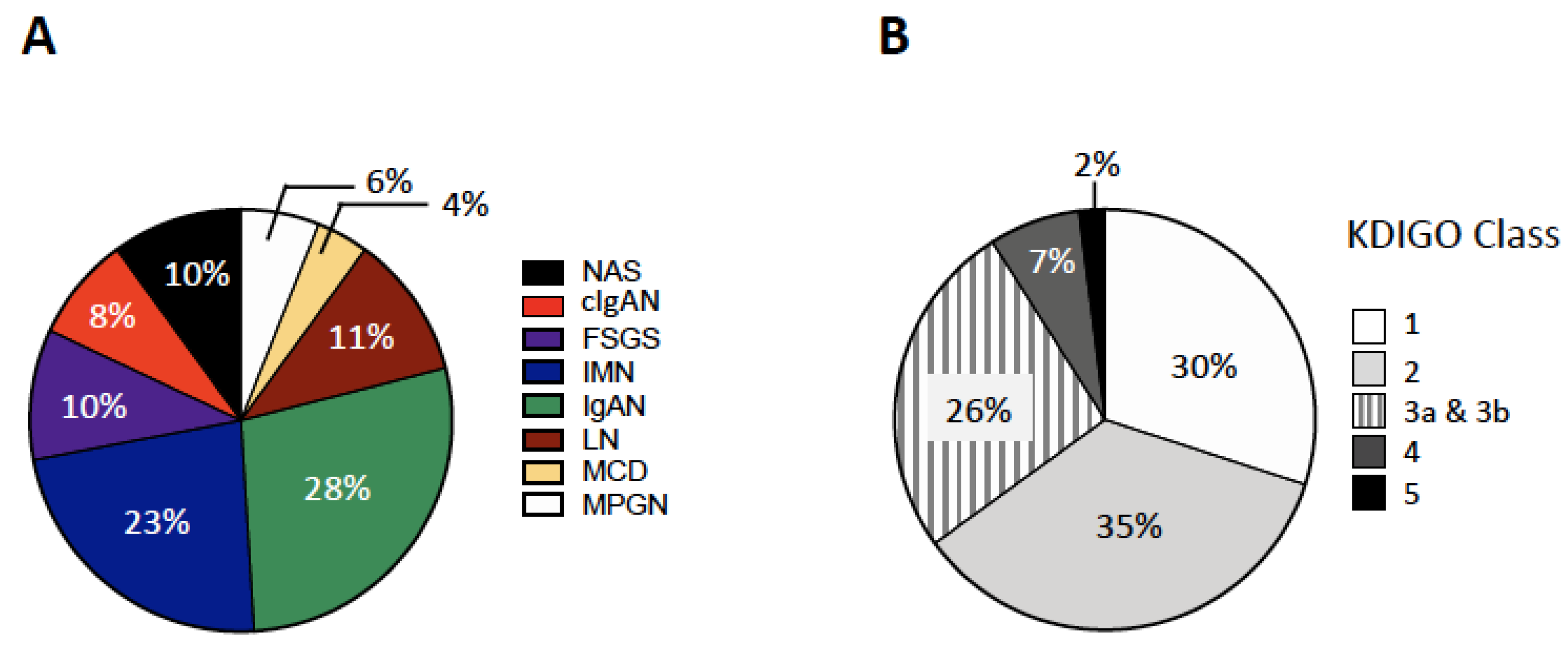
| Variable | All Patients | IgAN | IMN | LN | FSGS | cIgAN | MPGN | MCD | NAS |
|---|---|---|---|---|---|---|---|---|---|
| (n = 448) | (n = 127) | (n = 100) | (n = 49) | (n = 46) | (n = 37) | (n = 26) | (n = 18) | (n = 45) | |
| Sex | 260 (58) | 83 (65) | 61 (61) | 8 (16) *** | 24 (52) * | 24 (65) | 14 (54) | 11 (61) | 35 (78) |
| M (n, %) | |||||||||
| Age | 43 ± 17 | 42 ± 17 *** | 48 ± 18 * | 33 ± 14 *** | 39 ± 17 *** | 30 ± 11 *** | 39 ± 15 *** | 42 ± 20 ** | 57 ± 13 |
| (years) | |||||||||
| eGFR | 72 ± 30 | 71 ± 27 | 71 ± 30 | 78 ± 32 * | 82 ± 30 * | 62 ± 33 | 67 ± 38 | 90 ± 29 ** | 64 ± 22 |
| (mL/min/1.73 m2) | |||||||||
| Hypertension | 233 (52) | 55 (43.3) *** | 56 (56) *** | 22 (44.9) *** | 25 (54.3) *** | 20 (54.1) *** | 17 (65.4) * | 6 (33.3) *** | 40 (88.9) |
| (n, %) | |||||||||
| Nephrotic syndrome | 206 (46) | 2 (1.6) | 80 (80.0) *** | 29 (59.2) *** | 40 (87) *** | 15 (40.5) *** | 20 (76.9) *** | 18 (100) *** | 2 (4.4) |
| (n, %) |
| Variable | GN | p HT vs. NT | NAS | p HT vs. NT | ||
|---|---|---|---|---|---|---|
| HT (n = 201) | NT (n = 202) | HT (n = 40) | NT (n = 5) | |||
| Sex (M, %) | 125 (62) | 100 (50) | 0.01 | 32 (80) | 3 (60) | 0.30 |
| Age (years) | 43 ± 18 | 38 ± 17 | 0.01 | 57 ± 2 | 54 ± 7 | 0.58 |
| eGFR (mL/min/1.73 m2) | 56 (35–78) | 89 (72–102) | <0.001 | 62 ± 3 | 76 ± 22 | 0.20 |
| U-proteins/creatinine (mg/µg) | 2406 | 880 | <0.001 | 464 | 809 | 0.77 |
| (750–4978) | (220–2550) | (515–1027) | (0–1759) | |||
| U-IgG/creatinine (mg/µg) | 99 | 37 | <0.001 | 29 | 18 | 0.58 |
| (34–275) | (10–102) | (28–94) | (0–66) | |||
| U-albumin/creatinine (mg/µg) | 1948 | 626 | <0.001 | 350 | 495 | 0.61 |
| (588–4214) | (130–2398) | (389–845) | (0–1654) | |||
| U-α1-microglobulin/creatinine (mg/µg) | 26 | 9 | <0.001 | 9 | 15 | 0.57 |
| (11–55) | (4–21) | (11–25) | (4–22) | |||
| U-transferrin/creatinine (mg/µg) | 125 | 43 | <0.001 | 19 | 21 | 0.94 |
| (30–325) | (10–197) | (21–47) | (0–77) | |||
| U-NAG/creatinine (IU/g) | 9.76 | 5.9 | <0.001 | 5 | 7 | 0.45 |
| (4.69–20.96) | (2.93–11.41) | (5–8) | (3–13) | |||
| Model | Disease | Factors and Covariates | Test Parallel Lines | Goodness of Fit Test (Pearson P Deviance P) | Pseudo R-Square (Nagelkerke) | Associated Variable(s) | OR (95% CI) | Wald | p |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GN | Age | 0.02 | 0.610 0.362 | 0.11 | HT | 3.61 (2.35–5.55) | 34.43 | <0.001 |
