Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Statistical Considerations
3. Results
3.1. Baseline Features
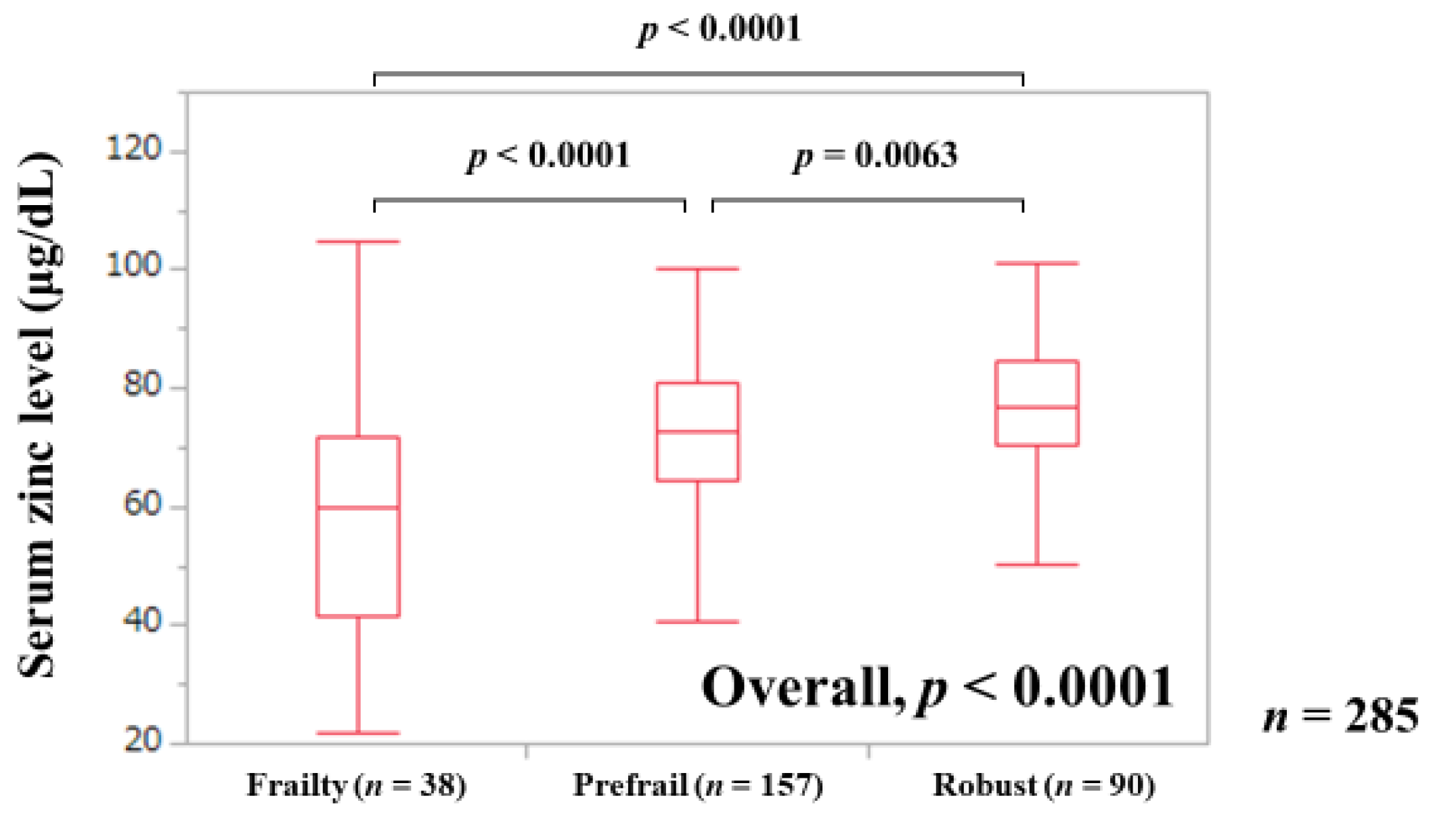
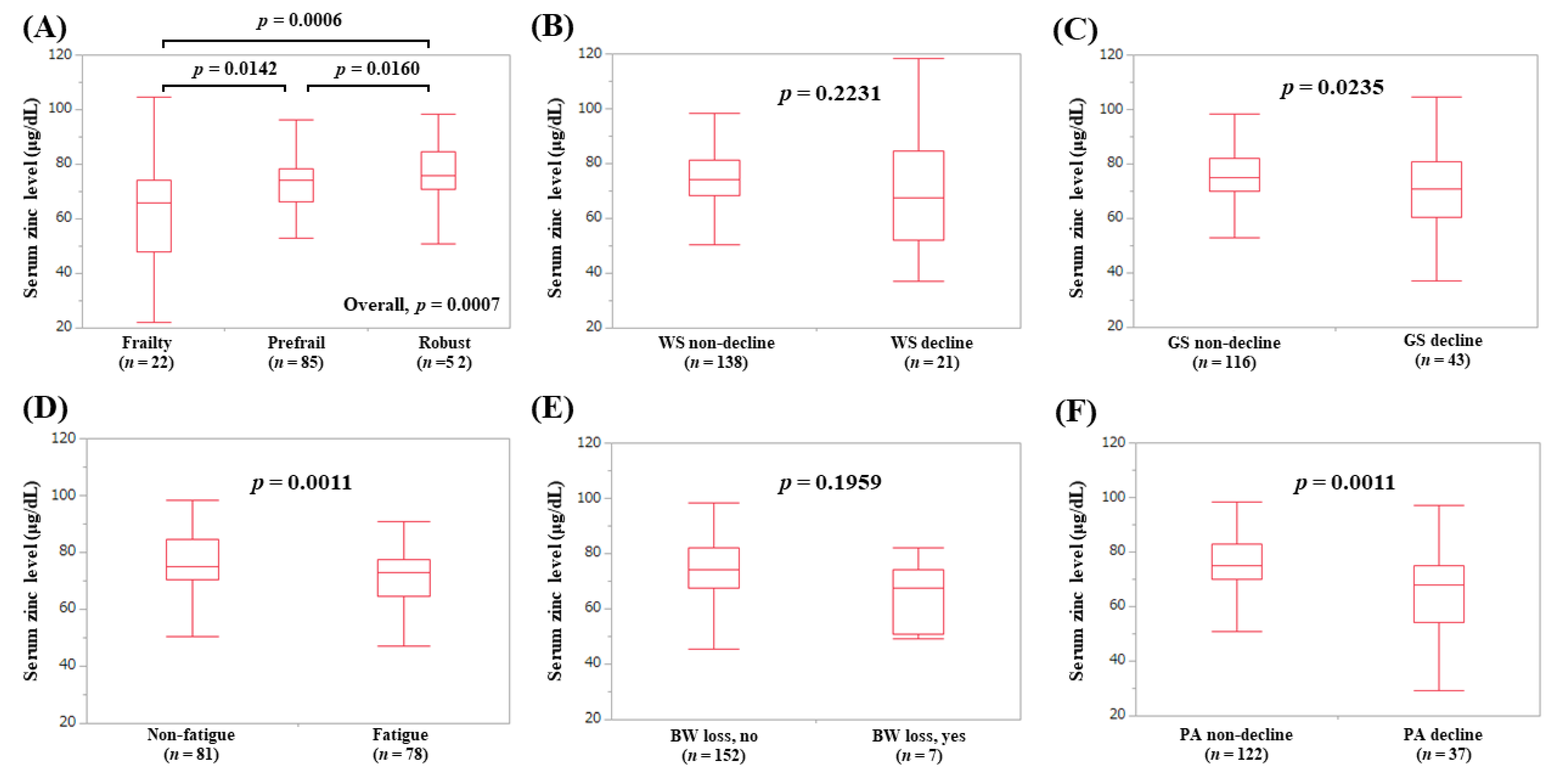
3.2. Serum Zn Level in Patients with Frailty, Prefrail, and Robust
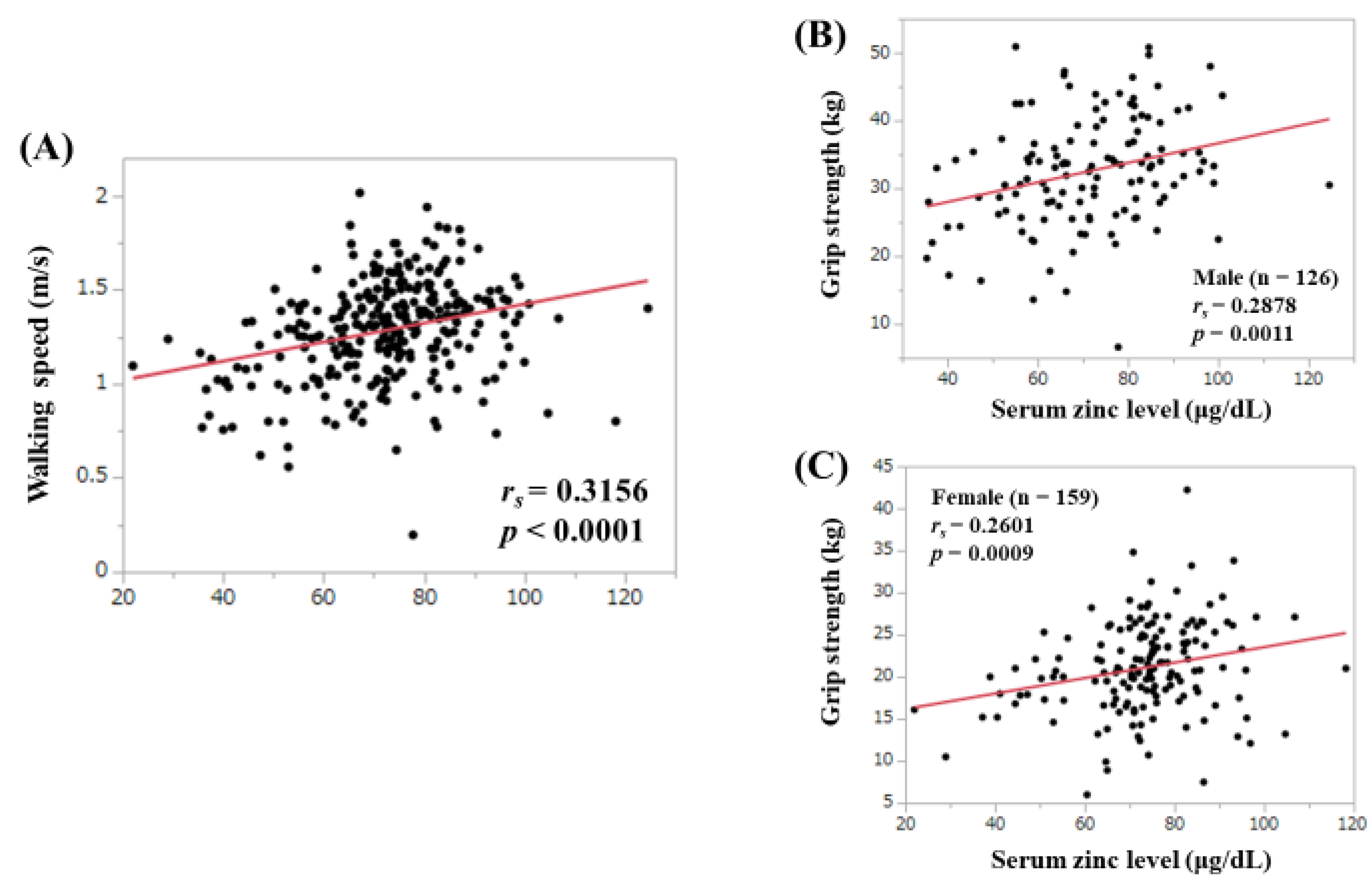
3.3. Correlation between Serum Zn Level and WS and GS
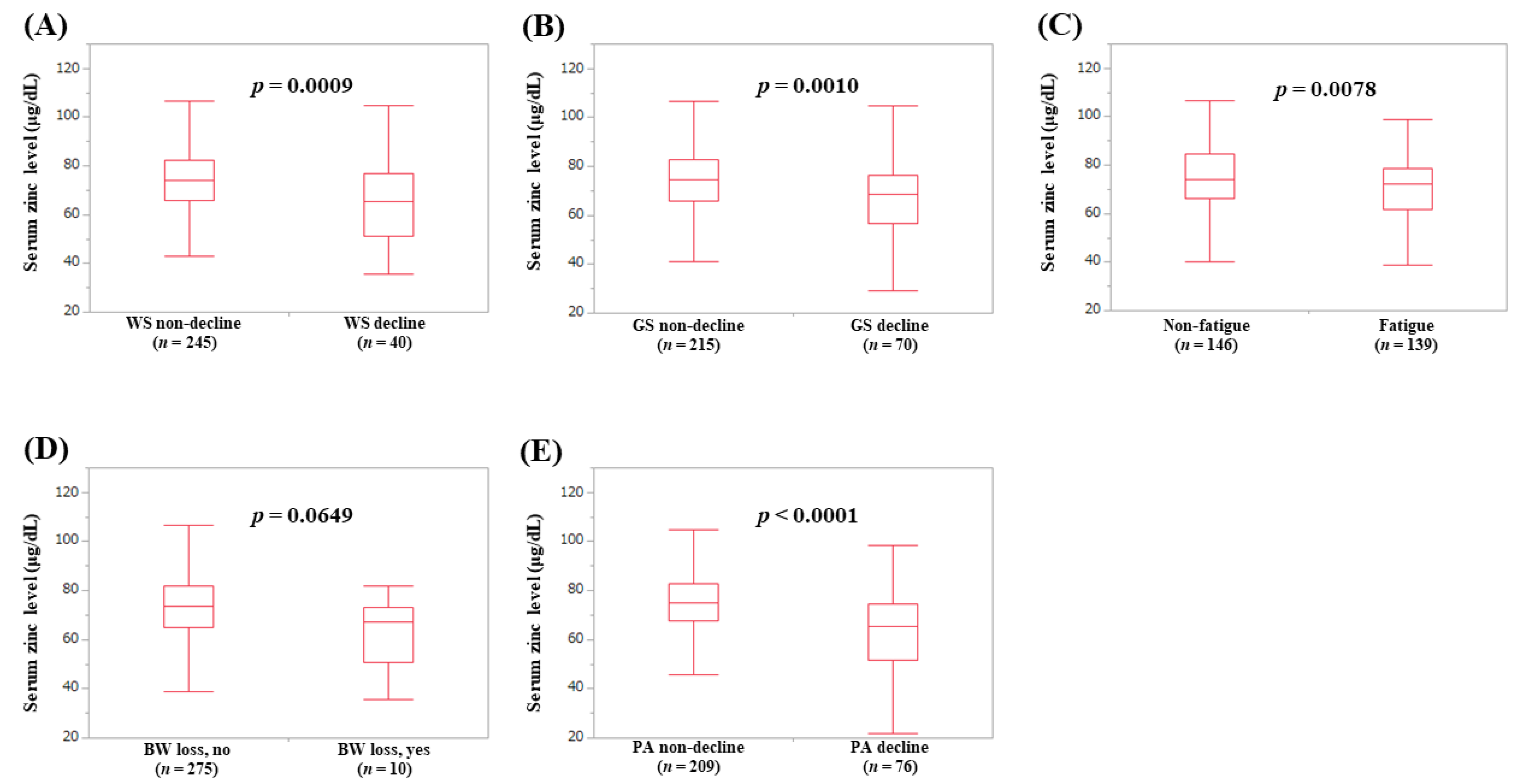
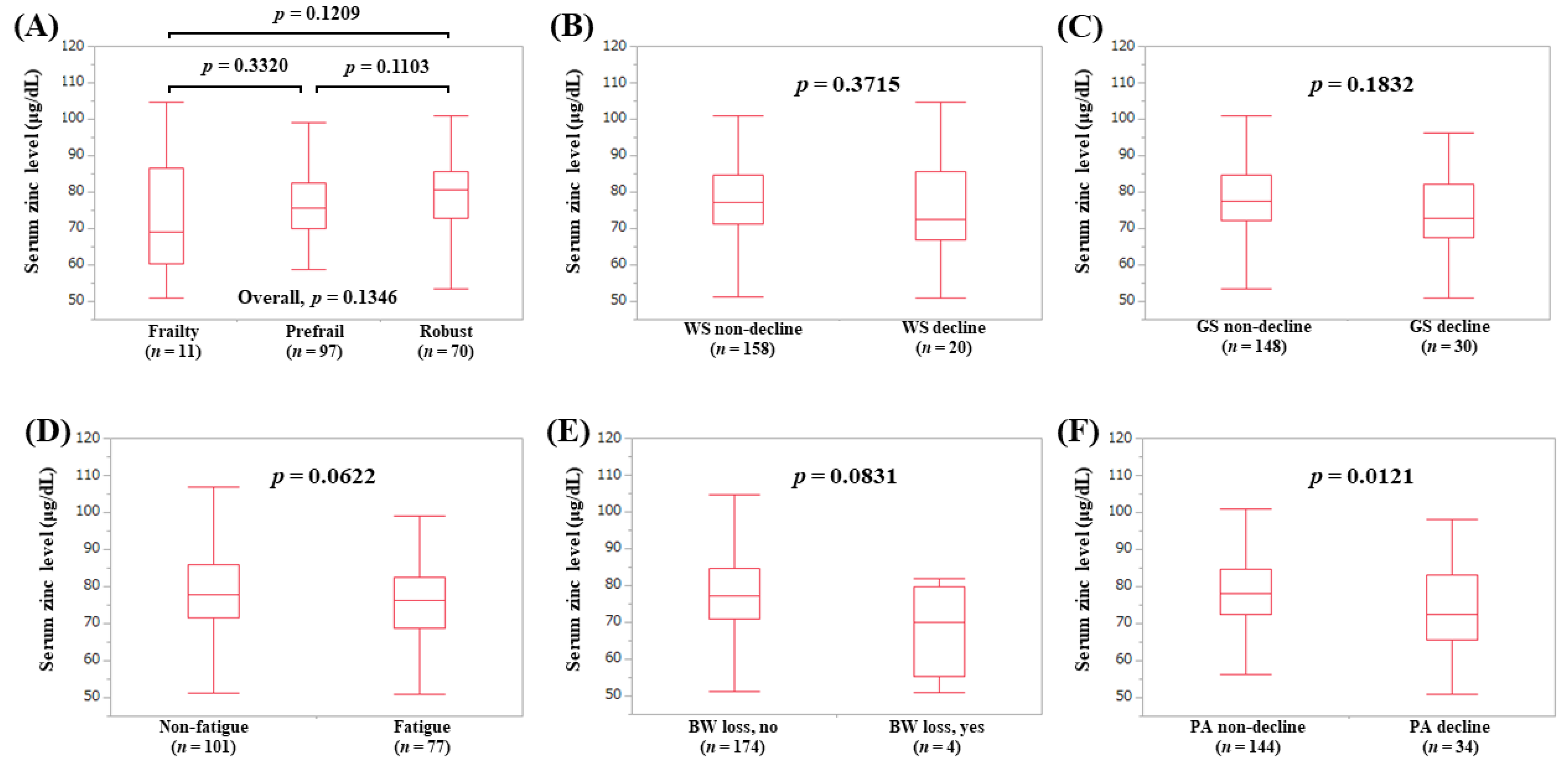
3.4. Serum Zn Level According to the Frailty Phenotypes
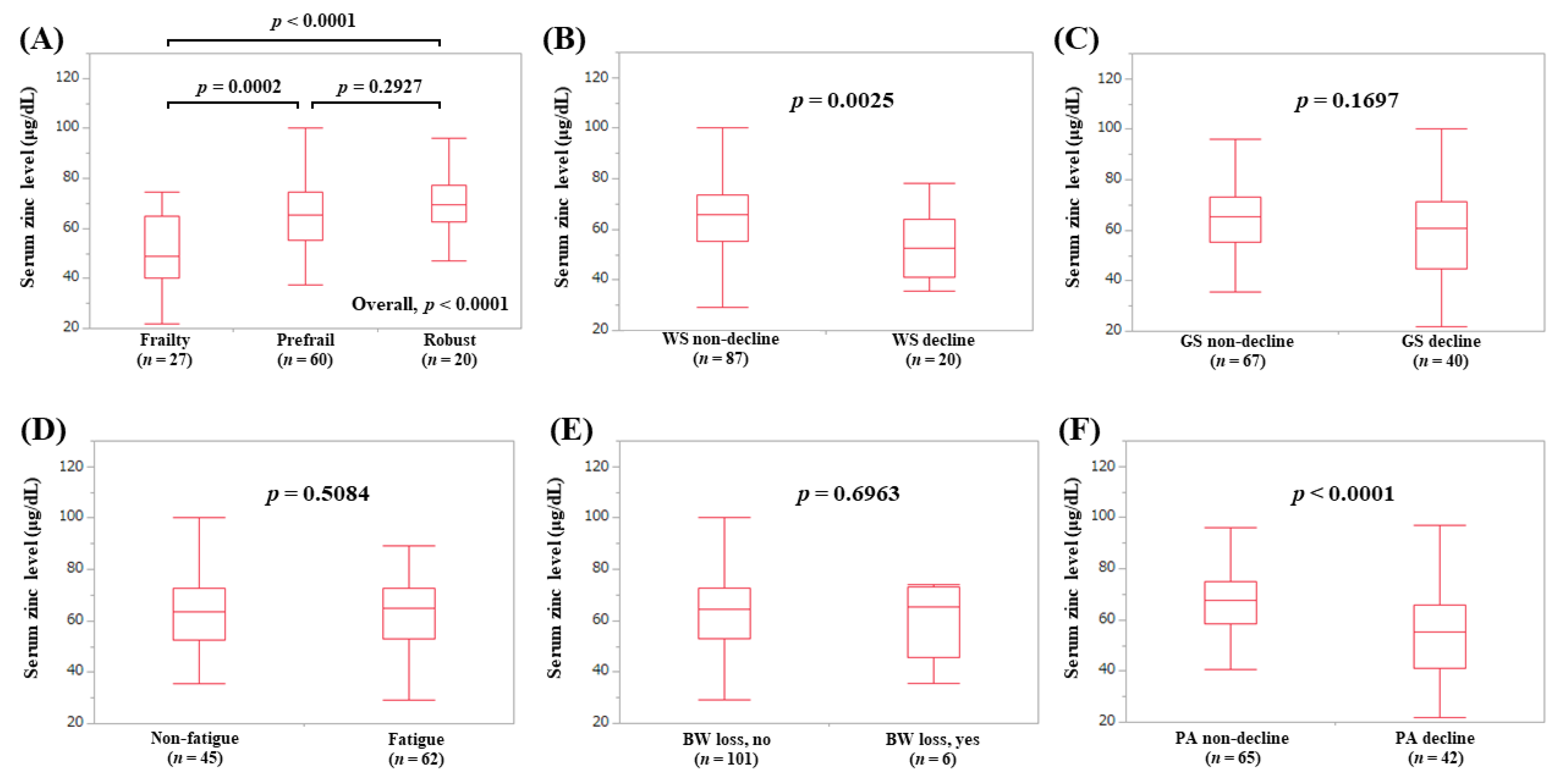
3.5. Serum Zn Level According to the Frailty Phenotypes in LC Patients (Subset Analysis 1)
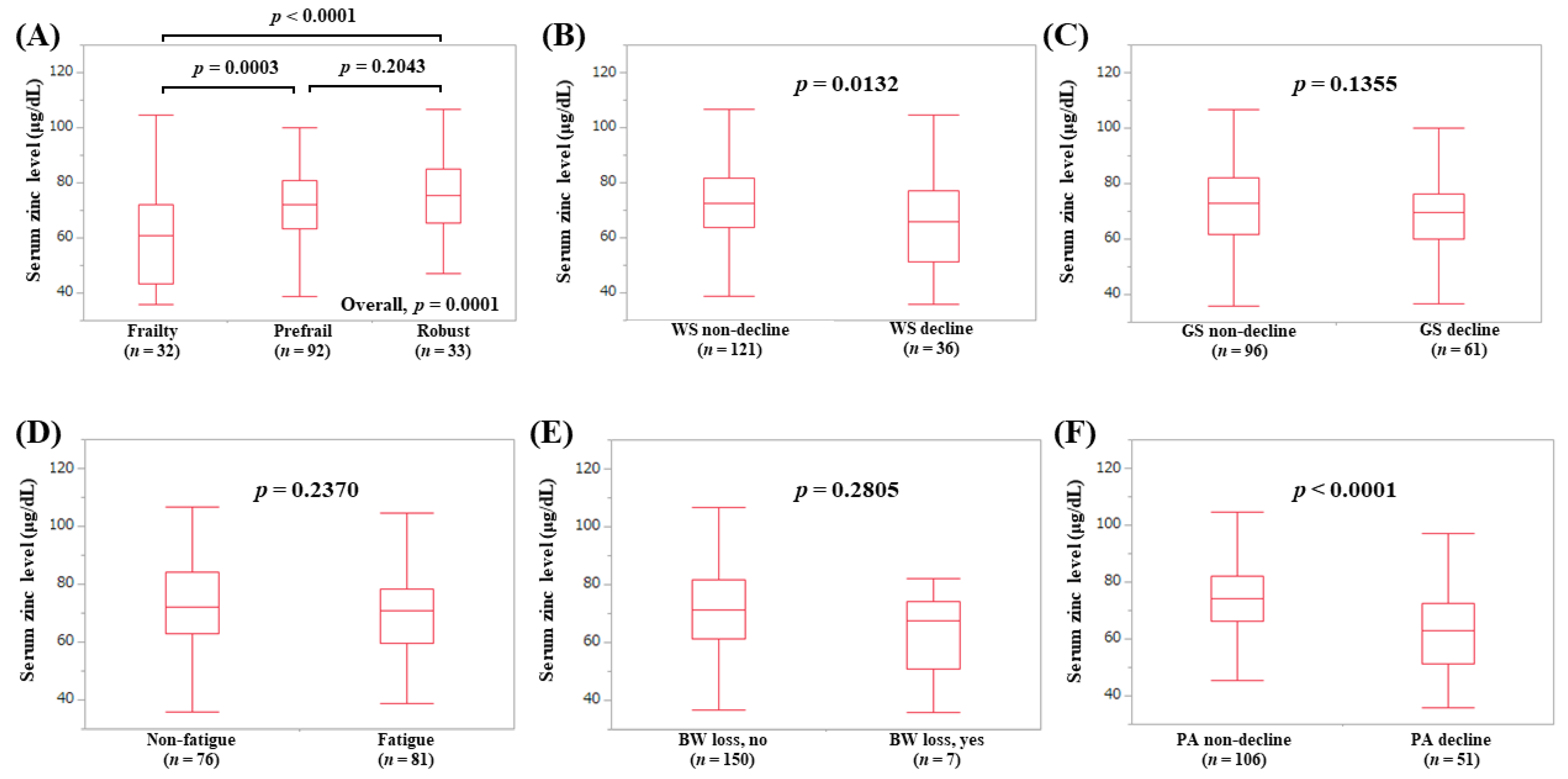
3.6. Serum Zn Level According to the Frailty Phenotypes in Non-LC Patients (Subset Analysis 2)
3.7. Serum Zn Level According to the Frailty Phenotypes in Elderly Patients (65 Years or More, Subset Analysis 3)
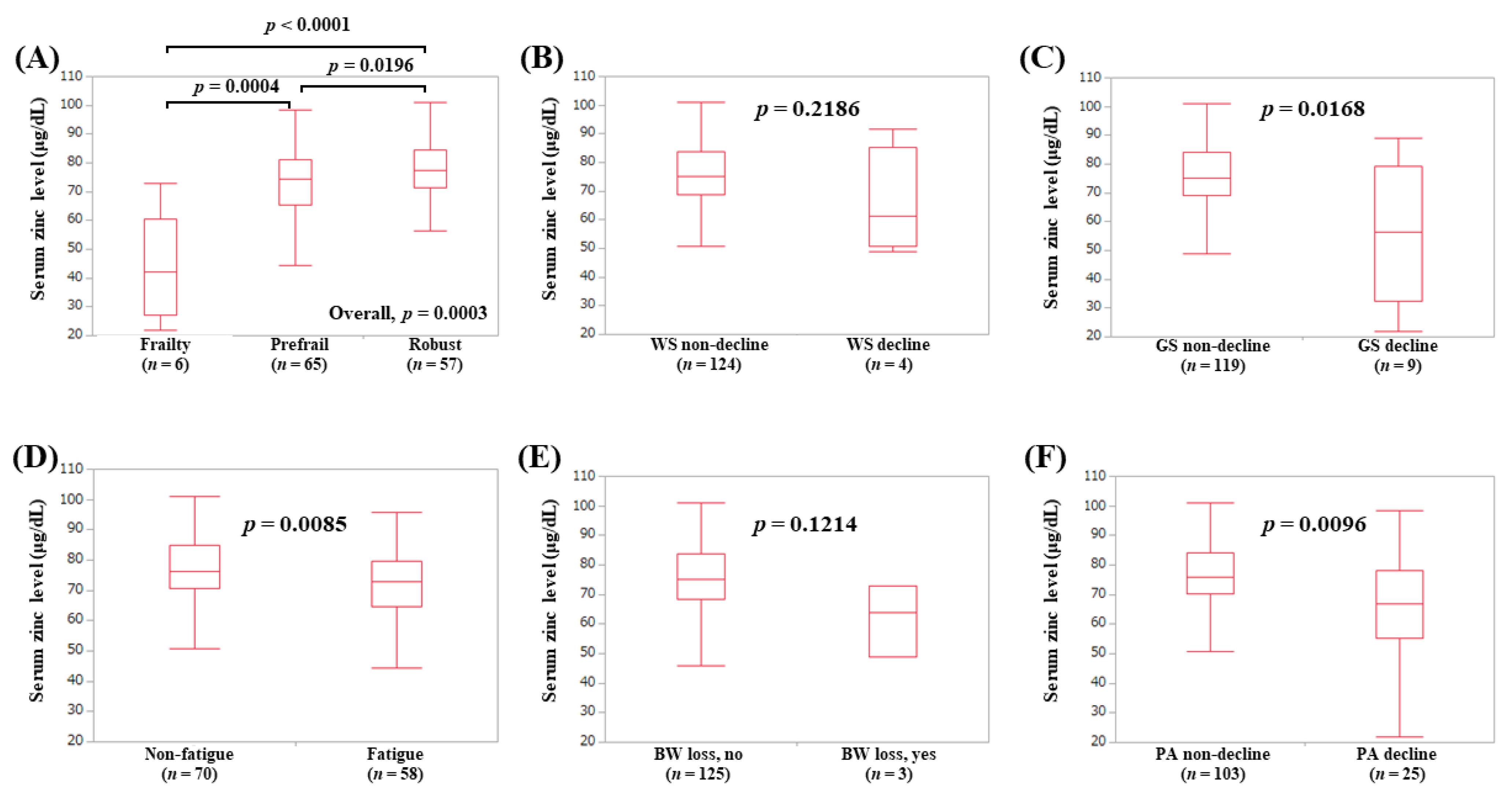
3.8. Serum Zn Level According to the Frailty Phenotypes in Younger Patients (Less than 65 Years, Subset Analysis 4)
3.9. Serum Zn Level According to the Frailty Phenotypes in Male Patients (Subset Analysis 5)
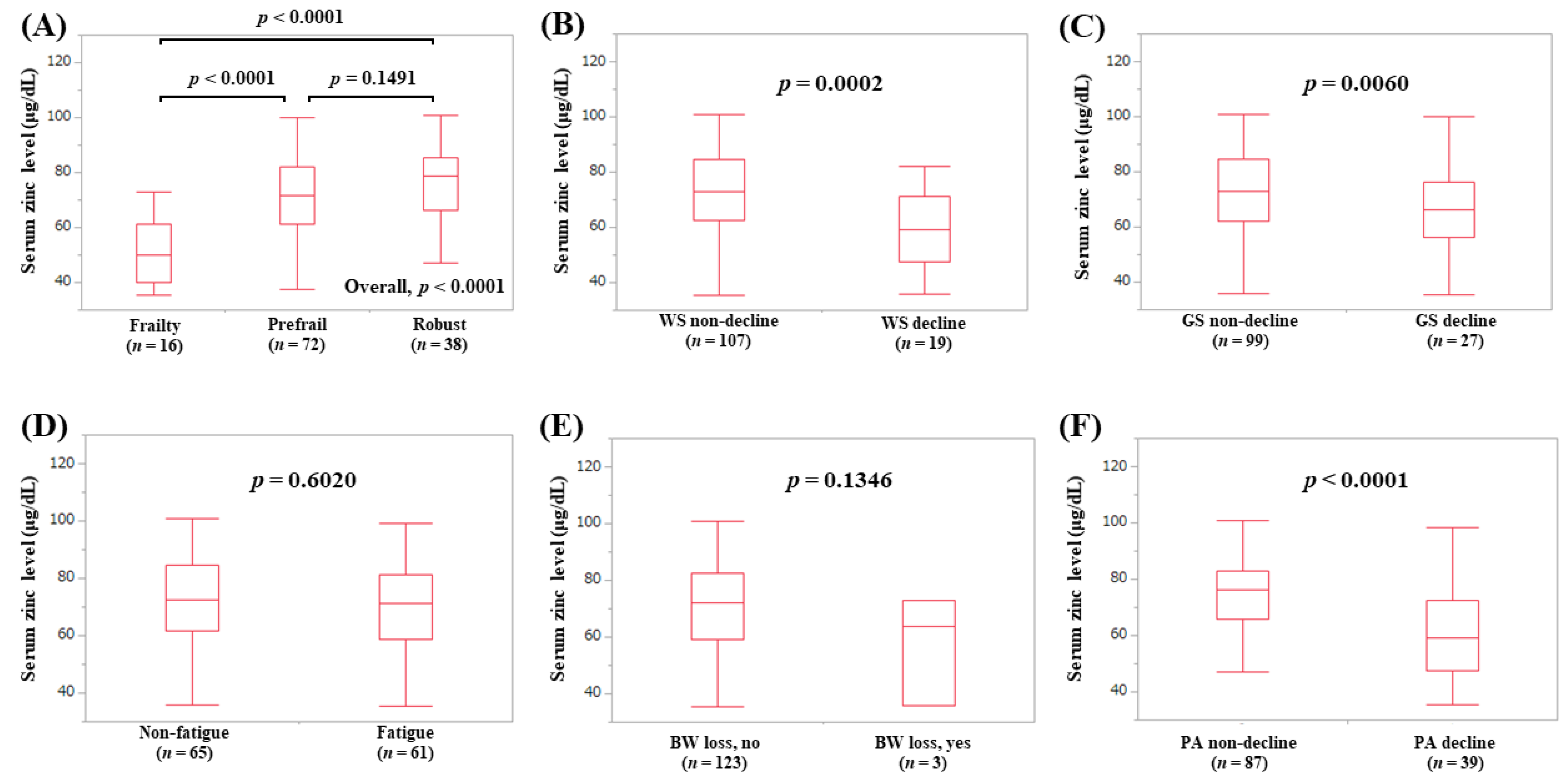
3.10. Serum Zn Level According to the Frailty Phenotypes in Female Patients (Subset Analysis 6)
3.11. Correlation between Frailty Score (0–5) and Baseline Numerical Variables
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | physical activity |
| BW | body weight |
| WS | walking speed |
| CLD | chronic liver disease |
| Zn | zinc |
| LC | liver cirrhosis |
| GS | grip strength |
| SMI | skeletal muscle index |
| BIA | bioimpedance analysis |
| IQR | interquartile range |
| ECW | extracellular water |
| TBW | total body water |
| IGF-1 | insulin-like growth factor-1 |
| GH | growth hormone |
References
