Preliminary Pilot Study of Combined Effects of Physical Activity and Achievement of LDL-Cholesterol Target on Coronary Plaque Volume Changes in Patients with Acute Coronary Syndrome
Abstract
1. Introduction
2. Methods
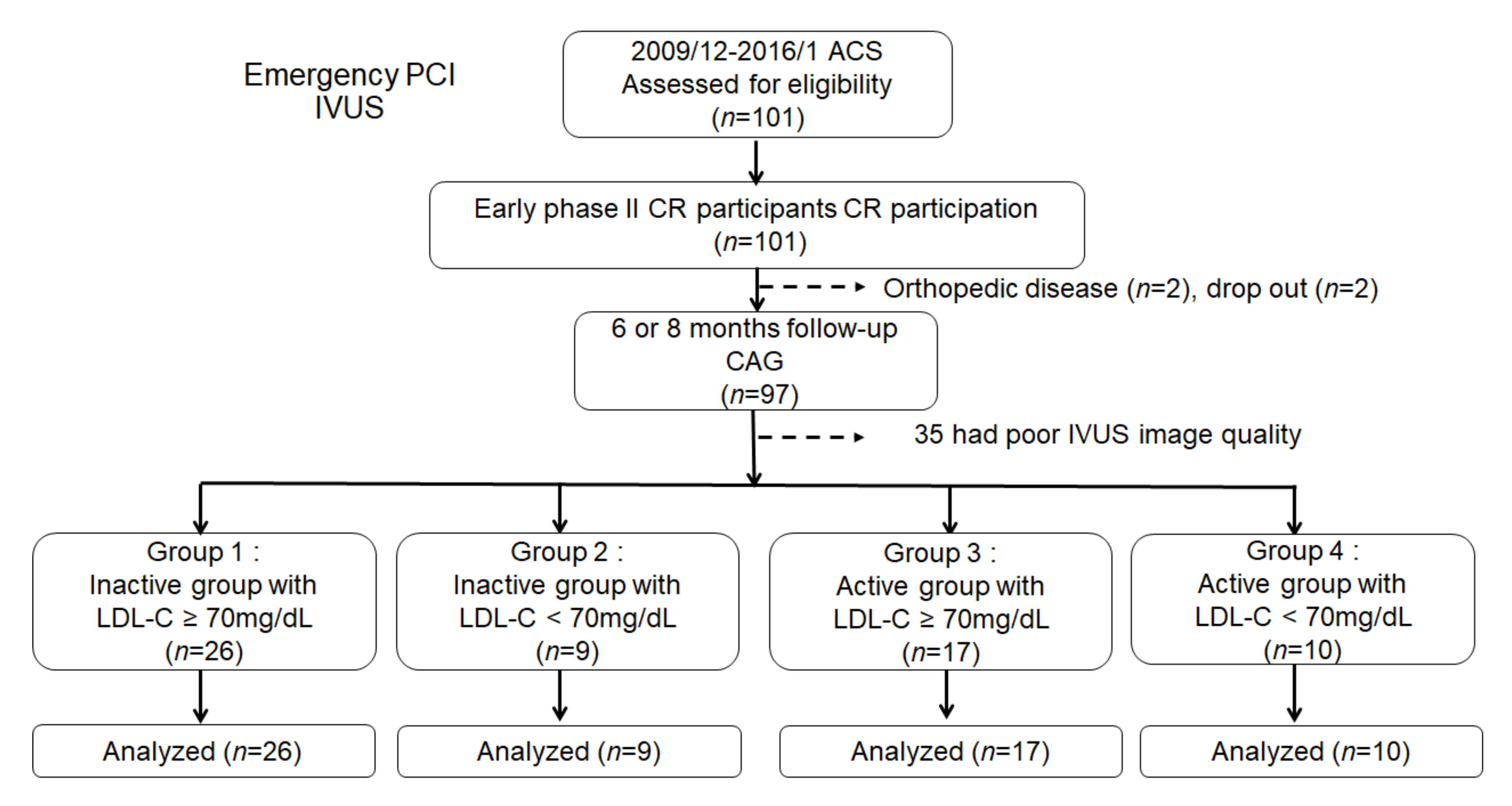
2.1. Study Subjects and Protocol
2.2. Early Phase II CR Protocol
2.3. Measurements
2.4. Statistical Analyses
3. Results
3.1. Study Population and Clinical Characteristics
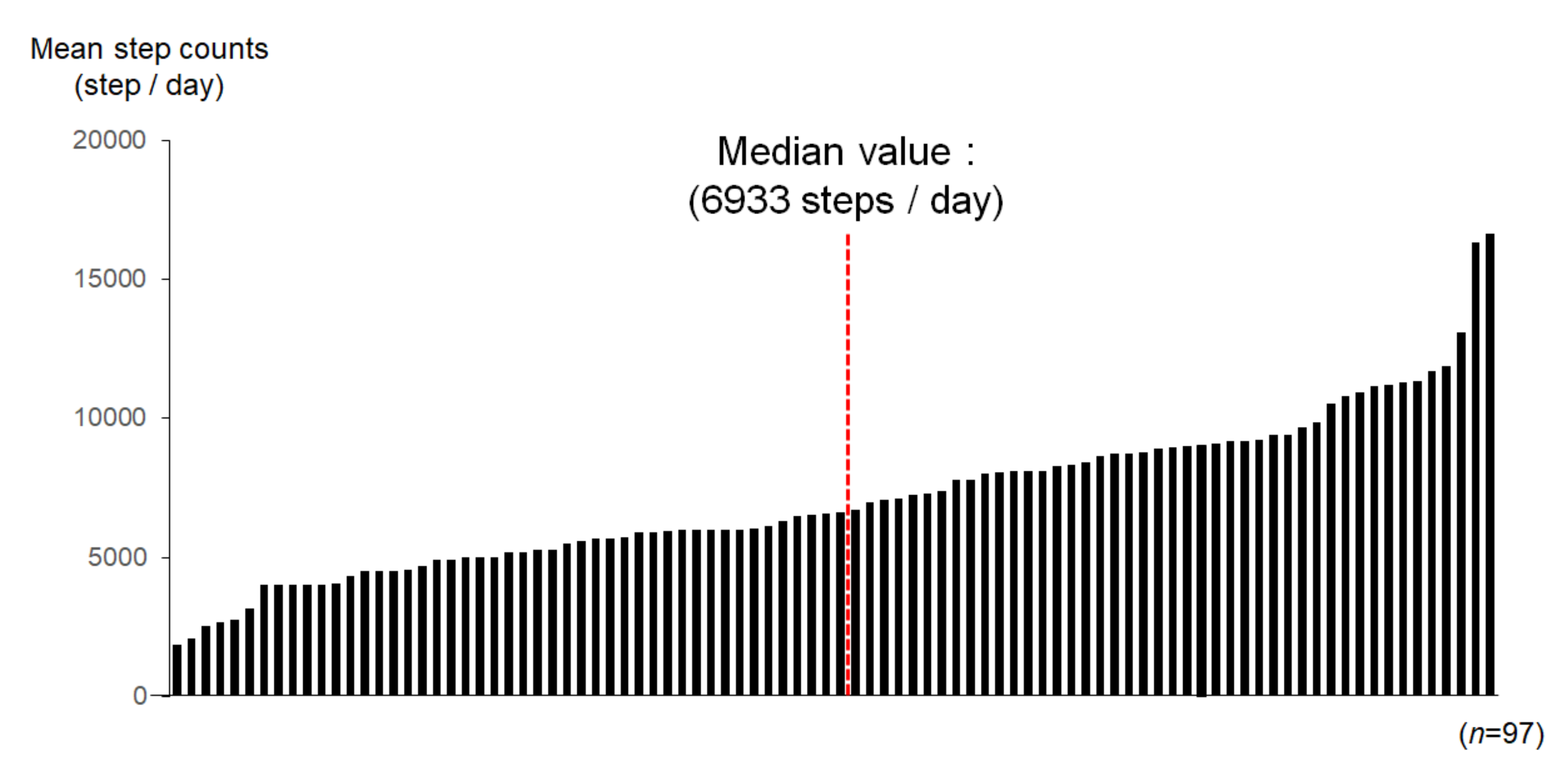
3.2. Physical Activity in Each Patient during Outpatient Clinic Visit
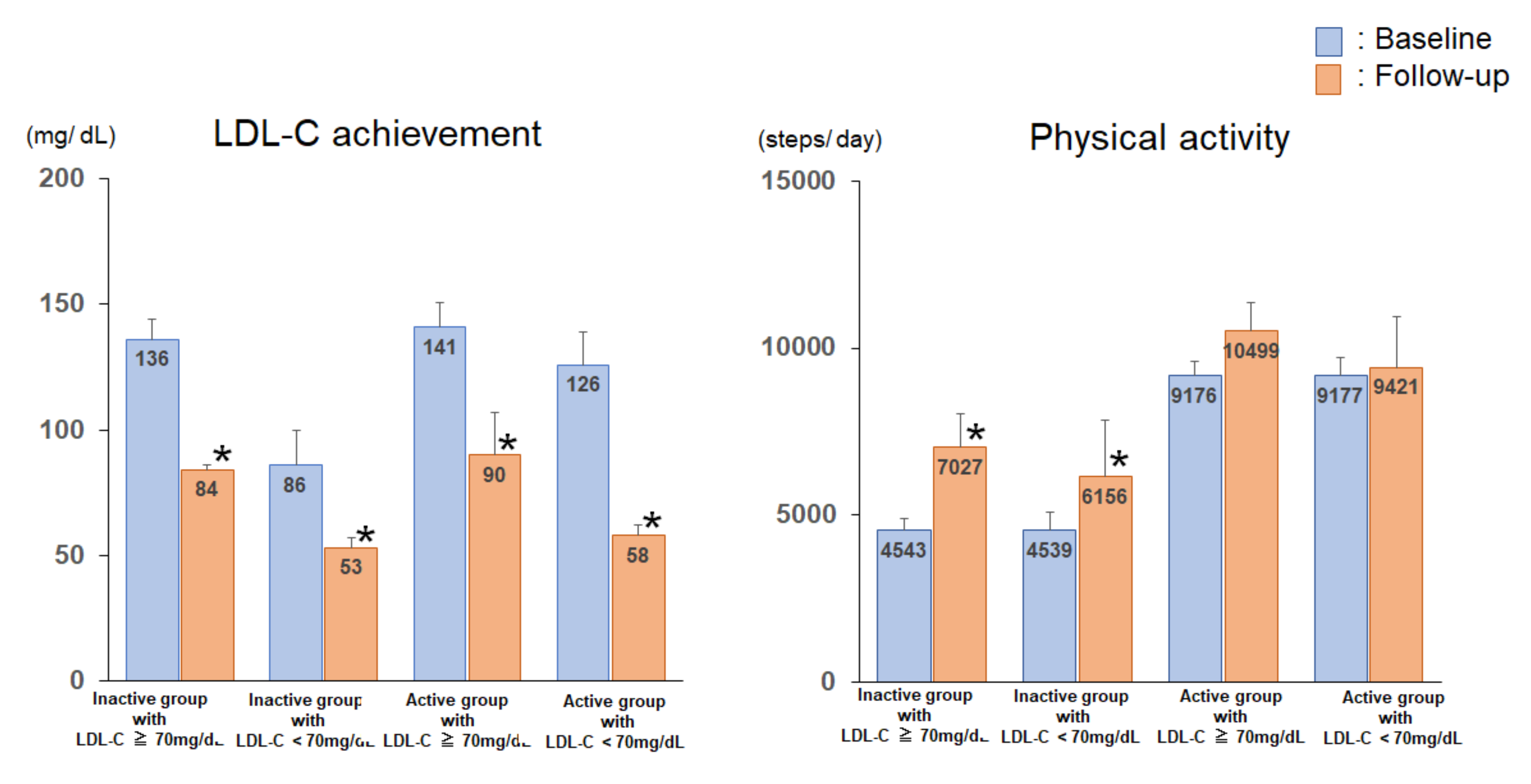
3.3. Serum Lipid Profile, Glucose Parameters, Anthropometric Parameters, and Exercise Tolerance of the Subjects of the 4 Groups at Baseline and Follow-Up
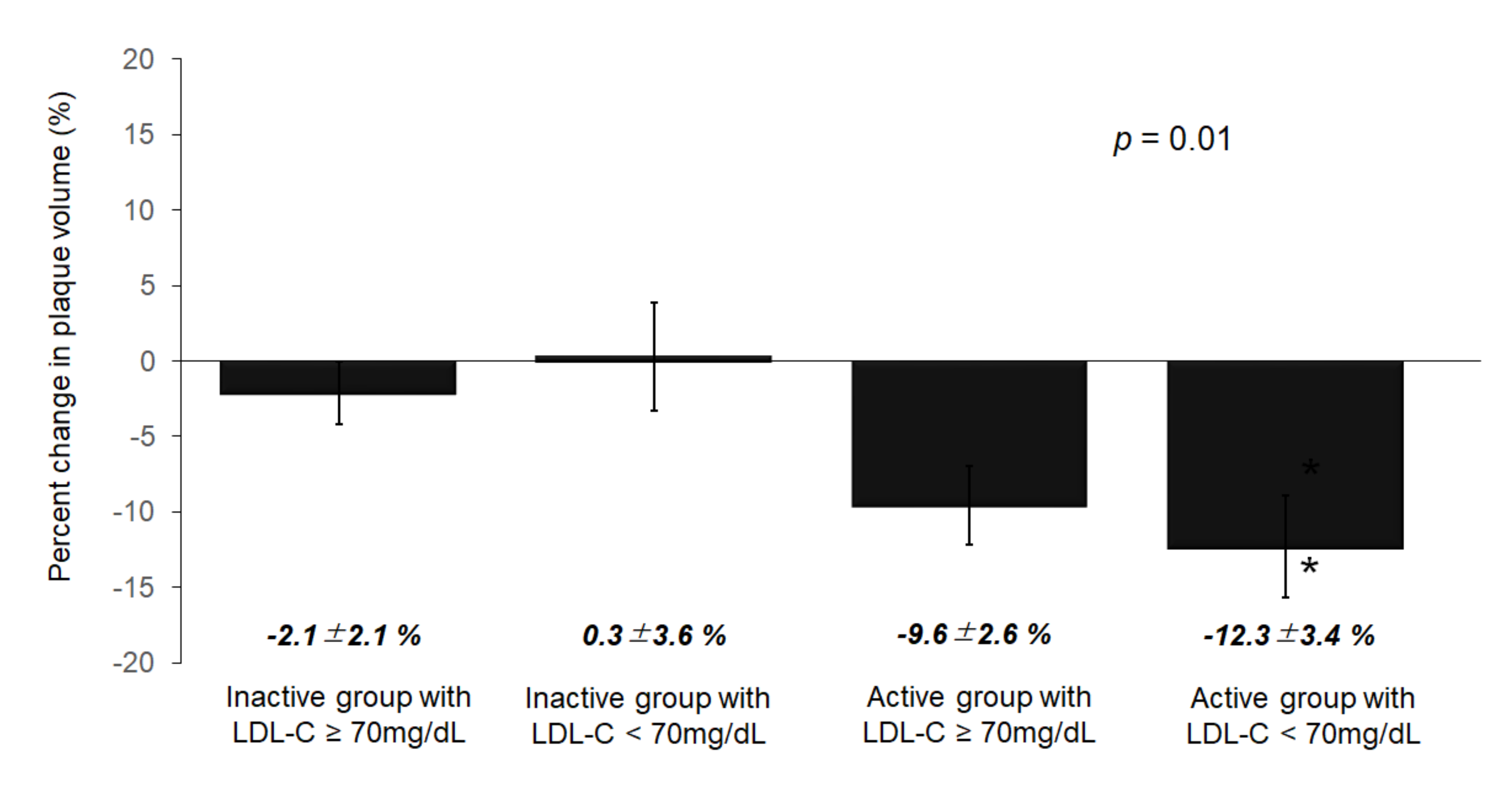
3.4. Volumetric Analysis of the IVUS Parameters
3.5. Correlations between Plaque Changes and PA
