Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants
Abstract
1. Introduction
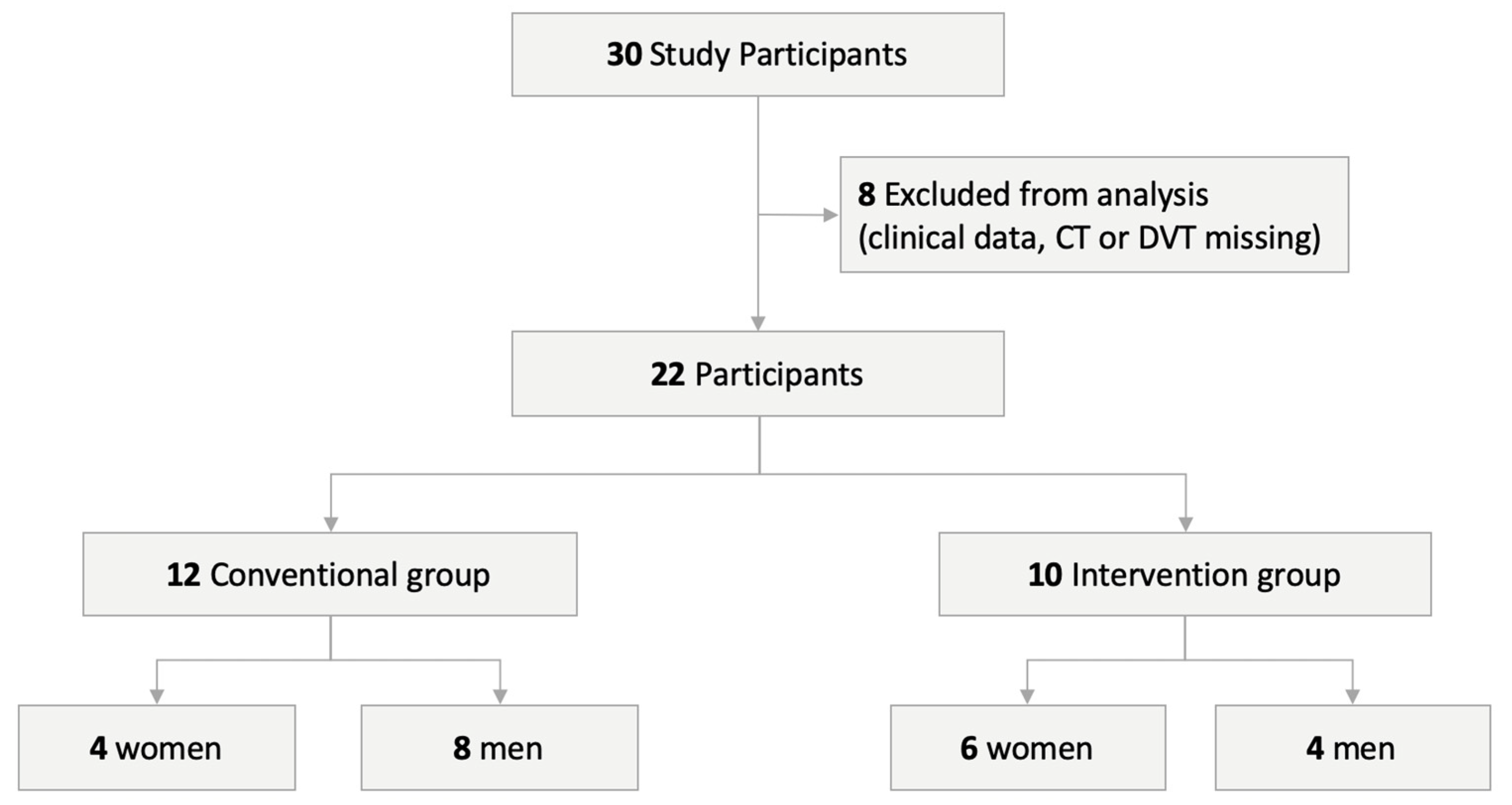
2. Experimental Section
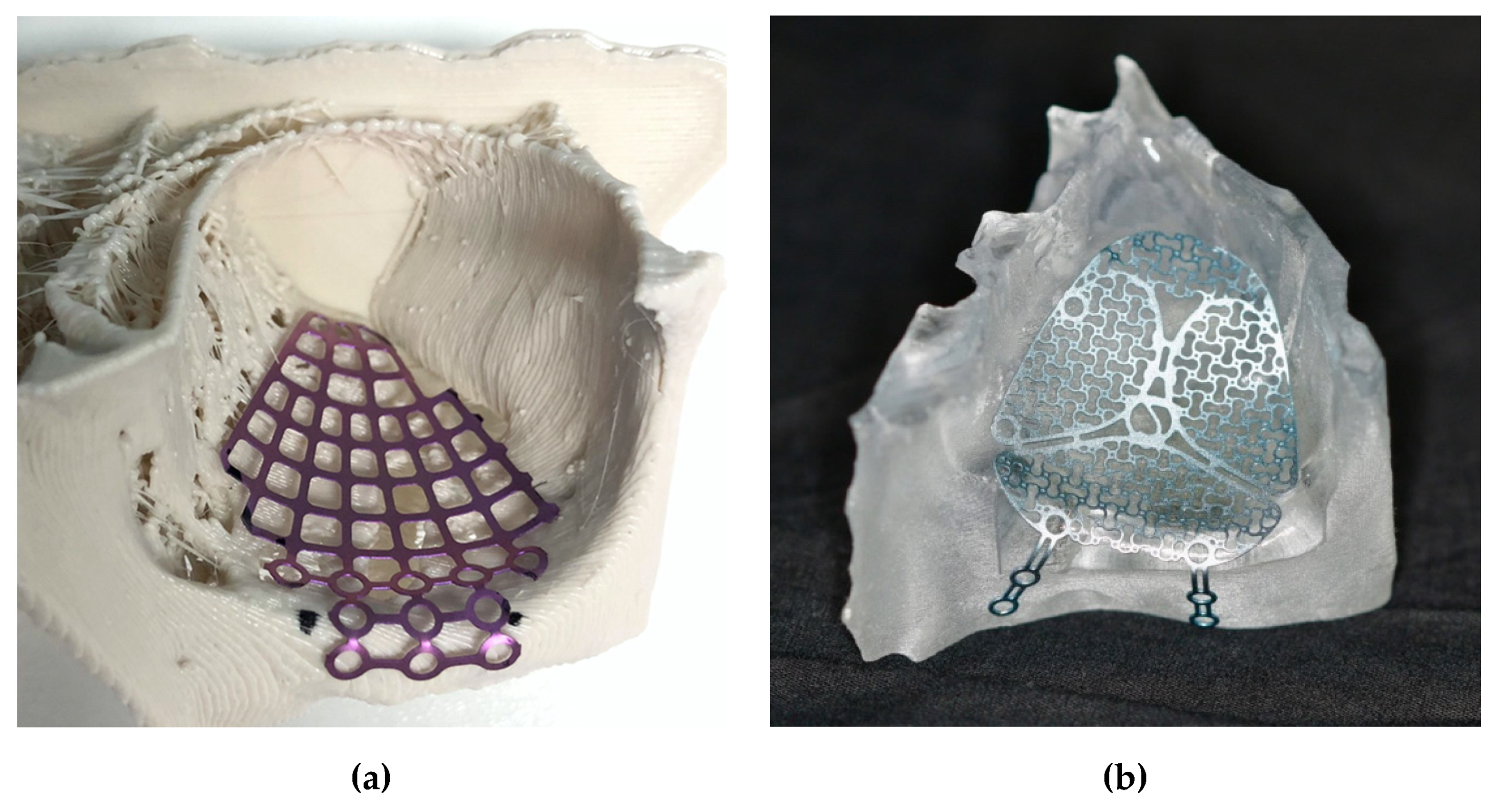
2.1. Surgical Procedure
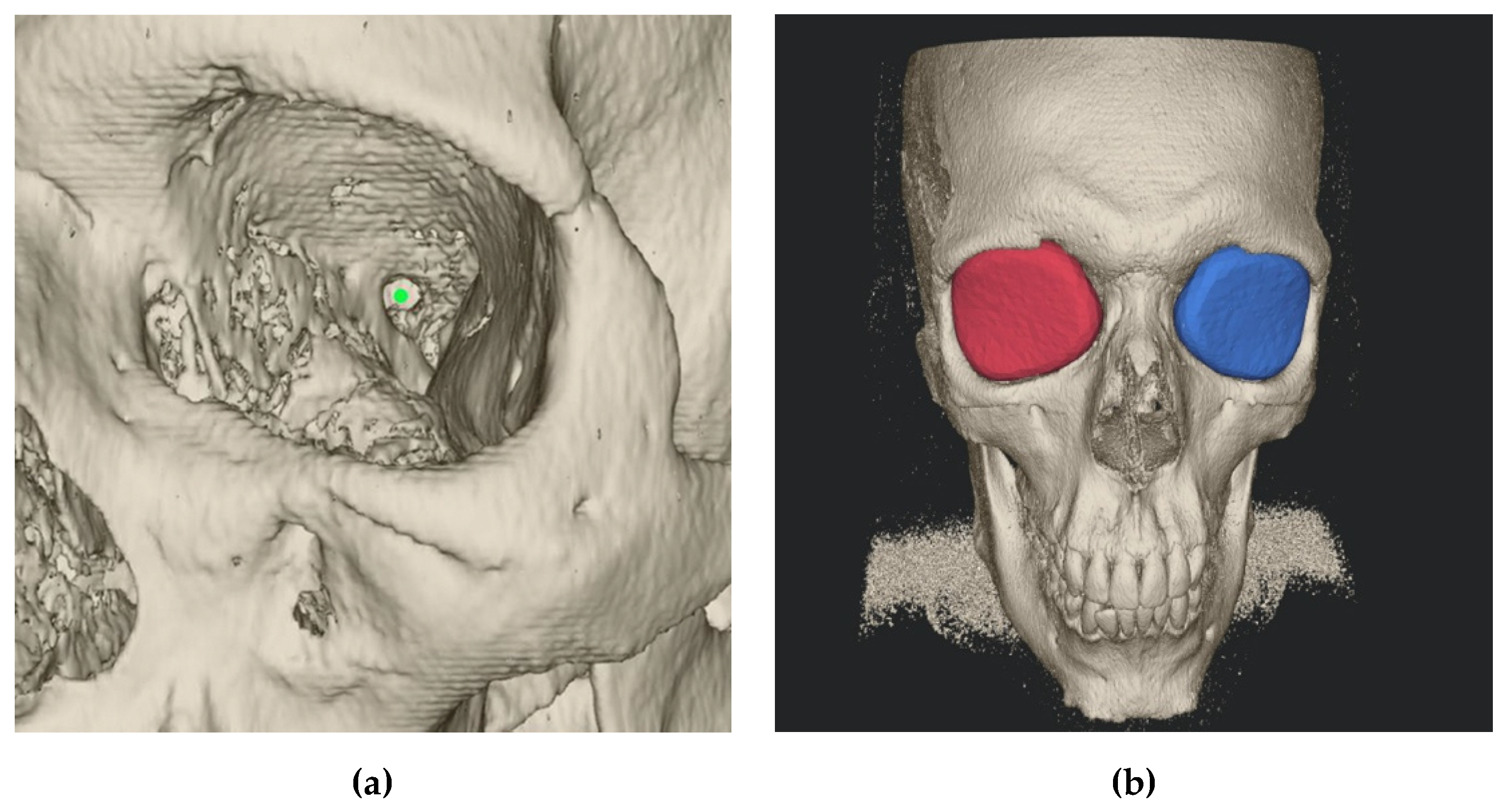
2.2. Semi-Automatic Orbital Fracture Analysis
2.3. Statistical Analysis
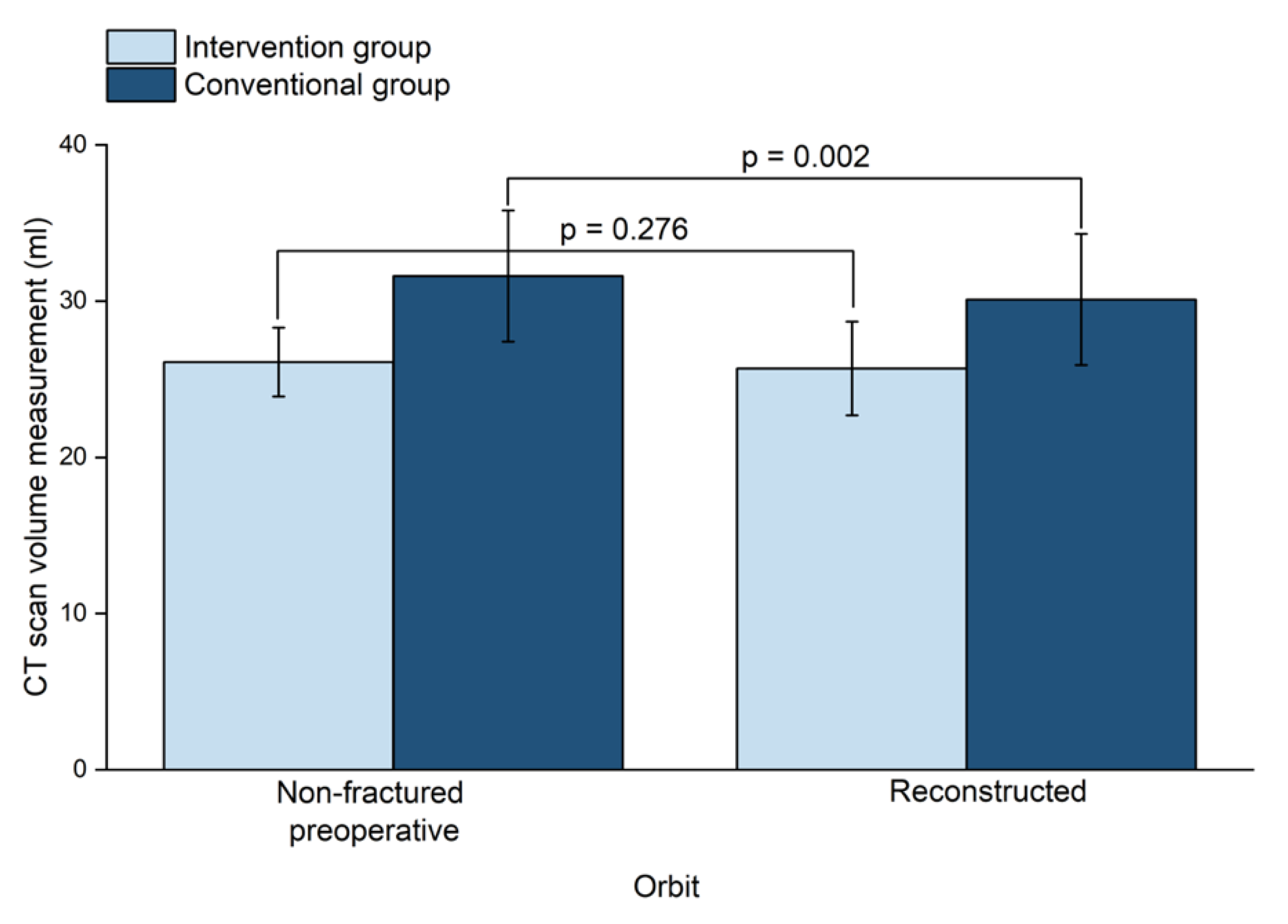
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A1 | Isolated defect of the orbital floor, or the medial wall, 1–2 cm2 |
| A2 | Defect of the orbital floor in the anterior two-third, or the medial wall, or both, >2 cm2, bony ledge preserved at medial margin of the infraorbital fissure |
| A3 | Defect of the orbital floor in the anterior two-third, or the medial wall, or both, >2 cm2, missing bony ledge medial to the infraorbital fissure |
| AOCMF | Arbeitsgemeinschaft für Osteosynthesefragen—Craniomaxillofacial Surgery |
| AOCOIAC | AO comprehensive injury automatic classifier software |
| CBCT | Cone-beam computed tomography |
| CMF | Craniomaxillofacial surgery |
| CT | Computed tomography |
| DICOM | Digital Imaging and Communications in Medicine |
| HU | Hounsfield units |
| PLA | Polylactic acid |
| SD | Standard deviation |
| 3D | Three-dimensional |
