Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience
Abstract
1. Introduction
2. Experimental Section
2.1. Patients
2.2. PDT Procedure
2.3. Outcome Measures
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Efficacy and Survival
3.3. Safety
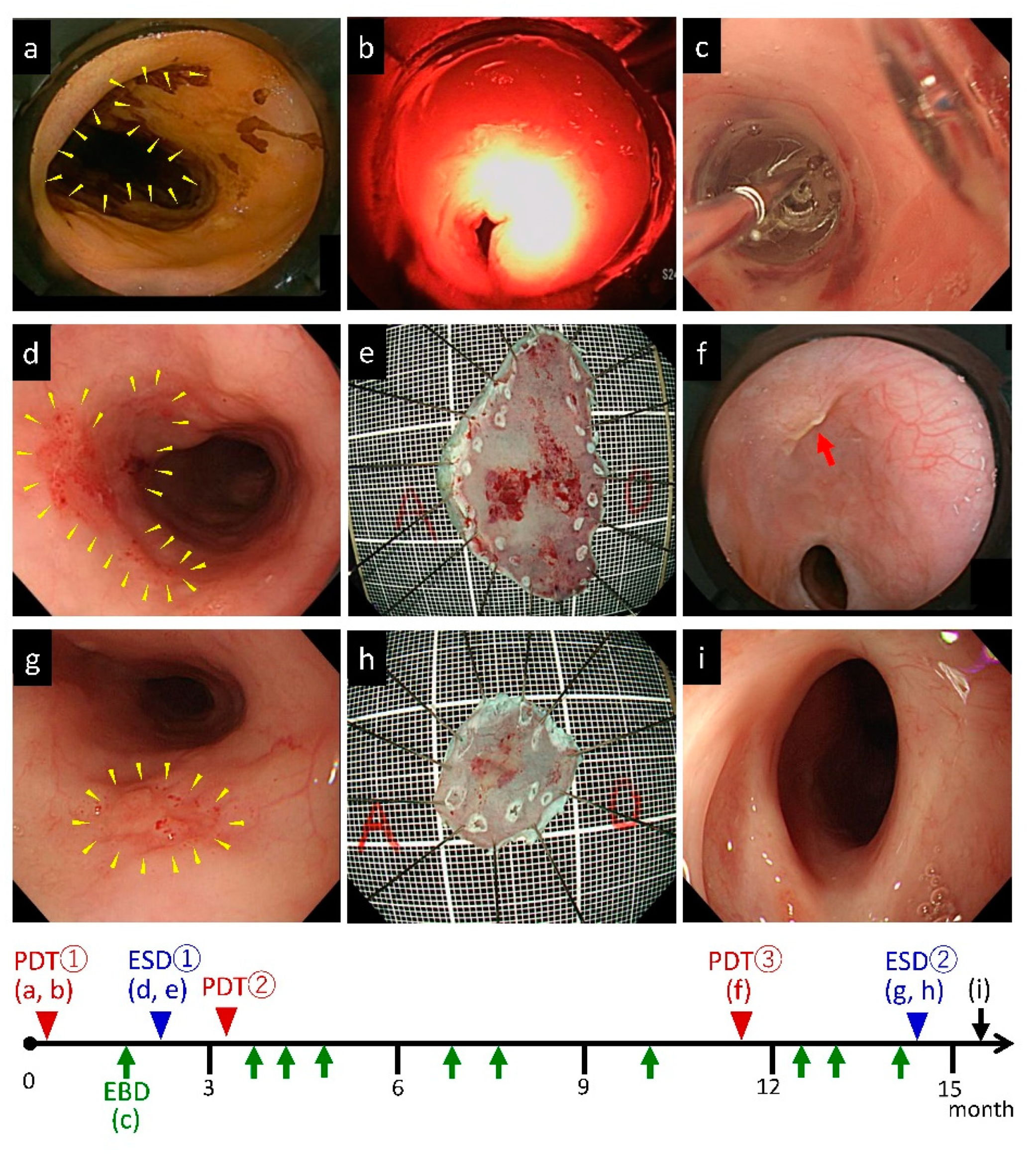
3.4. Combination Therapy of Repeated PDTs and ESDs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cooper, J.S.; Guo, M.D.; Herskovic, A.; Macdonald, J.S.; Martenson, J.A., Jr.; Al-Sarraf, M.; Byhardt, R.; Russell, A.H.; Beitler, J.J.; Spencer, S.; et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999, 281, 1623–1627. [Google Scholar] [CrossRef]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef]
- Kato, H.; Sato, A.; Fukuda, H.; Kagami, Y.; Udagawa, H.; Togo, A.; Ando, N.; Tanaka, O.; Shinoda, M.; Yamana, H.; et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn. J. Clin. Oncol. 2009, 39, 638–643. [Google Scholar] [CrossRef]
- Kato, K.; Muro, K.; Minashi, K.; Ohtsu, A.; Ishikura, S.; Boku, N.; Takiuchi, H.; Komatsu, Y.; Miyata, Y.; Fukuda, H.; et al. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 684–690. [Google Scholar] [CrossRef]
- Minsky, B.D.; Pajak, T.F.; Ginsberg, R.J.; Pisansky, T.M.; Martenson, J.; Komaki, R.; Okawara, G.; Rosenthal, S.A. and Kelsen, D.P. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J. Clin. Oncol. 2002, 20, 1167–1174. [Google Scholar] [CrossRef]
- Kato, K.; Eguchi Nakajima, T.; Ito, Y.; Katada, C.; Ishiyama, H.; Tokunaga, S.Y.; Tanaka, M.; Hironaka, S.; Hashimoto, T.; Ura, T.; et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn. J. Clin. Oncol. 2013, 43, 608–615. [Google Scholar] [CrossRef]
- Hatogai, K.; Yano, T.; Kojima, T.; Onozawa, M.; Fujii, S.; Daiko, H.; Yoda, Y.; Hombu, T.; Doi, T.; Kaneko, K.; et al. Local efficacy and survival outcome of salvage endoscopic therapy for local recurrent lesions after definitive chemoradiotherapy for esophageal cancer. Radiat. Oncol. 2016, 11, 31. [Google Scholar] [CrossRef]
- Chao, Y.K.; Chan, S.C.; Chang, H.K.; Liu, Y.H.; Wu, Y.C.; Hsieh, M.J.; Tseng, C.K.; Liu, H.P. Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur. J. Surg. Oncol. 2009, 35, 289–294. [Google Scholar] [CrossRef]
- Miyata, H.; Yamasaki, M.; Takiguchi, S.; Nakajima, K.; Fujiwara, Y.; Nishida, T.; Mori, M.; Doki, Y. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J. Surg. Oncol. 2009, 100, 442–446. [Google Scholar] [CrossRef]
- Tachimori, Y.; Kanamori, N.; Uemura, N.; Hokamura, N.; Igaki, H.; Kato, H. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2009, 137, 49–54. [Google Scholar] [CrossRef]
- Yano, T.; Hatogai, K.; Morimoto, H.; Yoda, Y.; Kaneko, K. Photodynamic therapy for esophageal cancer. Ann. Transl. Med. 2014, 2, 29. [Google Scholar] [PubMed]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Hatogai, K.; Yano, T.; Kojima, T.; Onozawa, M.; Daiko, H.; Nomura, S.; Yoda, Y.; Doi, T.; Kaneko, K.; Ohtsu, A. Salvage photodynamic therapy for local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Gastrointest. Endosc. 2016, 83, 1130–1139.e3. [Google Scholar] [CrossRef]