| Sex | |||||||||
| HT | |||||||||
| Nephrotic syndrome | |||||||||
| 2 | GN | HT | 0.327 | 0.329 | 0.10 | HT | 3.48 (2.31–5.25) | 35.21 | <0.001 |
| 0.327 | |||||||||
| 3 | GN | GGS | 0.240 | 0.981 | 0.44 | GGS | 1.11 (1.08–1.13) | 94.32 | <0.001 |
| HT | 0.981 | HT | 1.91 (1.22–3.00) | 7.99 | |||||
| 4 | GN | GGS | 0.088 | 0.867 | 0.42 | GGS | 1.11 (1.09–1.13) | 106.82 | <0.001 |
| 0.869 | |||||||||
| 5 | GN | GGS | 0.033 | 0.738 0.995 | 0.50 | GGS | 1.09 (1.07–1.11) | 63.61 | <0.001 |
| HT | HT | 1.64 (1.04–2.60) | 4.51 | 0.034 | |||||
| AH | AH | 0.055 (0.01–0.3) | 13.99 | <0.001 | |||||
| 1a | GN NT | GGS | 0.100 | 0.502 | 0.31 | GGS | 1.11 (1.08–1.50) | 40.50 | <0.001 |
| 0.638 | |||||||||
| 2a | GN NT | AH | 0.402 | 0.924 | 0.26 | AH | 5.35 (3.16–9.04) | 39.12 | <0.001 |
| 0.883 | |||||||||
| 1b | GN HT | GGS | 0.496 | 0.996 | 0.43 | GGS | 1.10 (1.07–1.13) | 53.73 | <0.001 |
| 0.991 | |||||||||
| 2b | GN HT | AH | 0.744 | 0.0.92 | 0.25 | AH | 3.46 (2.35–5.11) | 39.14 | <0.001 |
| 0.096 | |||||||||
| 1c | NAS | GGS | 0.058 | 0.851 | 0.13 | GGS | 1.04 (1.01–1.08) | 4.97 | 0.026 |
| 0.726 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazzi, C.; Seccia, T.M.; Napodano, P.; Campi, C.; Caroccia, B.; Cattarin, L.; Calò, L.A. High Blood Pressure Is Associated with Tubulointerstitial Damage along with Glomerular Damage in Glomerulonephritis. A large Cohort Study. J. Clin. Med. 2020, 9, 1656. https://doi.org/10.3390/jcm9061656
Bazzi C, Seccia TM, Napodano P, Campi C, Caroccia B, Cattarin L, Calò LA. High Blood Pressure Is Associated with Tubulointerstitial Damage along with Glomerular Damage in Glomerulonephritis. A large Cohort Study. Journal of Clinical Medicine. 2020; 9(6):1656. https://doi.org/10.3390/jcm9061656
Chicago/Turabian StyleBazzi, Claudio, Teresa M Seccia, Pietro Napodano, Cristina Campi, Brasilina Caroccia, Leda Cattarin, and Lorenzo A Calò. 2020. "High Blood Pressure Is Associated with Tubulointerstitial Damage along with Glomerular Damage in Glomerulonephritis. A large Cohort Study" Journal of Clinical Medicine 9, no. 6: 1656. https://doi.org/10.3390/jcm9061656
APA StyleBazzi, C., Seccia, T. M., Napodano, P., Campi, C., Caroccia, B., Cattarin, L., & Calò, L. A. (2020). High Blood Pressure Is Associated with Tubulointerstitial Damage along with Glomerular Damage in Glomerulonephritis. A large Cohort Study. Journal of Clinical Medicine, 9(6), 1656. https://doi.org/10.3390/jcm9061656