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, A.; Cannon, C.P.; Deedwania, P.C.; Labresh, K.A.; Smith, S.C., Jr.; Dai, D.; Hernandez, A.; Fonarow, G.C. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get With The Guidelines. Am. Heart J. 2009, 157, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Beringola, A.; Fitzsimons, D.; Pelliccia, A.; Moreno, G.; Martín-Asenjo, R.; Bueno, H. Planning secondary prevention: Room for improvement. Eur. J. Prev. Cardiol. 2017, 24 (Suppl. 3), 22–28. [Google Scholar] [CrossRef] [PubMed]
- Nishitani-Yokoyama, M.; Miyauchi, K.; Shimada, K.; Miyazaki, T.; Ogita, M.; Okazaki, S.; Shioya, M.; Koba, S.; Tsujita, H.; Kobayashi, Y.; et al. Effects of Phase II Comprehensive Cardiac Rehabilitation on Coronary Plaque Volume After Acute Coronary Syndrome. Int. Heart J. 2015, 56, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Nishitani-Yokoyama, M.; Miyauchi, K.; Shimada, K.; Yokoyama, T.; Ouchi, S.; Aikawa, T.; Kunimoto, M.; Yamada, M.; Honzawa, A.; Okazaki, S.; et al. Impact of physical activity on coronary plaque volume and components in acute coronary syndrome patients after early phase II cardiac rehabilitation. Circ. J. 2018, 83, 101–109. [Google Scholar] [CrossRef]
- Kinoshita, M.; Yokote, K.; Arai, H.; Iida, M.; Ishigaki, Y.; Ishibashi, S.; Umemoto, S.; Egusa, G.; Ohmura, H.; Okamura, T.; et al. Committee for Epidemiology and Clinical Management of Atherosclerosis. Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J. Atheroscler. Thromb. 2018, 25, 846–984. [Google Scholar] [CrossRef]
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef]
- Rioufol, G.; Finet, G.; Ginon, I.; André-Fouët, X.; Rossi, R.; Vialle, E.; Desjoyaux, E.; Convert, G.; Huret, J.F.; Tabib, A. Multiple atherosclerotic plaque rupture in acute coronary syndrome: A three-vessel intravascular ultrasound study. Circulation 2002, 106, 804–808. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP); Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Von Birgelen, C.; Hartmann, M.; Mintz, G.S.; van Houwelingen, K.G.; Deppermann, N.; Schmermund, A.; Böse, D.; Eggebrecht, H.; Neumann, T.; Gössl, M.; et al. Relationship between cardiovascular risk as predicted by established risk scores versus plaque progression as measured by serial intravascular ultrasound in left main coronary arteries. Circulation 2004, 110, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Boekholdt, S.M.; Hovingh, G.K.; Mora, S.; Arsenault, B.J.; Amarenco, P.; Pedersen, T.R.; LaRosa, J.C.; Waters, D.D.; DeMicco, D.A.; Simes, R.J.; et al. Very low levels of atherogenic lipoproteins and the risk for cardiovascular events: A meta-analysis of statin trials. J. Am. Coll. Cardiol. 2014, 64, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Yokoyama, T.; Miyauchi, K.; Shimada, K.; Kurata, T.; Sato, H.; Daida, H. Early statin treatment in patients with acute coronary syndrome: Demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: The ESTABLISH Study. Circulation 2004, 110, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Hiro, T.; Kimura, T.; Morimoto, T.; Miyauchi, K.; Nakagawa, Y.; Yamagishi, M.; Ozaki, Y.; Kimura, K.; Saito, S.; Yamaguchi, T.; et al. JAPAN-ACS Investigators: Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J. Am. Coll. Cardiol. 2009, 54, 293–302. [Google Scholar] [CrossRef]
- Daida, H.; Dohi, T.; Fukushima, Y.; Ohmura, H.; Miyauchi, K. The Goal of Achieving Atherosclerotic Plaque Regression with Lipid-Lowering Therapy: Insights from IVUS Trials. J. Atheroscler. Thromb. 2019, 26, 592–600. [Google Scholar] [CrossRef]
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016, 1, CD001800. [Google Scholar] [CrossRef]
- Guidelines for Rehabilitation in Patients with Cardiovascular Disease (JCS 2012). Available online: http://www.j-circ.or.jp/guideline/pdf/JCS2012_nohara_h.pdf (accessed on 25 March 2000).