- Ribeiro, A.R.; Howlett, S.E.; Fernandes, A. Frailty—A promising concept to evaluate disease vulnerability. Mech. Ageing Dev. 2020, 187, 111217. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. Implications of frailty screening in clinical practice. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, R.A.C.; Yamada, M.; Arai, H. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Arai, H. Chapter 1 Frailty: Definition, diagnosis, epidemiology. Geriatr. Gerontol. Int. 2020, 20, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Tanaka, S.; Iida, H.; Ueno, M.; Hirokawa, F.; Nomi, T.; Nakai, T.; Kaibori, M.; Ikoma, H.; Eguchi, H.; Shinkawa, H.; et al. Preoperative Risk Assessment for Loss of Independence Following Hepatic Resection in Elderly Patients. Ann. Surg. 2019. [Google Scholar] [CrossRef]
- Carey, E.J.; Steidley, D.E.; Aqel, B.A.; Byrne, T.J.; Mekeel, K.L.; Rakela, J.; Vargas, H.E.; Douglas, D.D. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transplant. 2010, 16, 1373–1378. [Google Scholar] [CrossRef]
- Dunn, M.A.; Rogal, S.S.; Duarte-Rojo, A.; Lai, J.C. Physical Function, Physical Activity and Quality of Life after Liver Transplantation: A Review. Liver Transplant. 2020. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, R.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Kok, B.; Whitlock, R.; Ferguson, T.; Bailey, R.J.; Burak, K.W.; Kowalczewski, J.; Tangri, N.; Tandon, P. Health-Related Quality of Life. Am. J. Gastroenterol. 2020, 115, 575–583. [Google Scholar] [CrossRef]
- Tapper, E.B. Frailty and Outcomes after Liver Transplantation. Curr. Transplant. Rep. 2019, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Dodge, J.L.; McCulloch, C.E.; Covinsky, K.E.; Singer, J.P. Frailty and the Burden of Concurrent and Incident Disability in Patients with Cirrhosis: A Prospective Cohort Study. Hepatol. Commun. 