References
- Shin, J.W.; Lim, J.S.; Yoo, G.; Byeon, J.H. An analysis of pure blowout fractures and associated ocular symptoms. J. Craniofacial Surg. 2013, 24, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.; El-Attar, A.; Moos, K.F. An Analysis of 2067 cases of zygomatico-orbital fracture. J. Oral Maxillofac. Surg. 1985, 43, 417–428. [Google Scholar] [CrossRef]
- Manson, P.N.; Grivas, A.N.; Rosenbaum, A.; Vannier, M.; Zinreich, J.; Iliff, N. Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast. Reconstr. Surg. 1986, 77, 203–2014. [Google Scholar] [CrossRef]
- Biesman, B.S.; Hornblass, A.; Lisman, R.; Kazlas, M. Diplopia after surgical repair of orbital floor fractures. Ophthalmic Plast. Reconstr. Surg. 1996, 12, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ewers, R.; Schicho, K.; Undt, G.; Wanschitz, F.; Truppe, M.; Seemann, R.; Wagner, A. Basic research and 12 years of clinical experience in computer-assisted navigation technology: A review. Int. J. Oral Maxillofac. Surg. 2005, 34, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hatz, C.R.; Msallem, B.; Aghlmandi, S.; Brantner, P.; Thieringer, F.M. Can an entry-level 3D printer create high-quality anatomical models? Accuracy assessment of mandibular models printed by a desktop 3D printer and a professional device. Int. J. Oral Maxillofac. Surg. 2019, 49, 143–148. [Google Scholar] [CrossRef]