- Tani, S.; Nagao, K.; Anazawa, T.; Kawamata, H.; Furuya, S.; Takahashi, H.; Iida, K.; Matsumoto, M.; Washio, T.; Kumabe, N.; et al. Coronary plaque regression and lifestyle modification in patients treated with pravastatin. Assessment mainly by daily aerobic exercise and an increase in the serum level of high-density lipoprotein cholesterol. Circ. J. 2010, 74, 954–961. [Google Scholar] [CrossRef]
- Yazdani, S.; Simon, A.D.; Vidhun, R.; Gulotta, C.; Schwartz, A.; Rabbani, L.E. Inflammatory profile in unstable angina versus stable angina in patients undergoing percutaneous interventions. Am. Heart J. 1998, 136, 357–361. [Google Scholar] [CrossRef]
- Hojo, Y.; Ikeda, U.; Takahashi, M.; Shimada, K. Increased levels of monocyte-related cytokines in patients with unstable angina. Atherosclerosis 2002, 161, 403–408. [Google Scholar] [CrossRef]
- Kurose, S.; Iwasaka, J.; Tsutsumi, H.; Yamanaka, Y.; Shinno, H.; Fukushima, Y.; Higurashi, K.; Imai, M.; Masuda, I.; Takeda, S.; et al. Effect of exercise-based cardiac rehabilitation on non-culprit mild coronary plaques in the culprit coronary artery of patients with acute coronary syndrome. Heart Vessels 2016, 31, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Onishi, T.; Shimada, K.; Sunayama, S.; Ohmura, H.; Sumide, T.; Masaki, Y.; Fukao, K.; Nishitani, M.; Kume, A.; Sato, H.; et al. Effects of cardiac rehabilitation in patients with metabolic syndrome after coronary artery bypass grafting. J. Cardiol. 2009, 53, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Adams, V.; Erbs, S.; Linke, A.; Kränkel, N.; Shu, Y.; Baither, Y.; Gielen, S.; Thiele, H.; Gummert, J.F.; et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003, 107, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Niebauer, J.; Marburger, C.; Grunze, M.; Kälberer, B.; Hauer, K.; Schlierf, G.; Kübler, W.; Schuler, G. Various intensities of leisure time physical activity in patients with coronary artery disease: Effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J. Am. Coll. Cardiol. 1993, 22, 468–477. [Google Scholar] [CrossRef]
- Takura, T.; Ebata-Kogure, N.; Goto, Y.; Kohzuki, M.; Nagayama, M.; Oikawa, K.; Koyama, T.; Itoh, H. Cost-Effectiveness of Cardiac Rehabilitation in Patients with Coronary Artery Disease: A Meta-Analysis. Cardiol. Res. Pract. 2019, 2019, 1840894. [Google Scholar] [CrossRef]
| Inactive Group with LDL-C ≥ 70 mg/dL (n = 26) | Inactive Group with LDL-C < 70 mg/dL (n = 9) | Active Group with LDL-C ≥ 70 mg/dL (n = 17) | Active Group with LDL-C < 70 mg/dL (n = 10) | p-Value | |
|---|---|---|---|---|---|
| Age, y | 62.5 ± 1.9 | 67.0 ± 3.2 | 56.6 ± 2.3 | 55.9 ± 3.0 | 0.02 |
| Male (%) | 23 (88) | 9 (100) | 17 (100) | 9 (90) | 0.37 |
| Hypertention (%) | 16 (62) | 6 (67) | 10 (59) | 7 (70) | 0.93 |
| Diabetes mellitus (%) | 9 (35) | 3 (33) | 10 (59) | 5 (50) | 0.39 |
| Dyslipidemia (%) | 22 (85) | 7 (78) | 17 (100) | 10 (100) | 0.13 |
| Current smoking (%) | 12 (46) | 4 (44) | 8 (47) | 7 (70) | 0.79 |
| Family history (%) | 10 (38) | 1 (11) | 6 (35) | 5 (50) | 0.33 |
