Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells
Abstract
1. Background
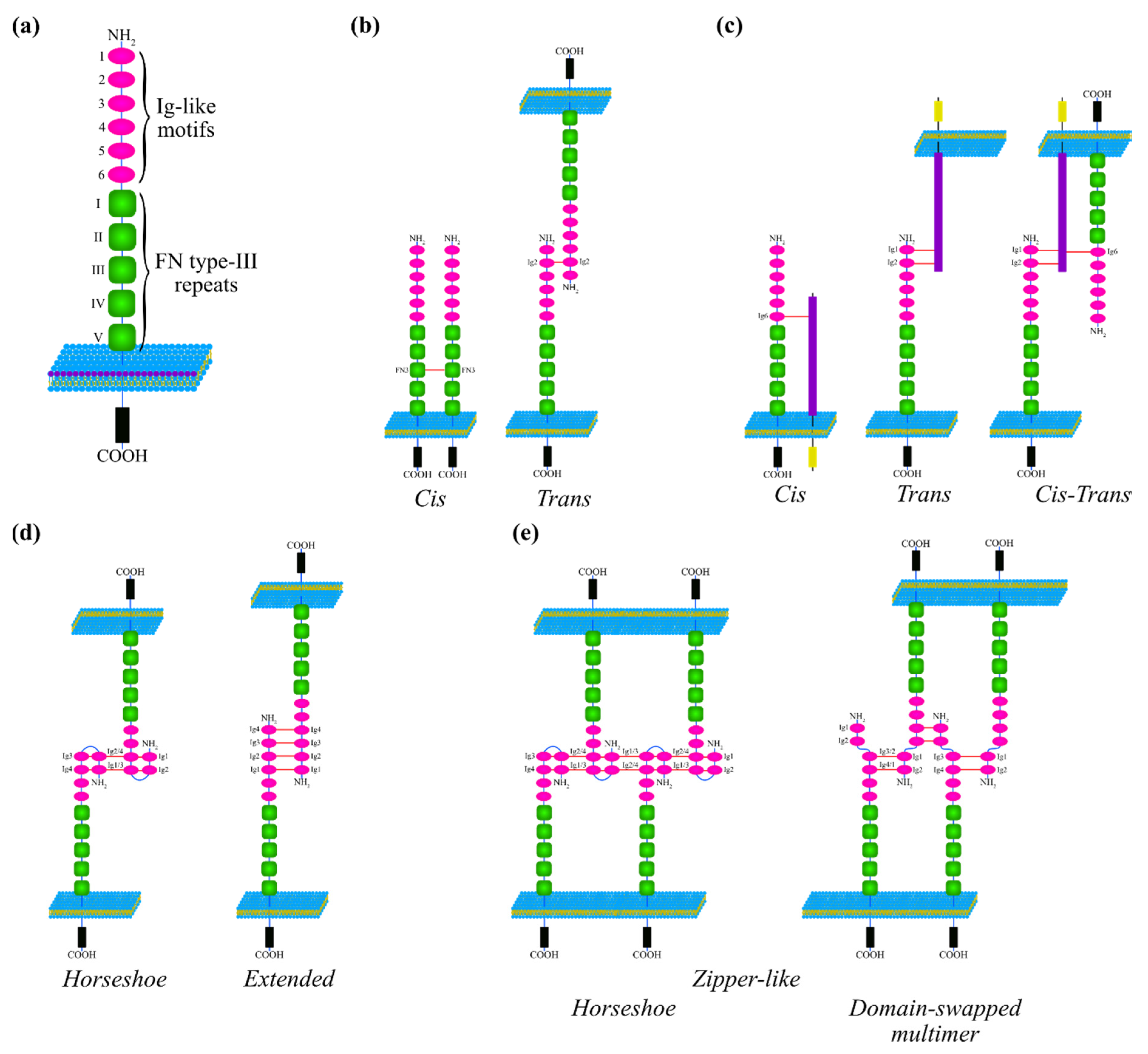
2. Molecular Characteristics of L1CAM
2.1. Structural Determinants of L1CAM Interactions
2.2. L1CAM Interactions
2.2.1. L1CAM Interacting Partners and Functional Implications
2.2.2. The Regulation of L1CAM Interactions via Phosphorylation of the Cytoplasmic Tail
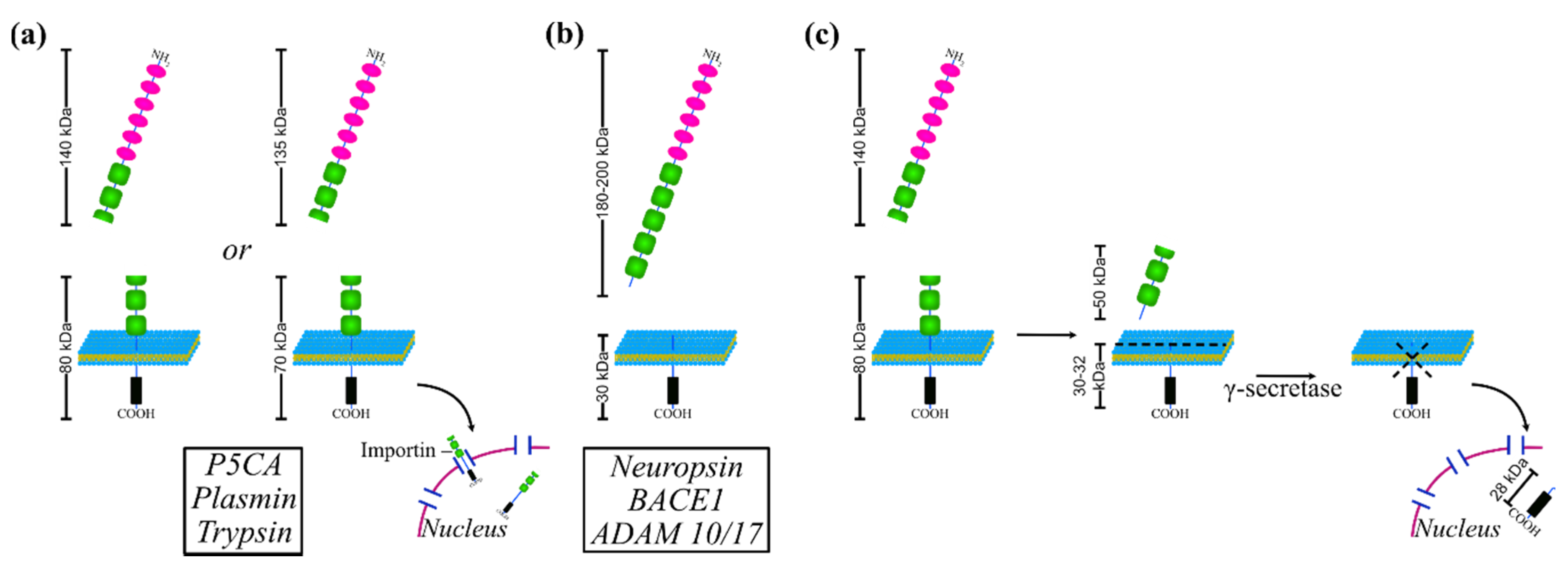
2.3. Proteolytic Processing of L1CAM
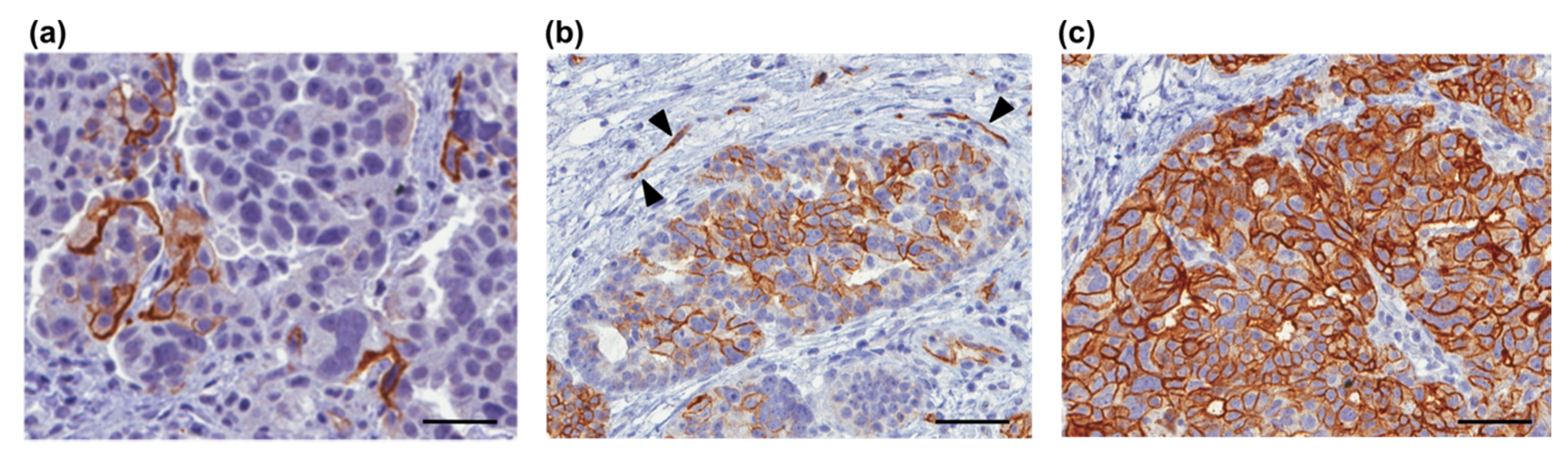
3. Clinical Relevance of L1CAM in Cancer Diagnosis and Prognosis
4. L1CAM Mechanism of Action in Stemness and in Stem-Related Processes
4.1. L1CAM Function in CSC and Its Contribution to Cancer Stemness-Associated Processes
4.1.1. Glioblastoma
4.1.2. Colorectal Cancer
4.1.3. Pancreatic Cancer
4.1.4. Gynecological Cancers
4.1.5. Retinoblastoma
4.1.6. L1CAM Impact on Stemness-Related Features of Tumor Microenvironment
4.1.7. L1CAM Expression Obtained by Omics Data Unveiled its Involvement in CSC Processes
4.2. Beyond Cancer: L1CAM in Normal Stem Cells
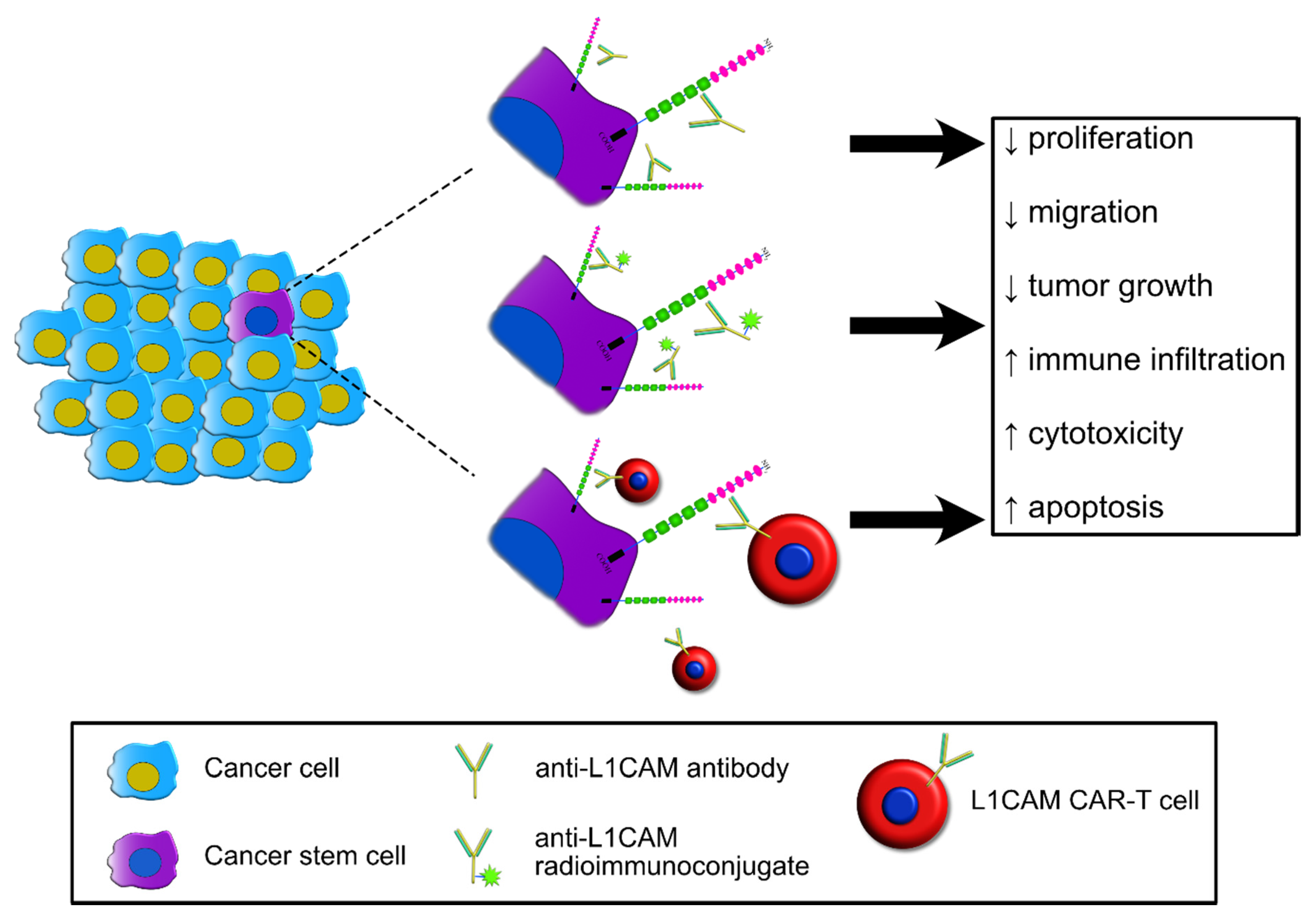
5. L1CAM as a Therapeutic Target
5.1. Monoclonal Antibodies
5.2. Radioimmunoconjugates
5.3. CAR-T Cells
6. Potential Implications and Clinical Perspectives of L1CAM in Cancer and CSC
Funding
Acknowledgments
Conflicts of Interest
References
- Rathjen, F.G.; Schachner, M. Immunocytological and biochemical characterization of a new neuronal cell surface component (L1 antigen) which is involved in cell adhesion. EMBO J. 1984, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brümmendorf, T.; Kenwrick, S.; Rathjen, F.G. Neural cell recognition molecule L1: From cell biology to human hereditary brain malformations. Curr. Opin. Neurobiol. 1998, 8, 87–97. [Google Scholar] [CrossRef]
- Patzke, C.; Acuna, C.; Giam, L.R.; Wernig, M.; Südhof, T.C. Conditional deletion of L1CAM in human neurons impairs both axonal and dendritic arborization and action potential generation. J. Exp. Med. 2016, 213, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.S.; Maness, P.F. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr. Opin. Neurobiol. 2008, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Fransen, E.; D’Hooge, R.; Van Camp, G.; Verhoye, M.; Sijbers, J.; Reyniers, E.; Soriano, P.; Kamiguchi, H.; Willemsen, R.; Koekkoek, S.K.E.; et al. L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum. Mol. Genet. 1998, 7, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Dahme, M.; Bartsch, U.; Martini, R.; Anliker, B.; Schachner, M.; Mantei, N. Disruption of the mouse L1 gene leads to malformations of the nervous system. Nat. Genet. 1997, 17, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Fransen, E.; Van Camp, G.; Vits, L.; Willems, P.J. L1-associated diseases: Clinical geneticists divide, molecular geneticists unite. Hum. Mol. Genet. 1997, 6, 1625–1632. [Google Scholar] [CrossRef]
- Maness, P.F.; Schachner, M. Neural recognition molecules of the immunoglobulin superfamily: Signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 2007, 10, 19–26. [Google Scholar] [CrossRef]
- Moos, M.; Tacke, R.; Scherer, H.; Teplow, D.; Früh, K.; Schachner, M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature 1988, 334, 701–703. [Google Scholar] [CrossRef]
- Castellani, V.; De Angelis, E.; Kenwrick, S.; Rougon, G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 2002, 21, 6348–6357. [Google Scholar] [CrossRef]
- Haspel, J.; Friedlander, D.R.; Ivgy-May, N.; Chickramane, S.; Roonprapunt, C.; Chen, S.; Schachner, M.; Grumet, M. Fast track—Critical and optimal ig domains for promotion of neurite outgrowth by L1/Ng-CAM. J. Neurobiol. 2000, 42, 287–302. [Google Scholar] [CrossRef]