- Msallem, B.; Beiglboeck, F.; Honigmann, P.; Jaquiéry, C.; Thieringer, F. Craniofacial reconstruction by a cost-efficient template-based process using 3D printing. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1582. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jeong, W.S.; Park, T.K.; Choi, J.W.; Koh, K.S.; Oh, T.S. The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction. J. Cranio-Maxillofac. Surg. 2017, 45, 928–936. [Google Scholar] [CrossRef]
- Raisian, S.; Fallahi, H.R.; Khiabani, K.S.; Heidarizadeh, M.; Azdoo, S. Customized titanium mesh based on the 3D printed model vs. manual intraoperative bending of titanium mesh for reconstructing of orbital bone fracture: A randomized clinical trial. Rev. Recent Clin. Trials 2017, 12, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Luo, T.Y.; Ma, J.; Dong, Z.; Cao, G.; Xu, J.K.; Liu, B.Y.; Zhang, Q.R.; Zhang, S.L. Application of three-dimensional printing technology in the orbital blowout fracture reconstruction. J. Craniofacial Surg. 2019, 30, 1825–1828. [Google Scholar] [CrossRef]
- Pang, S.S.Y.; Fang, C.; Chan, J.Y.W. Application of three-dimensional printing technology in orbital floor fracture reconstruction. Trauma Case Rep. 2018, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kwon, J.; Ahn, C.J.; Esmaeli, B.; Kim, G.B.; Kim, N.; Sa, H.S. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 2018, 32, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Kunz, C.; Sigron, G.R.; Jaquiéry, C. Functional outcome after non-surgical management of orbital fractures-the bias of decision-making according to size of defect: Critical review of 48 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 486–492. [Google Scholar] [CrossRef]
- Kunz, C.; Cornelius, C.-P.; Audigé, L.; Thieringer, F.; Buitrago-Téllez, C.; Metzger, M.; Wilde, F.; Prein, J. Orbitafrakturen nach der AO-CMF-Level-3-Trauma-KlassifikationOrbital fractures according to the AO-CMF level 3 trauma classification. Der MKG-Chirurg 2017, 10, 91–103. [Google Scholar] [CrossRef]
- Brucoli, M.; Arcuri, F.; Cavenaghi, R.; Benech, A. Analysis of complications after surgical repair of orbital fractures. J. Craniofacial Surg. 2011, 22, 1387–1390. [Google Scholar] [CrossRef]