| Classification of ACS (%) | |||||
| ST elevated MI (%) | 20 (77) | 6 (67) | 8 (47) | 5 (50) | 0.28 |
| Non-ST elevated MI (%) | 6 (23) | 2 (22) | 7 (41) | 3 (30) | |
| Unstable angina (%) | 0 (0) | 1 (11) | 2 (12) | 2 (20) | |
| Diseased vessels (%) | |||||
| Single | 16 (62) | 7 (78) | 13 (76) | 5 (50) | 0.29 |
| Double | 10 (38) | 2 (22) | 4 (24) | 4 (40) | |
| Triple | 0 (0) | 0 (0) | 0 (0) | 1 (10) | |
| Culprit vessel (%) | |||||
| RCA | 12 (46) | 3 (33) | 5 (29) | 2 (20) | 0.56 |
| LAD | 10 (39) | 6 (67) | 9 (53) | 7 (70) | |
| LCX | 4 (15) | 0 (0) | 3 (18) | 1 (10) | |
| Maximum CK, IU/L | 2008 ± 374 | 1799 ± 637 | 845 ± 463 | 1960 ± 604 | 0.24 |
| EF, % | 55.6 ± 1.9 | 56.6 ± 3.7 | 55.0 ± 2.3 | 55.1 ± 3.1 | 0.97 |
| Medications, n (%) | |||||
| DAPT (%) | 26 (100) | 9 (100) | 17 (100) | 10 (100) | 0.96 |
| ACE-I (%) | 16 (62) | 3 (33) | 13 (76) | 6 (60) | 0.05 |
| ARB (%) | 7 (27) | 5 (56) | 4 (24) | 3 (30) | 0.41 |
| β-blockers (%) | 19 (73) | 9 (100) | 15 (88) | 8 (80) | 0.08 |
| CCB (%) | 4 (15) | 1 (11) | 2 (12) | 2 (20) | 0.70 |
| Statin (%) | 25 (96) | 8 (89) | 16 (94) | 10 (100) | 0.71 |
| Ezetimibe (%) | 2 (10) | 0 (0) | 2 (12) | 3 (33) | 0.08 |
| α-GI (%) | 4 (15) | 0 (0) | 2 (12) | 0 (0) | 0.70 |
| SU (%) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 0.12 |
| Insulin (%) | 0 (0) | 1 (11) | 1 (6) | 1 (10) | 0.45 |
| Inactive Group with LDL-C ≥ 70 mg/dL (n = 26) | Inactive Group with LDL-C < 70 mg/dL (n = 9) | Active Group with LDL-C ≥ 70 mg/dL (n = 17) | Active Group with LDL-C < 70 mg/dL (n = 10) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | Baseline | Follow-Up | |
| Anthropometric parameters | ||||||||
| Body mass index (kg/m2) | 24.6 ± 0.6 | 23.7 ± 1.0 | 22.1± 1.1 | 24.3 ± 1.8 | 25.2 ± 0.8 | 24.9 ± 1.1 | 26.9 ± 1.0 | 21.4 ± 2.1 |
| Fat weight (%) | 27.8 ± 2.2 | 25.7 ± 1.9 | 18.9 ± 4.1 | 25.7 ± 3.6 | 23.8 ± 2.2 | 22.2 ± 2.0 | 32.0 ± 5.0 | 25.9 ± 4.4 |
| Lean body weight (kg) | 49.0 ± 2.8 | 51.2 ± 2.7 | 55.0 ± 5.2 | 52.3 ± 4.9 | 55.5 ± 2.8 | 53.5 ± 2.8 | 48.0 ± 6.3 | 50.6 ± 6.1 |
| Exercise tolerance | ||||||||
| Peak VO2, mL kg−1 min−1 | 15.4 ± 0.5 | 18.9 ± 1.1 * | 15.6 ± 0.9 | 19.1 ± 1.9 * | 16.4 ± 0.6 | 21.8 ± 1.1 * | 15.8 ± 0.8 | 20.0 ± 2.2 * |
| Lipid profile and glucose metabolism | ||||||||
| LDL-C, mg/dl | 136 ± 8 | 84 ± 2 * | 86 ± 14 | 53 ± 4 * | 141 ± 10 | 90 ± 3 * | 126 ± 13 | 58 ± 4 * |
| HDL-C, mg/dl | 41 ± 2 | 40 ± 2 | 46 ± 4 | 45 ± 4 | 44 ± 3 | 45 ± 3 | 42 ± 3 | 42 ± 3 |
| TG, mg/dl | 154 ± 16 | 158 ± 11 | 98 ± 26 | 111 ± 18 | 153 ± 19 | 127 ± 13 | 179 ± 25 | 127 ± 17 |
| FBS, mg/dl | 115 ± 7 | 98 ± 2 | 108 ± 11 | 101 ± 5 | 108 ± 9 | 98 ± 3 | 143 ± 11 | 100 ± 4 |
| Hemoglobin A1c, % | 5.8 ± 0.1 | 5.6 ± 0.1 | 5.6 ± 0.2 | 5.8 ± 0.2 | 5.7 ± 0.2 | 5.6 ± 0.1 | 6.4 ± 0.2 | 6.0 ± 0.1 |
| Inactive Group with LDL-C ≥ 70 mg/dL (n = 26) | Inactive Group with LDL-C < 70 mg/dL (n = 9) | Active Group with LDL-C ≥ 70 mg/dL (n = 17) | Active Group with LDL-C < 70 mg/dL (n = 10) | p-Value | |
|---|---|---|---|---|---|