- De Angelis, E. Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum. Mol. Genet. 2002, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Gomes, C.M.; Sousa, M.; Alves, P.M.; Costa, J. Kinetic analysis of L1 homophilic interaction: Role of the first four immunoglobulin domains and implications on binding mechanism. J. Biol. Chem. 2008, 283, 28038–28047. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jensen, G.J.; Bjorkman, P.J. Cryo-Electron Tomography of Homophilic Adhesion Mediated by the Neural Cell Adhesion Molecule L1. Structure 2009, 17, 460–471. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, X.; Siu, C.H. Colocalization of the homophilic binding site and the neuritogenic activity of the cell adhesion molecule L1 to its second Ig-like domain. J. Biol. Chem. 1995, 270, 29413–29421. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; MacFarlane, J.; Du, J.S.; Yeo, G.; Hicks, R.; Rathjen, F.G.; Kenwrick, S.; Brümmendorf, T. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 1999, 18, 4744–4753. [Google Scholar] [CrossRef]
- Silletti, S.; Mei, F.; Sheppard, D.; Montgomery, A.M.P. Plasmin-sensitive dibasic sequences in the third fibronectin-like domain of L1-cell adhesion molecule (CAM) facilitate homomultimerization and concomitant integrin recruitment. J. Cell Biol. 2000, 149, 1485–1501. [Google Scholar] [CrossRef]
- Heller, M.; Von der Ohe, M.; Kleene, R.; Mohajeri, M.H.; Schachner, M. The immunoglobulin-superfamily molecule basigin is a binding protein for oligomannosidic carbohydrates: An anti-idiotypic approach. J. Neurochem. 2003, 84, 557–565. [Google Scholar] [CrossRef]
- Kadmon, G.; Kowitz, A.; Altevogt, P.; Schachner, M. The neural cell adhesion molecule N-CAM enhances L1-dependent cell-cell interactions. J. Cell Biol. 1990, 110, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Oleszewski, M.; Beer, S.; Katich, S.; Geiger, C.; Zeller, Y.; Rauch, U.; Altevogt, P. Integrin and neurocan binding to L1 involves distinct Ig domains. J. Biol. Chem. 1999, 274, 24602–24610. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.M.P.; Becker, J.C.; Siu, C.H.; Lemmon, V.P.; Cheresh, D.A.; Pancook, J.D.; Zhao, X.; Reisfeld, R.A. Human neural cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J. Cell Biol. 1996, 132, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Felding-Habermann, B.; Silletti, S.; Mei, F.; Siu, C.H.; Yip, P.M.; Brooks, P.C.; Cheresh, D.A.; O’Toole, T.E.; Ginsberg, M.H.; Montgomery, A.M.P. A single immunoglobulin-like domain of the human neural cell adhesion molecule L1 supports adhesion by multiple vascular and platelet integrins. J. Cell Biol. 1997, 139, 1567–1581. [Google Scholar] [CrossRef]
- Hall, H.; Carbonetto, S.; Schachner, M. L1/HNK-1 Carbohydrate- and β1 Integrin-Dependent Neural Cell Adhesion to Laminin-1. J. Neurochem. 2002, 68, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Stoeck, A.; Schlich, S.; Issa, Y.; Gschwend, V.; Wenger, T.; Herr, I.; Marmé, A.; Bourbie, S.; Altevogt, P.; Gutwein, P. L1 on ovarian carcinoma cells is a binding partner for Neuropilin-1 on mesothelial cells. Cancer Lett. 2006, 239, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.Q.; Bennett, V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J. Biol. Chem. 1994, 269, 27163–27166. [Google Scholar] [PubMed]
- Hortsch, M.; Nagaraj, K.; Godenschwege, T.A. The interaction between L1-type proteins and ankyrins—A master switch for L1-type cam function. Cell. Mol. Biol. Lett. 2009, 14, 57–69. [Google Scholar] [CrossRef]
- Kulahin, N.; Li, S.; Hinsby, A.; Kiselyov, V.; Berezin, V.; Bock, E. Fibronectin type III (FN3) modules of the neuronal cell adhesion molecule L1 interact directly with the fibroblast growth factor (FGF) receptor. Mol. Cell. Neurosci. 2008, 37, 528–536. [Google Scholar] [CrossRef]
- Nagaraj, K.; Kristiansen, L.V.; Skrzynski, A.; Castiella, C.; Garcia-Alonso, L.; Hortsch, M. Pathogenic human L1-CAM mutations reduce the adhesion-dependent activation of EGFR. Hum. Mol. Genet. 2009, 18, 3822–3831. [Google Scholar] [CrossRef]
- Horstkorte, R.; Schachner, M.; Magyar, J.P.; Vorherr, T.; Schmitz, B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J. Cell Biol. 1993, 121, 1409–1422. [Google Scholar] [CrossRef]