- Gander, T.; Essig, H.; Metzler, P.; Lindhorst, D.; Dubois, L.; Rücker, M.; Schumann, P. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J. Cranio-Maxillofac. Surg. 2015, 43, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Dubois, L.; Schreurs, R.; Gooris, P.J.J.; Maal, T.J.J.; Beenen, L.F.; Becking, A.G. Should virtual mirroring be used in the preoperative planning of an orbital reconstruction? J. Oral Maxillofac. Surg. 2018, 76, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ploder, O.; Oeckher, M.; Klug, C.; Voracek, M.; Wagner, A.; Burggasser, G.; Baumann, A.; Czerny, C. Follow-up study of treatment of orbital floor fractures: Relation of clinical data and software-based CT-analysis. Int. J. Oral Maxillofac. Surg. 2003, 32, 257–262. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef]
- Lim, C.; Campbell, D.; Clucas, D. Rapid prototyping technology in orbital floor reconstruction: Application in three patients. Craniomaxillofac. Trauma Reconstr. 2014, 7, 143–146. [Google Scholar] [CrossRef]
- Park, S.W.; Choi, J.W.; Koh, K.S.; Oh, T.S. Mirror-imaged rapid prototype skull model and pre-molded synthetic scaffold to achieve optimal orbital cavity reconstruction. J. Oral Maxillofac. Surg. 2015, 73, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Schönegg, D.; Wagner, M.; Schumann, P.; Essig, H.; Seifert, B.; Rücker, M.; Gander, T. Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J. Cranio-Maxillofac. Surg. 2018, 46, 1544–1549. [Google Scholar] [CrossRef]