| IVUS profile at baseline | |||||
| Vessel volume, mm3 | 212.1 ± 26.4 | 163.6 ± 44.9 | 197.1 ± 32.7 | 173.4 ± 42.6 | 0.76 |
| Lumen volume, mm3 | 113.2 ± 14.6 | 91.3 ± 24.9 | 102.3 ± 18.1 | 88.6 ± 23.6 | 0.78 |
| Plaque volume, mm3 | 98.8 ± 12.8 | 72.2 ± 21.9 | 94.8 ± 15.9 | 84.7 ± 20.7 | 0.74 |
| IVUS profile at follow-up | |||||
| Vessel volume, mm3 | 208.1 ± 25.8 | 161.6 ± 43.9 | 186.8 ± 31.9 * | 150.0 ± 41.6 * | 0.69 |
| Lumen volume, mm3 | 111.4 ± 14.3 | 92.4 ± 24.3 | 101.6 ± 17.7 | 84.6 ± 23.1 | 0.76 |
| Plaque volume, mm3 | 96.6 ± 12.3 | 69.1 ± 20.9 | 85.2 ± 15.2 * | 74.4 ± 19.8 * | 0.63 |
| Percent change of VV, % | −2.4 ± 2.0 | 0.0 ± 3.4 | −6.4 ± 2.5 | −8.5 ± 3.3 | 0.20 |
| Percent change of LV, % | −1.5 ± 3.3 | 1.9 ± 5.6 | −4.0 ± 4.1 | −3.6 ± 5.3 | 0.84 |
| Percent change of PV, % | −2.1 ± 2.1 | 0.3 ± 3.6 | −9.6 ± 2.6 | −12.3 ± 3.4 | 0.01 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Age | 0.3 | 0.01 | ||
| Body mass index | −0.09 | 0.46 | ||
| Diabetes (absent-present) | −0.07 | 0.54 | ||
| Fasting glucose | 0.04 | 0.76 | ||
| Delta LDL-C | 0.3 | 0.01 | 0.3 | 0.03 |
| Delta HDL-C | −0.21 | 0.1 | ||
| Physical activity | −0.45 | 0.0003 | −0.34 | 0.02 |
| Delta peak VO2 | −0.09 | 0.44 | ||
| Plaque volume at baseline | 0.09 | 0.50 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishitani-Yokoyama, M.; Miyauchi, K.; Shimada, K.; Yokoyama, T.; Ouchi, S.; Aikawa, T.; Kunimoto, M.; Yamada, M.; Honzawa, A.; Okazaki, S.; et al. Preliminary Pilot Study of Combined Effects of Physical Activity and Achievement of LDL-Cholesterol Target on Coronary Plaque Volume Changes in Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 1578. https://doi.org/10.3390/jcm9051578
Nishitani-Yokoyama M, Miyauchi K, Shimada K, Yokoyama T, Ouchi S, Aikawa T, Kunimoto M, Yamada M, Honzawa A, Okazaki S, et al. Preliminary Pilot Study of Combined Effects of Physical Activity and Achievement of LDL-Cholesterol Target on Coronary Plaque Volume Changes in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine. 2020; 9(5):1578. https://doi.org/10.3390/jcm9051578
Chicago/Turabian StyleNishitani-Yokoyama, Miho, Katsumi Miyauchi, Kazunori Shimada, Takayuki Yokoyama, Shohei Ouchi, Tatsuro Aikawa, Mitsuhiro Kunimoto, Miki Yamada, Akio Honzawa, Shinya Okazaki, and et al. 2020. "Preliminary Pilot Study of Combined Effects of Physical Activity and Achievement of LDL-Cholesterol Target on Coronary Plaque Volume Changes in Patients with Acute Coronary Syndrome" Journal of Clinical Medicine 9, no. 5: 1578. https://doi.org/10.3390/jcm9051578
APA StyleNishitani-Yokoyama, M., Miyauchi, K., Shimada, K., Yokoyama, T., Ouchi, S., Aikawa, T., Kunimoto, M., Yamada, M., Honzawa, A., Okazaki, S., Tsujita, H., Koba, S., & Daida, H. (2020). Preliminary Pilot Study of Combined Effects of Physical Activity and Achievement of LDL-Cholesterol Target on Coronary Plaque Volume Changes in Patients with Acute Coronary Syndrome. Journal of Clinical Medicine, 9(5), 1578. https://doi.org/10.3390/jcm9051578