- Oleszewski, M.; Gutwein, P.; Von Der Lieth, W.; Rauch, U.; Altevogt, P. Characterization of the L1-neurocan-binding site. Implications for L1-L1 homophilic binding. J. Biol. Chem. 2000, 275, 34478–34485. [Google Scholar] [CrossRef]
- Blaess, S.; Kammerer, R.A.; Hall, H. Structural analysis of the sixth immunoglobulin-like domain of mouse neural cell adhesion molecule L1 and its interactions with alpha(v)beta3, alpha(IIb)beta3, and alpha5beta1 integrins. J. Neurochem. 1998, 71, 2615–2625. [Google Scholar] [CrossRef] [PubMed]
- Buhusi, M.; Schlatter, M.C.; Demyanenko, G.P.; Thresher, R.; Maness, P.F. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J. Neurosci. 2008, 28, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.J.; Furness, J.; Walsh, F.S.; Doherty, P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 1994, 13, 583–594. [Google Scholar] [CrossRef]
- Kulahin, N.; Li, S.; Kiselyov, V.; Bock, E.; Berezin, V. Identification of neural cell adhesion molecule L1-derived neuritogenic ligands of the fibroblast growth factor receptor. J. Neurosci. Res. 2009, 87, 1806–1812. [Google Scholar] [CrossRef]
- Donier, E.; Gomez-Sanchez, J.A.; Grijota-Martinez, C.; Lakomá, J.; Baars, S.; Garcia-Alonso, L.; Cabedo, H. L1CAM binds ErbB receptors through Ig-like domains coupling cell adhesion and neuregulin signalling. PLoS ONE 2012, 7, e40674. [Google Scholar] [CrossRef]
- Islam, R.; Kristiansen, L.V.; Romani, S.; Garcia-Alonso, L.; Hortsch, M. Activation of EGF Receptor Kinase by L1-mediated Homophilic Cell Interactions. Mol. Biol. Cell 2004, 15, 2003–2012. [Google Scholar] [CrossRef]
- Schaefer, A.W.; Kamiguchi, H.; Wong, E.V.; Beach, C.M.; Landreth, G.; Lemmon, V. Activation of the MAPK signal cascade by the neural cell adhesion molecule L1 requires L1 internalization. J. Biol. Chem. 1999, 274, 37965–37973. [Google Scholar] [CrossRef]
- Schaefer, A.W.; Kamei, Y.; Kamiguchi, H.; Wong, E.V.; Rapoport, I.; Kirchhausen, T.; Beach, C.M.; Landreth, G.; Lemmon, S.K.; Lemmon, V. L1 endocytosis is controlled by a phosphorylation-dephosphorylation cycle stimulated by outside-in signaling by L1. J. Cell Biol. 2002, 157, 1223–1232. [Google Scholar] [CrossRef]
- Kamiguchi, H.; Long, K.E.; Pendergast, M.; Schaefer, A.W.; Rapoport, I.; Kirchhausen, T.; Lemmon, V. The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway. J. Neurosci. 1998, 18, 5311–5321. [Google Scholar] [CrossRef]
- Chen, M.M.; Lee, C.Y.; Leland, H.A.; Lin, G.Y.; Montgomery, A.M.; Silletti, S. Inside-out regulation of L1 conformation, integrin binding, proteolysis, and concomitant cell migration. Mol. Biol. Cell 2010, 21, 1671–1685. [Google Scholar] [CrossRef]
- Chen, M.M.; Leland, H.A.; Lee, C.Y.; Silletti, S. Tyrosine and serine phosphorylation regulate the conformation and subsequent threonine phosphorylation of the L1 cytoplasmic domain. Biochem. Biophys. Res. Commun. 2009, 389, 257–264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiefel, H.; Bondong, S.; Hazin, J.; Ridinger, J.; Schirmer, U.; Riedle, S.; Altevogt, P. L1CAM: A major driver for tumor cell invasion and motility. Cell Adhes. Migr. 2012, 6, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Kalus, I.; Schnegelsberg, B.; Seidah, N.G.; Kleene, R.; Schachner, M. The proprotein convertase PC5A and a metalloprotease are involved in the proteolytic processing of the neural adhesion molecule L1. J. Biol. Chem. 2003, 278, 10381–10388. [Google Scholar] [CrossRef]
- Nayeem, N.; Silletti, S.; Yang, X.M.; Lemmon, V.P.; Reisfeld, R.A.; Stallcup, W.B.; Montgomery, A.M.P. A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J. Cell Sci. 1999, 112, 4739–4749. [Google Scholar] [PubMed]