- Oh, S.A.; Aum, J.H.; Kang, D.H.; Gu, J.H. Change of the orbital volume ratio in pure blow-out fractures depending on fracture location. J. Craniofacial Surg. 2013, 24, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Callahan, A.B.; Campbell, A.A.; Petris, C.; Kazim, M. Low-cost 3D printing orbital implant templates in secondary orbital reconstructions. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.F.; Evans, P.L.; Bocca, A.; Patton, D.W.; Sugar, A.W.; Baxter, P.W. Customized titanium reconstruction of post-traumatic orbital wall defects: A review of 22 cases. Int. J. Oral Maxillofac. Surg. 2011, 40, 1357–1362. [Google Scholar] [CrossRef]
- Zieliński, R.; Malińska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J. Cranio-Maxillofac. Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- Zweifel, D.F.; Simon, C.; Hoarau, R.; Pasche, P.; Broome, M. Are virtual planning and guided surgery for head and neck reconstruction economically viable? J. Oral Maxillofac. Surg. 2015, 73, 170–175. [Google Scholar] [CrossRef]
- Haddock, N.T.; Monaco, C.; Weimer, K.A.; Hirsch, D.L.; Levine, J.P.; Saadeh, P.B. Increasing bony contact and overlap with computer-designed offset cuts in free fibula mandible reconstruction. J. Craniofacial Surg. 2012, 23, 1592–1595. [Google Scholar] [CrossRef]
| Variable | Intervention Group | Conventional Group |
|---|---|---|
| Number of Patients (%) | 10 (45.45%) | 12 (54.55%) |
| Age in Years, Mean (Range) | 47.5 (20–83) | 51.8 (21–79) |
| Sex | ||
| Male | 4 | 8 |
| Female | 6 | 4 |
| Cause of Injury | ||
| Fall | 5 | 7 |
| Assaults | 2 | 4 |
| Sports Injuries | 2 | 1 |
| Work-Related Injuries | 1 | 0 |
| Days Between Trauma and Surgery | 2.8 ± 2.5 | 4.1 ± 3.1 |
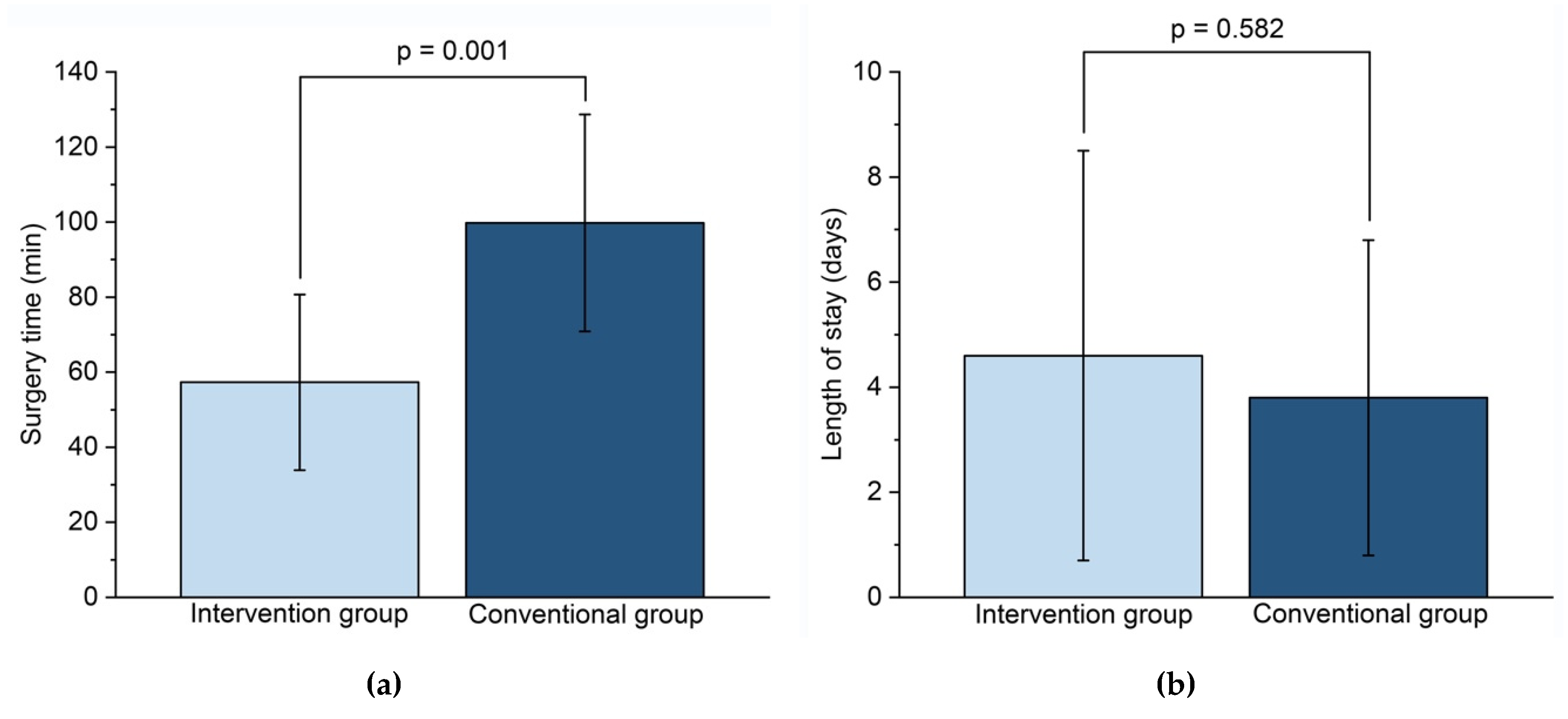
| Surgery Time (Min) | 57.3 ± 23.4 | 99.8 ± 28.9 |
| Length of Stay (Days) | 4.6 ± 3.9 | 3.8 ± 3.0 |
| Fracture Classification | ||
| AO CMF | ||
| 92 m.OiW2(i) | 5 | 10 |
| 92 Oi.mW2(i) | 5 | 2 |
| Kunz et al. | ||
| A1 | 3 | 7 |
| A2 | 6 | 5 |
| A3 | 1 | 0 |
| Non-Fractured Orbit (mL) | Fractured orbit (mL) | ||
|---|---|---|---|
| Conventional Group | |||
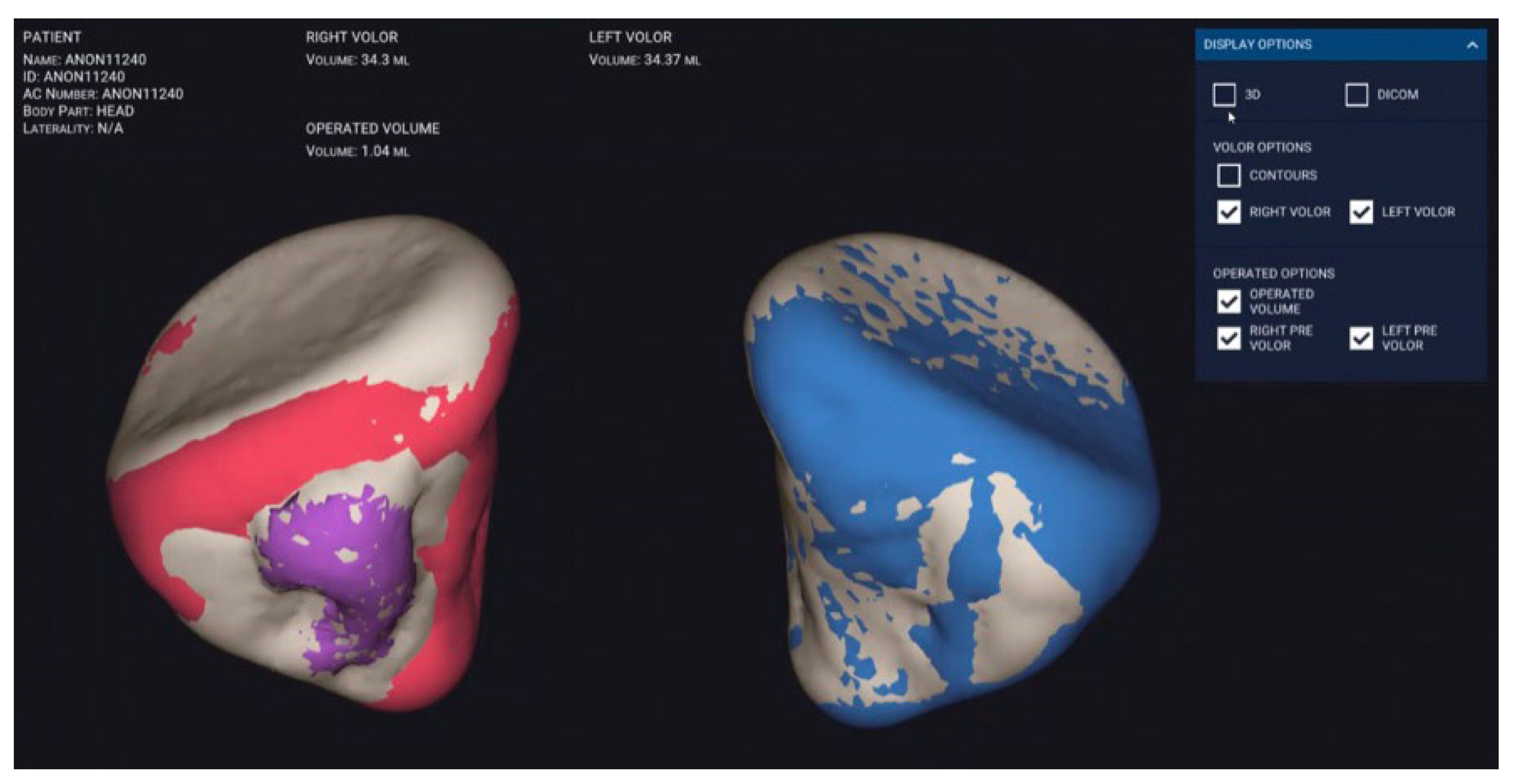
| Preoperative | 31.6 ± 4.2 | 33.1 ± 4.7 | |
| Postoperative | 31.4 ± 4.3 | 30.1 ± 4.2 | |
| Absolut Volume Difference (mL) | 1.6 ± 1.2 * | ||
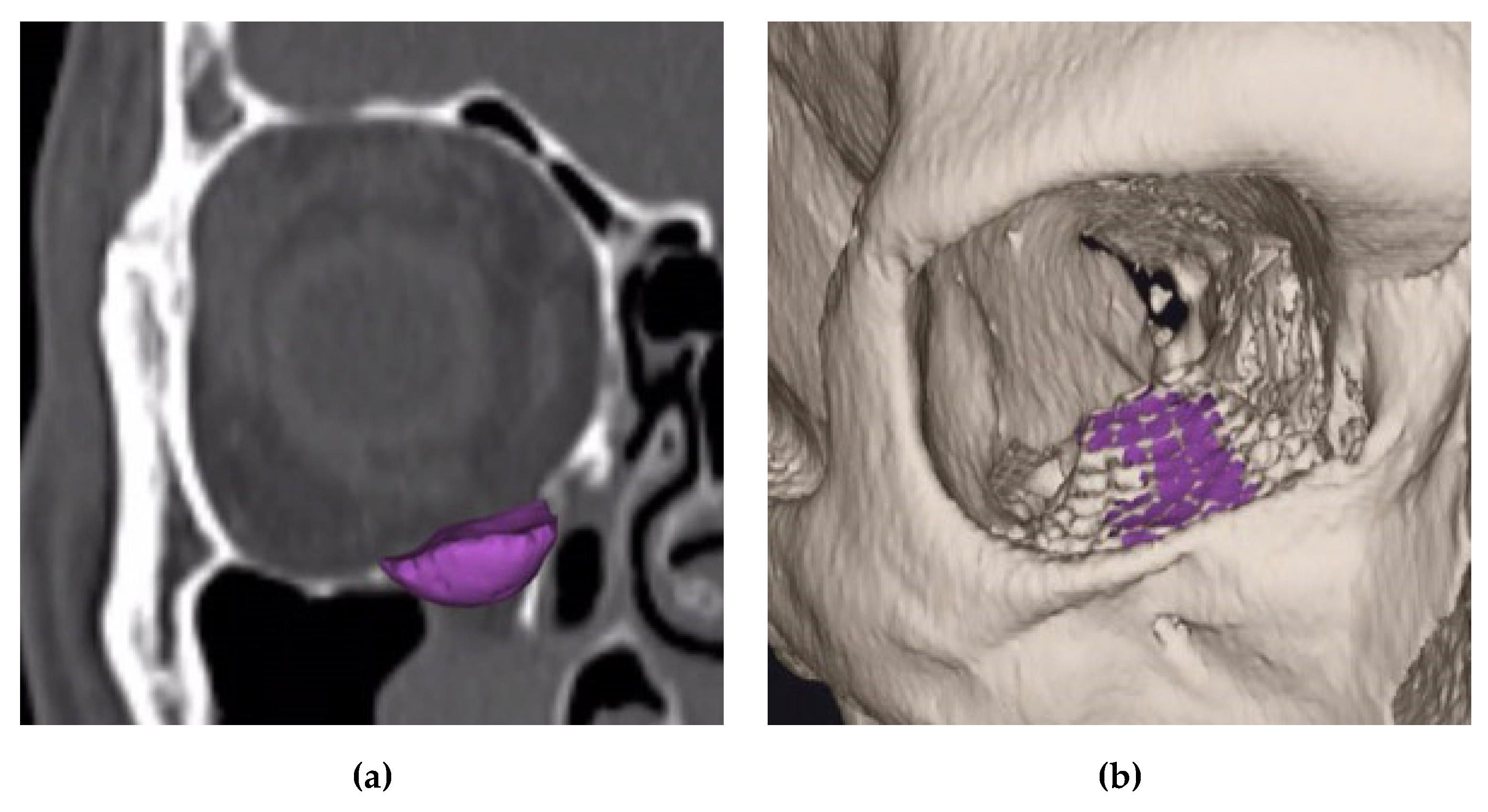