2019, 4, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Sonnenday, C.J.; Tapper, E.B.; Duarte-Rojo, A.; Dunn, M.A.; Bernal, W.; Carey, E.J.; Dasarathy, S.; Kamath, B.M.; Kappus, M.R.; et al. Frailty in liver transplantation: An expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Arab. Archaeol. Epigr. 2019, 19, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 2019, 51, 64–77. [Google Scholar] [CrossRef]
- Kok, B.; Tandon, P. Frailty in Patients with Cirrhosis. Curr. Treat. Options Gastroenterol. 2018, 16, 215–225. [Google Scholar] [CrossRef]
- Grüngreiff, K. Zinc in liver disease. J. Trace Elements Exp. Med. 2002, 15, 67–78. [Google Scholar] [CrossRef]
- Alker, W.; Haase, H. Zinc and Sepsis. Nutrients 2018, 10, 976. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Korczak, B.B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Gao, H.; Dai, W.; Zhao, L.; Min, J.; Wang, F. The Role of Zinc and Zinc Homeostasis in Macrophage Function. J. Immunol. Res. 2018, 2018, 6872621. [Google Scholar] [CrossRef]
- Kogan, S.; Sood, A.; Garnick, M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds 2017, 29, 102–106. [Google Scholar]
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nissensohn, M.; Sanchez-Villegas, A.; Lugo, D.F.; Sánchez, P.H.; Alonso, J.D.; Quintana, L.P.; Ruano, C.; Lowe, N.L.; Moran, V.H.; Skinner, A.L.; et al. Effect of Zinc Intake on Growth in Infants: A Meta-analysis. Crit. Rev. Food Sci. Nutr. 2014, 56, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Himoto, T.; Masaki, T. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Yoh, K.; Iwata, Y.; Sakai, Y.; Kishino, K.; Ikeda, N.; Takashima, T.; Aizawa, N.; Takata, R.; et al. Serum Zinc Level Classification System: Usefulness in Patients with Liver Cirrhosis. J. Clin. Med. 2019, 8, 2057. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, I.; Arakawa, T.; Suii, H.; Tatsumi, R.; Yamaguchi, M.; Nakajima, T.; Kuwata, Y.; Toyota, J.; Karino, Y. Zinc deficiency in patients with chronic liver disease in Japan. Hepatol. Res. 2020, 50, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Saito, H.; Ueno, Y.; Uto, H.; Obara, K.; Sakaida, I.; Shibuya, A.; Seike, M.; Nagoshi, S.; Segawa, M.; et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 2016, 51, 629–650. [Google Scholar] [CrossRef]
- Lurie, Y.; Webb, M.; Cytter-Kuint, R.; Shteingart, S.; Lederkremer, G.Z. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J. Gastroenterol. 2015, 21, 11567–11583. [Google Scholar] [CrossRef]
- Zarski, J.P.; Sturm, N.; Guéchot, J.; Paris, A.; Zafrani, E.S.; Asselah, T.; Boisson, R.C.; Bosson, J.L.; Guyader, D.; Renversez, J.C.; et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: The ANRS HCEP-23 study. J. Hepatol. 2012, 56, 55–62. [Google Scholar] [CrossRef]
- Romanelli, R.; Stasi, C. Recent Advancements in Diagnosis and Therapy of Liver Cirrhosis. Curr. Drug Targets 2016, 17, 1804–1817. [Google Scholar] [CrossRef]
- Kariyama, K.; Nouso, K.; Toyoda, H.; Tada, T.; Hiraoka, A.; Tsuji, K.; Itobayashi, E.; Ishikawa, T.; Wakuta, A.; Oonishi, A.; et al. Utility of FIB4-T as a Prognostic Factor for Hepatocellular Carcinoma. Cancers 2019, 11, 203. [Google Scholar] [CrossRef]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Takata, R.; Hasegawa, K.; Nakano, C.; et al. Development of a simple predictive model for decreased skeletal muscle mass in patients with compensated chronic liver disease. Hepatol. Res. 2017, 47, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Buchard, B.; Boirie, Y.; Cassagnes, L.; Lamblin, G.; Coilly, A.; Abergel, A. Assessment of Malnutrition, Sarcopenia and Frailty in Patients with Cirrhosis: Which Tools Should We Use in Clinical Practice? Nutrients 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.J.; Lee, D.S.; Kim, W.K.; Han, B.S.; Lee, S.C.; Bae, K.H. Metabolic Adaptation in Obesity and Type II Diabetes: Myokines, Adipokines