- Sadoul, K.; Sadoul, R.; Faissner, A.; Schachner, M. Biochemical Characterization of Different Molecular Forms of the Neural Cell Adhesion Molecule L1. J. Neurochem. 1988, 50, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.; Wolters-Eisfeld, G.; Joshi, G.; Djogo, N.; Jakovcevski, I.; Schachner, M.; Kleene, R. Generation and nuclear translocation of sumoylated transmembrane fragment of cell adhesion molecule L1. J. Biol. Chem. 2012, 287, 17161–17175. [Google Scholar] [CrossRef]
- Matsumoto-Miyai, K.; Ninomiya, A.; Yamasaki, H.; Tamura, H.; Nakamura, Y.; Shiosaka, S. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J. Neurosci. 2003, 23, 7727–7736. [Google Scholar] [CrossRef]
- Zou, Y.; Uddin, M.M.; Padmanabhan, S.; Zhu, Y.; Bu, P.; Vancura, A.; Vancurova, I. The proto-oncogene Bcl3 induces immune checkpoint PD-L1 expression, mediating proliferation of ovarian cancer cells. J. Biol. Chem. 2018, 293, 15483–15496. [Google Scholar] [CrossRef]
- Maretzky, T.; Schulte, M.; Ludwig, A.; Rose-John, S.; Blobel, C.; Hartmann, D.; Altevogt, P.; Saftig, P.; Reiss, K. L1 Is Sequentially Processed by Two Differently Activated Metalloproteases and Presenilin/ -Secretase and Regulates Neural Cell Adhesion, Cell Migration, and Neurite Outgrowth. Mol. Cell. Biol. 2005, 25, 9040–9053. [Google Scholar] [CrossRef]
- Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Gast, D.; Joumaa, S.; Zentgraf, H.; Fogel, M.; Altevogt, D.P. ADAM10-mediated cleavage of L1 adhesion molecule at the cell surface and in released membrane vesicles. FASEB J. 2003, 17, 292–294. [Google Scholar] [CrossRef]
- Riedle, S.; Kiefel, H.; Gast, D.; Bondong, S.; Wolterink, S.; Gutwein, P.; Altevogt, P. Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/γ-secretase activity. Biochem. J. 2009, 420, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Angiolini, F.; Belloni, E.; Giordano, M.; Campioni, M.; Forneris, F.; Paronetto, M.P.; Lupia, M.; Brandas, C.; Pradella, D.; Di Matteo, A.; et al. A novel L1CAM isoform with angiogenic activity generated by NOVA2-mediated alternative splicing. Elife 2019, 8, e44305. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, P.; Doberstein, K.; Fogel, M. L1CAM in human cancer. Int. J. Cancer 2016, 138, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Ben-Shmuel, A.; Raveh, S.; Ben-Ze’ev, A. L1-CAM in cancerous tissues. Expert Opin. Biol. Ther. 2008, 8, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Nout, R.A.; Stelloo, E.; Dreef, E.; Nijman, H.W.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Creutzberg, C.L.; Smit, V.T.H.B.M. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: Pooled PORTEC trial results. Eur. J. Cancer 2014, 50, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Smogeli, E.; Davidson, B.; Cvancarova, M.; Holth, A.; Katz, B.; Risberg, B.; Kristensen, G.; Lindemann, K. L1CAM as a prognostic marker in stage I endometrial cancer: A validation study. BMC Cancer 2016, 16, 596. [Google Scholar] [CrossRef]
- Tangen, I.L.; Kopperud, R.K.; Visser, N.C.M.; Staff, A.C.; Tingulstad, S.; Marcickiewicz, J.; Amant, F.; Bjørge, L.; Pijnenborg, J.M.A.; Salvesen, H.B.; et al. Expression of L1CAM in curettage or high L1CAM level in preoperative blood samples predicts lymph node metastases and poor outcome in endometrial cancer patients. Br. J. Cancer 2017, 117, 840–847. [Google Scholar] [CrossRef]
- Corrado, G.; Laquintana, V.; Loria, R.; Carosi, M.; De Salvo, L.; Sperduti, I.; Zampa, A.; Cicchillitti, L.; Piaggio, G.; Cutillo, G.; et al. Endometrial cancer prognosis correlates with the expression of L1CAM and miR34a biomarkers. J. Exp. Clin. Cancer Res. 2018, 37, 139. [Google Scholar] [CrossRef] [PubMed]
- Van Der Putten, L.J.M.; Visser, N.C.M.; Van De Vijver, K.; Santacana, M.; Bronsert, P.; Bulten, J.; Hirschfeld, M.; Colas, E.; Gil-Moreno, A.; Garcia, A.; et al. L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br. J. Cancer 2016, 115, 716–724. [Google Scholar] [CrossRef]