| Fracture Area (mm2) | 408.5 ± 137.5 | ||
| Fracture Max. Collapse (mm) | 6.9 ± 2.3 | ||
| Intervention Group | |||
| Preoperative | 26.1 ± 2.2 | 28.4 ± 4.0 | |
| Postoperative | 26.1 ± 2.2 | 25.7 ± 3.0 | |
| Absolut Volume Difference (mL) | 1.0 ± 0.7 | ||
| Fracture Area (mm2) | 389.4 ± 135.1 | ||
| Fracture Max. Collapse (mm) | 8.6 ± 5.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigron, G.R.; Rüedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. https://doi.org/10.3390/jcm9051579
Sigron GR, Rüedi N, Chammartin F, Meyer S, Msallem B, Kunz C, Thieringer FM. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. Journal of Clinical Medicine. 2020; 9(5):1579. https://doi.org/10.3390/jcm9051579
Chicago/Turabian StyleSigron, Guido R., Nathalie Rüedi, Frédérique Chammartin, Simon Meyer, Bilal Msallem, Christoph Kunz, and Florian M. Thieringer. 2020. "Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants" Journal of Clinical Medicine 9, no. 5: 1579. https://doi.org/10.3390/jcm9051579
APA StyleSigron, G. R., Rüedi, N., Chammartin, F., Meyer, S., Msallem, B., Kunz, C., & Thieringer, F. M. (2020). Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. Journal of Clinical Medicine, 9(5), 1579. https://doi.org/10.3390/jcm9051579