and Hepatokines. Int. J. Mol. Sci. 2016, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Gaier, E.D.; Kleppinger, A.; Ralle, M.; Mains, R.E.; Kenny, A.M.; Eipper, B.A. High serum Cu and Cu/Zn ratios correlate with impairments in bone density, physical performance and overall health in a population of elderly men with frailty characteristics. Exp. Gerontol. 2012, 47, 491–496. [Google Scholar] [CrossRef]
- Tannou, T.; Koeberle, S.; Manckoundia, P.; Aubry, R. Multifactorial immunodeficiency in frail elderly patients: Contributing factors and management. Med. Mal. Infect. 2019, 49, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.E.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sportsmed 2017, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Dyar, K.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Lauretani, F.; Butto, V.; Bondi, G.; Cattabiani, C.; Nouvenne, A.; Meschi, T.; Dall’Aglio, E.; Ceda, G. IGF-1, the Cross Road of the Nutritional, Inflammatory and Hormonal Pathways to Frailty. Nutrients 2013, 5, 4184–4205. [Google Scholar] [CrossRef]
- Rocha, E.D.; De Brito, N.J.; Dantas, M.M.; Silva, A.A.; Almeida, M.D.; Brandão-Neto, J. Effect of Zinc Supplementation on GH, IGF1, IGFBP3, OCN, and ALP in Non-Zinc-Deficient Children. J. Am. Coll. Nutr. 2015, 34, 290–299. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular Water to Total Body Water Ratio in Viral Liver Diseases: A Study Using Bioimpedance Analysis. Nutrients 2018, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Hadjihambi, A.; Arias, N.; Sheikh, M.; Jalan, R. Hepatic encephalopathy: A critical current review. Hepatol. Int. 2017, 12, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Pasternak, O.; Liu, S.; Loke, Y.M.; Choo, B.L.; Hilal, S.; Xu, X.; Ikram, M.K.; Venketasubramanian, N.; Chen, C.L.; et al. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer’s disease with and without cerebrovascular disease. Alzheimer’s Res. Ther. 2017, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Kehayias, J.J.; Ribeiro, S.M.L.; Skahan, A.; Itzkowitz, L.; Dallal, G.; Rogers, G.; Khodeir, M. Water homeostasis, frailty and cognitive function in the nursing home. J. Nutr. Health Aging 2012, 16, 35–39. [Google Scholar] [CrossRef]
- Meleleo, D.; Bartolomeo, N.; Cassano, L.; Nitti, A.; Susca, G.; Mastrototaro, G.; Armenise, U.; Zito, A.; DeVito, F.; Scicchitano, P.; et al. Evaluation of body composition with bioimpedence. A comparison between athletic and non-athletic children. Eur. J. Sport Sci. 2017, 17, 710–719. [Google Scholar] [CrossRef]
- Huang, X.; Liu, X.; Yu, Y. Depression and Chronic Liver Diseases: Are There Shared Underlying Mechanisms? Front. Mol. Neurosci. 2017, 10, 134. [Google Scholar] [CrossRef]
- Lee, Y.P.; Chang, C.H.; Liu, H.H.; Chen, C.Y.; Chen, C.Y.; Hsu, C.C.; Chang, C.I.; Lin, Y.T.; Lee, C.S.; Tsai, J.S. Plasma zinc alpha2-glycoprotein levels correlate positively with frailty severity in female elders. Medicine 2016, 95, e4753. [Google Scholar] [CrossRef]
| Variables | All Cases (n = 285) |
|---|---|
| Age (years) | 66 (55.5, 72) |
| Gender, male/female | 126/159 |
| Liver disease etiology HCV-related/HBV-related/HBV- and HCV-related/NBNC-related | 157/49/7/72 |
| Presence of frailty, yes/no | 38/247 |
| Presence of LC, yes/no | 107/178 |
| Body mass index (kg/m2) | 22.9 (20.5, 25.75) |
| Walking speed (m/s) | 1.314 (1.111, 1.447) |
| Grip strength (kg), male | 33.0 (27.25, 37.075) |
| Grip strength (kg), female | 20.8 (17.7, 24.7) |
| SMI (kg/m2), male | 7.435 (6.82, 8.0) |
| SMI (kg/m2), female | 5.94 (5.49, 6.48) |
| ECW to TBW ratio | 0.39 (0.384, 0.3955) |
| Total bilirubin (mg/dL) | 0.8 (0.6, 1.1) |
| Serum albumin (g/dL) | 4.2 (3.9, 4.5) |
| Prothrombin time (%) | 90.5 (79.6, 98.2) |