- Vizza, E.; Mancini, E.; Laquintana, V.; Loria, R.; Carosi, M.; Baiocco, E.; Cicchillitti, L.; Piaggio, G.; Patrizi, L.; Sperduti, I.; et al. The prognostic significance of positive peritoneal cytology in endometrial cancer and its correlations with L1-CAM biomarker. Surg. Oncol. 2019, 28, 151–157. [Google Scholar] [CrossRef]
- Fogel, M.; Gutwein, P.; Mechtersheimer, S.; Riedle, S.; Stoeck, A.; Smirnov, A.; Edler, L.; Ben-Arie, A.; Huszar, M.; Altevogt, P. L1 expression as a predictor of progression and survival in patients with uterine and ovarian carcinomas. Lancet 2003, 362, 869–875. [Google Scholar] [CrossRef]
- Zecchini, S.; Bianchi, M.; Colombo, N.; Fasani, R.; Goisis, G.; Casadio, C.; Viale, G.; Liu, J.; Herlyn, M.; Godwin, A.K.; et al. The differential role of L1 in ovarian carcinoma and normal ovarian surface epithelium. Cancer Res. 2008, 68, 1110–1118. [Google Scholar] [CrossRef]
- Soovares, P.; Pasanen, A.; Bützow, R.; Lassus, H. L1CAM expression associates with poor outcome in endometrioid, but not in clear cell ovarian carcinoma. Gynecol. Oncol. 2017, 146, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bondong, S.; Kiefel, H.; Hielscher, T.; Zeimet, A.G.; Zeillinger, R.; Pils, D.; Schuster, E.; Castillo-Tong, D.C.; Cadron, I.; Vergote, I.; et al. Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-κB activation. Ann. Oncol. 2012, 23, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Thies, A.; Schachner, M.; Moll, I.; Berger, J.; Schulze, H.J.; Brunner, G.; Schumacher, U. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur. J. Cancer 2002, 38, 1708–1716. [Google Scholar] [CrossRef]
- Wu, J.D.; Hong, C.Q.; Huang, W.H.; Wei, X.L.; Zhang, F.; Zhuang, Y.X.; Zhang, Y.Q.; Zhang, G.J. L1 Cell Adhesion Molecule and Its Soluble Form sL1 Exhibit Poor Prognosis in Primary Breast Cancer Patients. Clin. Breast Cancer 2018, 18, e851–e861. [Google Scholar] [CrossRef]
- Ichikawa, T.; Okugawa, Y.; Toiyama, Y.; Tanaka, K.; Yin, C.; Kitajima, T.; Kondo, S.; Shimura, T.; Ohi, M.; Araki, T.; et al. Clinical significance and biological role of L1 cell adhesion molecule in gastric cancer. Br. J. Cancer 2019, 121, 1058–1068. [Google Scholar] [CrossRef]
- Boo, Y.J.; Park, J.M.; Kim, J.; Chae, Y.S.; Min, B.W.; Um, J.W.; Moon, H.Y. L1 expression as a marker for poor prognosis, tumor progression, and short survival in patients with colorectal cancer. Ann. Surg. Oncol. 2007, 14, 1703–1711. [Google Scholar] [CrossRef]
- Ben, Q.W.; Wang, J.C.; Liu, J.; Zhu, Y.; Yuan, F.; Yao, W.Y.; Yuan, Y.Z. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2010, 17, 2213–2221. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, P.; Li, D.; Li, W. L1CAM-positive expression is associated with poorer survival outcomes in resected non-small cell lung cancer patients. Int. J. Clin. Exp. Pathol. 2019, 12, 2665–2671. [Google Scholar]
- Doberstein, K.; Wieland, A.; Lee, S.B.B.; Blaheta, R.A.A.; Wedel, S.; Moch, H.; Schraml, P.; Pfeilschifter, J.; Kristiansen, G.; Gutwein, P. L1-CAM expression in ccRCC correlates with shorter patients survival times and confers chemoresistance in renal cell carcinoma cells. Carcinogenesis 2011, 32, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Pasanen, A.; Loukovaara, M.; Tuomi, T.; Bützow, R. Preoperative risk stratification of endometrial carcinoma: L1CAM as a biomarker. Int. J. Gynecol. Cancer 2017, 27, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Kasimir-Bauer, S.; Wimberger, P.; Kimmig, R.; Heubner, M. Utility of mesothelin, L1CAM and afamin as biomarkers in primary ovarian cancer. Anticancer Res. 2013, 33, 329–336. [Google Scholar] [PubMed]
- Bajaj, J.; Diaz, E.; Reya, T. Stem cells in cancer initiation and progression. J. Cell Biol. 2020, 219, e201911053. [Google Scholar] [CrossRef] [PubMed]
- Turdo, A.; Veschi, V.; Gaggianesi, M.; Chinnici, A.; Bianca, P.; Todaro, M.; Stassi, G. Meeting the challenge of targeting cancer stem cells. Front. Cell Dev. Biol. 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Sainz, B. Pancreatic cancer stem cells: A state or an entity? Semin. Cancer Biol. 2018, 53, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Rowan, K. Are cancer stem cells real? After four decades, debate still simmers. J. Natl. Cancer Inst. 2009, 101, 546–547. [Google Scholar] [CrossRef][Green Version]