- Yano, T.; Muto, M.; Minashi, K.; Iwasaki, J.; Kojima, T.; Fuse, N.; Doi, T.; Kaneko, K.; Ohtsu, A. Photodynamic therapy as salvage treatment for local failure after chemoradiotherapy in patients with esophageal squamous cell carcinoma: A phase II study. Int. J. Cancer 2012, 131, 1228–1234. [Google Scholar] [CrossRef]
- Yano, T.; Muto, M.; Minashi, K.; Ohtsu, A.; Yoshida, S. Photodynamic therapy as salvage treatment for local failures after definitive chemoradiotherapy for esophageal cancer. Gastrointest. Endosc. 2005, 62, 31–36. [Google Scholar] [CrossRef]
- Yano, T.; Muto, M.; Minashi, K.; Onozawa, M.; Nihei, K.; Ishikura, S.; Kaneko, K.; Ohtsu, A. Long-term results of salvage photodynamic therapy for patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2011, 43, 657–663. [Google Scholar] [CrossRef]
- Kato, H.; Furukawa, K.; Sato, M.; Okunaka, T.; Kusunoki, Y.; Kawahara, M.; Fukuoka, M.; Miyazawa, T.; Yana, T.; Matsui, K.; et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003, 42, 103–111. [Google Scholar] [CrossRef]
- Yano, T.; Muto, M.; Yoshimura, K.; Niimi, M.; Ezoe, Y.; Yoda, Y.; Yamamoto, Y.; Nishisaki, H.; Higashino, K.; Iishi, H. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat. Oncol. 2012, 7, 113. [Google Scholar] [CrossRef]
- Yano, T.; Kasai, H.; Horimatsu, T.; Yoshimura, K.; Teramukai, S.; Morita, S.; Tada, H.; Yamamoto, Y.; Kataoka, H.; Kakushima, N.; et al. A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget 2017, 8, 22135–22144. [Google Scholar] [CrossRef]
- Yano, T.; Wang, K.K. Photodynamic therapy for gastrointestinal cancer. Photochem. Photobiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Minamide, T.; Yoda, Y.; Hori, K.; Shinmura, K.; Oono, Y.; Ikematsu, H.; Yano, T. Advantages of salvage photodynamic therapy using talaporfin sodium for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Surg. Endosc. 2020, 34, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Yano, T.; Kaneko, K.; Tsuruta, S.; Oono, Y.; Kojima, T.; Minashi, K.; Ikematsu, H.; Ohtsu, A. Endoscopic balloon dilatation for benign fibrotic strictures after curative nonsurgical treatment for esophageal cancer. Surg. Endosc. 2012, 26, 2877–2883. [Google Scholar] [CrossRef] [PubMed]
- Ezoe, Y.; Muto, M.; Horimatsu, T.; Morita, S.; Miyamoto, S.I.; Mochizuki, S.; Minashi, K.; Yano, T.; Ohtsu, A.; Chiba, T. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J. Clin. Gastroenterol. 2011, 45, 222–227. [Google Scholar] [CrossRef]
- Hanaoka, N.; Ishihara, R.; Takeuchi, Y.; Uedo, N.; Higashino, K.; Ohta, T.; Kanzaki, H.; Hanafusa, M.; Nagai, K.; Matsui, F.; et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: A controlled prospective study. Endoscopy 2012, 44, 1007–1011. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kobayashi, M.; Takeuchi, M.; Sato, Y.; Narisawa, R.; Aoyagi, Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest. Endosc. 2011, 74, 1389–1393. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Isomoto, H.; Nakayama, T.; Hayashi, T.; Nishiyama, H.; Ohnita, K.; Takeshima, F.; Shikuwa, S.; Kohno, S.; Nakao, K.; et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest. Endosc. 2011, 73, 1115–1121. [Google Scholar] [CrossRef]