| Platelet count (×104/mm3) | 17.3 (12.35, 21.15) |
| AST (IU/L) | 24 (19, 34) |
| ALT (IU/L) | 18 (13, 30.5) |
| Total cholesterol (mg/dL) | 182 (150, 214) |
| Triglyceride (mg/dL) | 85 (63.5, 116.5) |
| HbA1c (NGSP) | 5.7 (5.4, 6.0) |
| eGFR (ml/min/1.73 m2) | 81 (67.5, 94.5) |
| Serum sodium (mmol/L) | 140 (139, 141) |
| Serum zinc (μg/dL) | 73 (64.25, 82) |
| Serum ammonia (μg/dL) | 20 (30, 39) |
| Branched-chain amino acid to tyrosine ratio | 5.64 (4.17, 6.795) |
| All Cases | Male | Female | ||||
|---|---|---|---|---|---|---|
| rs | p-Value | rs | p-Value | rs | p-Value | |
| Age | 0.3570 | <0.0001 | 0.2740 | 0.0019 | 0.4237 | <0.0001 |
| Body mass index | −0.0768 | 0.1964 | −0.1444 | 0.1067 | −0.0212 | 0.7910 |
| Total bilirubin | 0.0009 | 0.9874 | −0.0736 | 0.4128 | 0.0528 | 0.5088 |
| Serum albumin | −0.3212 | <0.0001 | −0.3746 | <0.0001 | −0.2774 | 0.0004 |
| Prothrombin time | −0.1045 | 0.0783 | −0.1161 | 0.1954 | −0.0860 | 0.2869 |
| Platelet count | −0.1933 | 0.0010 | −0.1396 | 0.1191 | −0.2290 | 0.0037 |
| AST | 0.1013 | 0.0879 | 0.1112 | 0.2153 | 0.0908 | 0.2551 |
| ALT | 0.0142 | 0.8118 | −0.0813 | 0.3654 | 0.0949 | 0.2342 |
| Total cholesterol | −0.1813 | 0.0021 | −0.0720 | 0.4231 | −0.2518 | 0.0014 |
| Triglyceride | 0.0467 | 0.4326 | −0.0630 | 0.4836 | 0.1227 | 0.1233 |
| HbA1c (NGSP) | 0.0047 | 0.9375 | 0.0423 | 0.6378 | −0.0191 | 0.8114 |
| eGFR | −0.0409 | 0.4921 | −0.0306 | 0.7338 | −0.0495 | 0.5341 |
| SMI | NA | NA | −0.2155 | 0.0164 | −0.2345 | 0.0029 |
| ECW to TBW ratio | 0.4386 | <0.0001 | 0.3775 | <0.0001 | 0.5196 | <0.0001 |
| BTR | −0.2374 | <0.0001 | −0.3379 | 0.0002 | −0.1514 | 0.0652 |
| Serum zinc | −0.3406 | <0.0001 | −0.3641 | <0.0001 | −0.3214 | <0.0001 |
| Serum ammonia | 0.0632 | 0.3007 | 0.2050 | 0.0259 | 0.0374 | 0.6471 |
| Estimates | Standard Error | p-Value | |
|---|---|---|---|
| Age | −0.00472 | 0.0110 | 0.6693 |