- Jordan, C.T. Cancer Stem Cells: Controversial or Just Misunderstood? Cell Stem Cell 2009, 4, 203–205. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Weinberg, R.A. Tackling the cancer stem cells-what challenges do they pose? Nat. Rev. Drug Discov. 2014, 13, 497–512. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Bujko, K.; Mack, A.; Kucia, M.; Ratajczak, J. Cancer from the perspective of stem cells and misappropriated tissue regeneration mechanisms. Leukemia 2018, 32, 2519–2526. [Google Scholar] [CrossRef]
- Nimmakayala, R.K.; Batra, S.K.; Ponnusamy, M.P. Unraveling the journey of cancer stem cells from origin to metastasis. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 50–63. [Google Scholar] [CrossRef]
- Lupia, M.; Cavallaro, U. Ovarian cancer stem cells: Still an elusive entity? Mol. Cancer 2017, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; Li, Z.; Sathornsumetee, S.; Wang, H.; McLendon, R.E.; Hjelmeland, A.B.; Rich, J.N. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008, 68, 6043–6048. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wu, Q.; Huang, Z.; Guryanova, O.A.; Huang, Q.; Shou, W.; Rich, J.N.; Bao, S. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J. 2011, 30, 800–813. [Google Scholar] [CrossRef]
- Maréchal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Mills, B.N.; Albert, G.P.; Halterman, M.W. Expression Profiling of the MAP Kinase Phosphatase Family Reveals a Role for DUSP1 in the Glioblastoma Stem Cell Niche. Cancer Microenviron. 2017, 10, 57–68. [Google Scholar] [CrossRef]
- Erhart, F.; Blauensteiner, B.; Zirkovits, G.; Printz, D.; Soukup, K.; Klingenbrunner, S.; Fischhuber, K.; Reitermaier, R.; Halfmann, A.; Lötsch, D.; et al. Gliomasphere marker combinatorics: Multidimensional flow cytometry detects CD44+/CD133+/ITGA6+/CD36+ signature. J. Cell. Mol. Med. 2019, 23, 281–292. [Google Scholar] [CrossRef]
- Brescia, P.; Richichi, C.; Pelicci, G. Current strategies for identification of glioma stem cells: Adequate or unsatisfactory? J. Oncol. 2012, 2012, 376894. [Google Scholar] [CrossRef]
- Held-Feindt, J.; Schmelz, S.; Hattermann, K.; Mentlein, R.; Mehdorn, H.M.; Sebens, S. The neural adhesion molecule L1CAM confers chemoresistance in human glioblastomas. Neurochem. Int. 2012, 61, 1183–1191. [Google Scholar] [CrossRef]
- Geismann, C.; Morscheck, M.; Koch, D.; Bergmann, F.; Ungefroren, H.; Arlt, A.; Tsao, M.S.; Bachem, M.G.; Altevogt, P.; Sipos, B.; et al. Up-regulation of L1CAM in pancreatic duct cells is transforming growth factor β1- and slug-dependent: Role in malignant transformation of pancreatic cancer. Cancer Res. 2009, 69, 4517–4526. [Google Scholar] [CrossRef] [PubMed]
- Geismann, C.; Arlt, A.; Bauer, I.; Pfeifer, M.; Schirmer, U.; Altevogt, P.; Müerköster, S.S.; Schäfer, H. Binding of the transcription factor Slug to the L1CAM promoter is essential for transforming growth factor-β1 (TGF-β)-induced L1CAM expression in human pancreatic ductal adenocarcinoma cells. Int. J. Oncol. 2011, 38, 257–266. [Google Scholar] [PubMed]
- Gavert, N.; Conacci-Sorrell, M.; Gast, D.; Schneider, A.; Altevogt, P.; Brabletz, T.; Ben-Ze’Ev, A. L1, a novel target of β-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J. Cell Biol. 2005, 168, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Conacci-Sorrell, M.E.; Ben-Yedidia, T.; Shtutman, M.; Feinstein, E.; Einat, P.; Ben-Ze’ev, A. Nr-CAM is a target gene of the β-catenin/LEF-1 pathway in melanoma and colon cancer and its expression enhances motility and confers tumorigenesis. Genes Dev. 2002, 16, 2058–2072. [Google Scholar] [CrossRef]