- Aoyama, T.; Hara, K.; Kazama, K.; Atsumi, Y.; Tamagawa, H.; Tamagawa, A.; Machida, D.; Komori, K.; Maezawa, Y.; Kano, K.; et al. The Short- and Long-term Outcomes of Esophagectomy for Esophageal Cancer in Patients Older than 75 Years. Anticancer Res. 2020, 40, 1087–1093. [Google Scholar] [CrossRef]
- Bollschweiler, E.; Plum, P.; Monig, S.P.; Holscher, A.H. Current and future treatment options for esophageal cancer in the elderly. Expert Opin. Pharmacother. 2017, 18, 1001–1010. [Google Scholar] [CrossRef]
- Kumar, M.U.; Swamy, K.; Supe, S.S.; Anantha, N. Influence of intraluminal brachytherapy dose on complications in the treatment of esophageal cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 1069–1072. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Jiang, S.; Shang, Y.; Wu, Y. Evaluating the optimal re-irradiation dose for locally recurrent esophageal squamous cell carcinoma after definitive radiotherapy. Radiat. Oncol. 2019, 14, 191. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients | n = 12 | |
|---|---|---|
| Sex (male:female), n (%) | 7:5 (58.3:41.7) | |
| Age (mean ± SD, range, year) at PDT | 74.7 ± 8.3 (63–87) | |
| Tumor location | upper, n (%) | 4 (33.3) |
| middle, n (%) | 6 (50.0) | |
| lower, n (%) | 2 (16.7) | |
| Histological type | SCC | |
| cStage at prior treatment | cStage I, n (%) | 8 (66.7) |
| cStage II, n (%) | 2 (16.7) | |
| cStage III, n (%) | 1 (8.3) | |
| cStageIVA, n (%) | 1 (8.3) | |
| Prior treatment (CRT: RT), n (%) | 8:4 (66.7:33.3) | |
| Regimen of chemotherapy | CDDP + 5-FU | 7 |
| CDGP + 5-FU | 1 | |
| Total dose of radiotherapy (median, range, Gy) | 60 (50.4–70) | |
| n = 12 | ||
|---|---|---|
| Tumor status | recurrent tumor | 10 |
| residual tumor | 2 | |
| Number of lesions | single | 10 |
| multiple | 2 | |
| Invasion depth at PDT | T1a | 3 |
| T1b | 9 | |
| Circumference of lesion at PDT | ≤1/4 | 6 |
| >1/4, ≤1/2 | 4 | |
| >1/2, ≤3/4 | 1 | |
| >3/4, ≤1 | 1 | |
| Longitudinal lesion length (mean ± SD, range, cm) | 1.67 ± 0.86 (1–4) | |
| Interval between CRT/RT and PDT (median, range, months) | 9 (3–192) | |
| n = 12 | ||
|---|---|---|
| Total dose of irradiation (median, range, J) | 400 (200–800) | |
| Total number of PDT (median, range, times) | 1 (1–3) | |
| Hospital stay (median, range, days) | 19 (17–28) | |
| Local efficacy | L-CR, n (%) | 10 (83.3) |
| L-nonCR, n (%) | 2 (16.7) | |
| Adverse Event | n = 12 | Treatment |
|---|---|---|
| Skin phototoxicity, n (%) | 0 (0.0) | |
| Esophageal stricture, n (%) | 5 (41.7) | All patients needed EBD |
| Esophagobronchial fistula, n (%) | 1 (8.3) | PEG 7 months after PDT |