| Serum albumin | −0.216 | 0.272 | 0.4289 |
| SMI | −0.238 | 0.102 | 0.0223 |
| BTR | −0.00292 | 0.0648 | 0.6536 |
| Serum zinc | −0.00847 | 0.00821 | 0.0903 |
| Serum ammonia | 0.00494 | 0.00361 | 0.1738 |
| ECW to TBW ratio | 21.607 | 14.091 | 0.0128 |
| Estimates | Standard Error | p-Value | |
|---|---|---|---|
| Age | 0.00672 | 0.00773 | 0.3860 |
| Serum albumin | −0.109 | 0.189 | 0.5648 |
| SMI | −0.150 | 0.105 | 0.1567 |
| Platelet count | 0.0135 | 0.0117 | 0.2509 |
| Serum zinc | −0.0131 | 0.00635 | 0.0406 |
| Total cholesterol | −0.00237 | 0.00182 | 0.1939 |
| ECW to TBW ratio | 61.248 | 11.351 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, H.; Yoh, K.; Enomoto, H.; Iwata, Y.; Sakai, Y.; Kishino, K.; Shimono, Y.; Ikeda, N.; Takashima, T.; Aizawa, N.; et al. Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases. J. Clin. Med. 2020, 9, 1570. https://doi.org/10.3390/jcm9051570
Nishikawa H, Yoh K, Enomoto H, Iwata Y, Sakai Y, Kishino K, Shimono Y, Ikeda N, Takashima T, Aizawa N, et al. Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases. Journal of Clinical Medicine. 2020; 9(5):1570. https://doi.org/10.3390/jcm9051570
Chicago/Turabian StyleNishikawa, Hiroki, Kazunori Yoh, Hirayuki Enomoto, Yoshinori Iwata, Yoshiyuki Sakai, Kyohei Kishino, Yoshihiro Shimono, Naoto Ikeda, Tomoyuki Takashima, Nobuhiro Aizawa, and et al. 2020. "Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases" Journal of Clinical Medicine 9, no. 5: 1570. https://doi.org/10.3390/jcm9051570
APA StyleNishikawa, H., Yoh, K., Enomoto, H., Iwata, Y., Sakai, Y., Kishino, K., Shimono, Y., Ikeda, N., Takashima, T., Aizawa, N., Takata, R., Hasegawa, K., Koriyama, T., Yuri, Y., Nishimura, T., Nishiguchi, S., & Iijima, H. (2020). Serum Zinc Level Is Associated with Frailty in Chronic Liver Diseases. Journal of Clinical Medicine, 9(5), 1570. https://doi.org/10.3390/jcm9051570