- Gavert, N.; Vivanti, A.; Hazin, J.; Brabletz, T.; Ben-Ze’ev, A. L1-Mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol. Cancer Res. 2011, 9, 14–24. [Google Scholar] [CrossRef]
- Shapiro, B.; Tocci, P.; Haase, G.; Gavert, N.; Ben-Ze’ev, A. Clusterin, a gene enriched in intestinal stem cells, is required for L1-mediated colon cancer metastasis. Oncotarget 2015, 6, 34389–34401. [Google Scholar] [CrossRef]
- Van der Flier, L.G.; van Gijn, M.E.; Hatzis, P.; Kujala, P.; Haegebarth, A.; Stange, D.E.; Begthel, H.; van den Born, M.; Guryev, V.; Oving, I.; et al. Transcription Factor Achaete Scute-Like 2 Controls Intestinal Stem Cell Fate. Cell 2009, 136, 903–912. [Google Scholar] [CrossRef]
- Schuijers, J.; Junker, J.P.; Mokry, M.; Hatzis, P.; Koo, B.K.; Sasselli, V.; Van Der Flier, L.G.; Cuppen, E.; Van Oudenaarden, A.; Clevers, H. Ascl2 acts as an R-spondin/wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 2015, 16, 158–170. [Google Scholar] [CrossRef]
- Basu, S.; Gavert, N.; Brabletz, T.; Ben-Ze’ev, A. The intestinal stem cell regulating gene ASCL2 is required for L1-mediated colon cancer progression. Cancer Lett. 2018, 424, 9–18. [Google Scholar] [CrossRef]
- Ganesh, K.; Basnet, H.; Kaygusuz, Y.; Laughney, A.M.; He, L.; Sharma, R.; O’Rourke, K.P.; Reuter, V.P.; Huang, Y.-H.; Turkekul, M.; et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 2020, 1, 28–45. [Google Scholar] [CrossRef]
- Fang, Q.-X.; Zheng, X.-C.; Zhao, H.-J. L1CAM is involved in lymph node metastasis via ERK1/2 signaling in colorectal cancer. Am. J. Transl. Res. 2020, 12, 837–846. [Google Scholar] [PubMed]
- Lund, K.; Dembinski, J.L.; Solberg, N.; Urbanucci, A.; Mills, I.G.; Krauss, S. Slug-dependent upregulation of L1CAM is responsible for the increased invasion potential of pancreatic cancer cells following long-term 5-FU treatment. PLoS ONE 2015, 10, e0123684. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, N.; Jacob, F.; Peitzsch, C.; Dubrovska, A.; Krudewig, C.; Huang, Y.L.; Heinzelmann-Schwarz, V.; Schibli, R.; Béhé, M.; Grünberg, J. L1 cell adhesion molecule confers radioresistance to ovarian cancer and defines a new cancer stem cell population. Cancers 2020, 12, 217. [Google Scholar] [CrossRef]
- Roberts, C.M.; Tran, M.A.; Pitruzzello, M.C.; Wen, W.; Loeza, J.; Dellinger, T.H.; Mor, G.; Glackin, C.A. TWIST1 drives cisplatin resistance and cell survival in an ovarian cancer model, via upregulation of GAS6, L1CAM, and Akt signalling. Sci. Rep. 2016, 6, 37652. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, F.; Liu, N. L1CAM promotes epithelial to mesenchymal transition and formation of cancer initiating cells in human endometrial cancer. Exp. Ther. Med. 2018, 15, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Wolf, M.; Staebler, A.; Buchweitz, O.; Kelsch, R.; Schüring, A.N.; Kiesel, L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J. Pathol. 2008, 215, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Lee, K.; Kim, J.H.; Jun, H.O.; Kim, Y.; Cho, Y.L.; Yu, Y.S.; Min, J.K.; Kim, J.H. L1 increases adhesion-mediated proliferation and chemoresistance of retinoblastoma. Oncotarget 2017, 8, 15441–15452. [Google Scholar] [CrossRef][Green Version]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Chang, G. Multidrug resistance ABC transporters. FEBS Lett. 2003, 555, 102–105. [Google Scholar] [CrossRef]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; LLeonart, M.E. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Magrini, E.; Villa, A.; Angiolini, F.; Doni, A.; Mazzarol, G.; Rudini, N.; Maddaluno, L.; Komuta, M.; Topal, B.; Prenen, H.; et al. Endothelial deficiency of L1 reduces tumor angiogenesis and promotes vessel normalization. J. Clin. Investig. 2014, 124, 4335–4350. [Google Scholar] [CrossRef]