| Stricture n = 5 | Non-Stricture n = 7 | p-Value | |
|---|---|---|---|
| Age (mean ± SD, range, years) | 73.8 ± 6.22 | 75.3 ± 9.98 | 0.78 |
| Sex (male:female) | 3:2 | 5:2 | 0.56 |
| Invasion depth at prior treatment (T1:T2, 3, 4) | 3:2 | 6:1 | 0.52 |
| Prior treatment (CRT:RT) | 5:0 | 4:3 | 0.08 |
| Radiation dose (mean ± SD, range, Gy) | 62.0 ± 4.47 (60–70) | 61.5 ± 6.79 (50.4–70) | 0.89 |
| Invasion depth at PDT (T1:T2, 3, 4) | 5:0 | 7:0 | |
| Circumference of lesion (mean ± SD, range) | 0.42 ± 0.21 (1/4–1) | 0.35 ± 0.11 (1/4–1/2) | 0.46 |
| Longitudinal lesion length (mean ± SD, range, cm) | 2.00 ± 1.22 (1–4) | 1.43 ± 0.45 (1–2) | 0.28 |
| Total irradiation dose per round (mean ± SD, range, J) | 475 ± 196 (227–700) | 457 ± 181 (200–800) | 0.87 |
| Black attachment use, n (%) | 1 (20.0) | 4 (57.1) | 0.22 |
| Alb at PDT (mean ± SD, range, g/dL) | 4.16 ± 0.05 (4.1–4.2) | 3.74 ± 0.60 (2.8–4.4) | 0.16 |
| Hb at PDT (mean ± SD, range, g/dL) | 11.5 ± 1.02 (10.1–12.6) | 11.9 ± 1.81 (9.6–14.4) | 0.64 |
| CRP at PDT (mean ± SD, range, mg/dL) | 0.07 ± 0.03 (0.03–0.12) | 1.33 ± 2.35 (0.03–6.57) | 0.27 |
| Circumferential ulcer 1 week after PDT (mean ± SD, range) | 1.00 ± 0 (1) | 0.75 ± 0.29 (1/4–3/4) | 0.06 |
| Patient | Age, Year | Sex | PDT | Tumor Location | Circumference of Lesion at PDT | Tumor Depth | Total Dose of Irradiation, J | Outcomes | Adverse Effects |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | 1st | Upper | 1 | SM | 400 | --- | Stricture |
| 2nd | Upper | 1/3 | M | 300 | --- | Stricture | |||
| 3rd | Upper | ≤1/4 | M | 400 | L-CR | Stricture | |||
| 2 | 65 | F | 1st | Lower | 2/3 | SM | 500 | --- | Non |
| 2nd | Lower | 1/3 | SM | 700 | L-CR | Non |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, N.; Osawa, S.; Miyazu, T.; Kaneko, M.; Tamura, S.; Tani, S.; Yamade, M.; Iwaizumi, M.; Hamaya, Y.; Furuta, T.; et al. Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience. J. Clin. Med. 2020, 9, 1509. https://doi.org/10.3390/jcm9051509
Ishida N, Osawa S, Miyazu T, Kaneko M, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Furuta T, et al. Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience. Journal of Clinical Medicine. 2020; 9(5):1509. https://doi.org/10.3390/jcm9051509
Chicago/Turabian StyleIshida, Natsuki, Satoshi Osawa, Takahiro Miyazu, Masanao Kaneko, Satoshi Tamura, Shinya Tani, Mihoko Yamade, Moriya Iwaizumi, Yasushi Hamaya, Takahisa Furuta, and et al. 2020. "Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience" Journal of Clinical Medicine 9, no. 5: 1509. https://doi.org/10.3390/jcm9051509
APA StyleIshida, N., Osawa, S., Miyazu, T., Kaneko, M., Tamura, S., Tani, S., Yamade, M., Iwaizumi, M., Hamaya, Y., Furuta, T., & Sugimoto, K. (2020). Photodynamic Therapy Using Talaporfin Sodium for Local Failure after Chemoradiotherapy or Radiotherapy for Esophageal Cancer: A Single Center Experience. Journal of Clinical Medicine, 9(5), 1509. https://doi.org/10.3390/jcm9051509