- Medici, D. Endothelial-Mesenchymal Transition in Regenerative Medicine. Stem Cells Int. 2016, 2016, 6962801. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Campos, B.; Bageritz, J.; Lorenzo Bermejo, J.; Becker, N.; Engel, F.; Acker, T.; Momma, S.; Herold-Mende, C.; Lichter, P.; et al. LGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cells. Brain Pathol. 2013, 23, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Okawa, S.; Gagrica, S.; Blin, C.; Ender, C.; Pollard, S.M.; Krijgsveld, J. Proteome and Secretome Characterization of Glioblastoma-Derived Neural Stem Cells. Stem Cells 2017, 35, 967–980. [Google Scholar] [CrossRef]
- Gemei, M.; Corbo, C.; D’Alessio, F.; Di Noto, R.; Vento, R.; Del Vecchio, L. Surface proteomic analysis of differentiated versus stem-like osteosarcoma human cells. Proteomics 2013, 13, 3293–3297. [Google Scholar] [CrossRef]
- Liang, L.; Aiken, C.; McClelland, R.; Morrison, L.C.; Tatari, N.; Remke, M.; Ramaswamy, V.; Issaivanan, M.; Ryken, T.; Del Bigio, M.R.; et al. Characterization of novel biomarkers in selecting for subtype specific medulloblastoma phenotypes. Oncotarget 2015, 6, 38881–38900. [Google Scholar] [CrossRef]
- Son, Y.S.; Seong, R.H.; Ryu, C.J.; Cho, Y.S.; Bae, K.H.; Chung, S.J.; Lee, B.; Min, J.K.; Hong, H.J. Brief report: L1 cell adhesion molecule, a novel surface molecule of human embryonic stem cells, is essential for self-renewal and pluripotency. Stem Cells 2011, 29, 2094–2099. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; An, Y.; Ren, F.; Yang, Z.Z.; Zhu, H.; Zhou, L.; He, X.; Schachner, M.; Xiao, Z.; et al. Cell recognition molecule L1 promotes embryonic stem cell differentiation through the regulation of cell surface glycosylation. Biochem. Biophys. Res. Commun. 2013, 440, 405–412. [Google Scholar] [CrossRef]
- Pusey, M.A.; Pace, K.; Fascelli, M.; Linser, P.J.; Steindler, D.A.; Galileo, D.S. Ectopic expression of L1CAM ectodomain alters differentiation and motility, but not proliferation, of human neural progenitor cells. Int. J. Dev. Neurosci. 2019, 78, 49–64. [Google Scholar] [CrossRef]
- Arlt, M.J.E.; Novak-Hofer, I.; Gast, D.; Gschwend, V.; Moldenhauer, G.; Grünberg, J.; Honer, M.; Schubiger, P.A.; Altevogt, P.; Krüger, A. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006, 66, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Wolterink, S.; Moldenhauer, G.; Fogel, M.; Kiefel, H.; Pfeifer, M.; Lüttgau, S.; Gouveia, R.; Costa, J.; Endell, J.; Moebius, U.; et al. Therapeutic antibodies to human L1CAM: Functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res. 2010, 70, 2504–2515. [Google Scholar] [CrossRef] [PubMed]
- Doberstein, K.; Harter, P.N.; Haberkorn, U.; Bretz, N.P.; Arnold, B.; Carretero, R.; Moldenhauer, G.; Mittelbronn, M.; Altevogt, P. Antibody therapy to human L1CAM in a transgenic mouse model blocks local tumor growth but induces EMT. Int. J. Cancer 2015, 136, E326–E339. [Google Scholar] [CrossRef]
- Lee, E.S.; Jeong, M.S.; Singh, R.; Jung, J.; Yoon, H.; Min, J.K.; Kim, K.H.; Hong, H.J. A chimeric antibody to L1 cell adhesion molecule shows therapeutic effect in an intrahepatic cholangiocarcinoma model. Exp. Mol. Med. 2012, 44, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.; Dieckmann, C.; Korniienko, O.; Moldenhauer, G.; Kiefel, H.; Salnikov, A.; Krüger, A.; Altevogt, P.; Sebens, S. Combined treatment of L1CAM antibodies and cytostatic drugs improve the therapeutic response of pancreatic and ovarian carcinoma. Cancer Lett. 2012, 319, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, T.S.; Song, I.H.; Kim, A.R.; Lee, Y.J.; Kim, H.; Hwang, H.; Jeong, M.S.; Kang, S.G.; Hong, H.J. Combination of anti-L1 cell adhesion molecule antibody and gemcitabine or cisplatin improves the therapeutic response of intrahepatic cholangiocarcinoma. PLoS ONE 2017, 12, e0170078. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.; Grünberg, J.; Cohrs, S.; Hohn, A.; Waldner-Knogler, K.; Jeger, S.; Zimmermann, K.; Novak-Hofer, I.; Schibli, R. L1-CAM-targeted antibody therapy and 177Lu-radioimmunotherapy of disseminated ovarian cancer. Int. J. Cancer 2012, 130, 2715–2721. [Google Scholar] [CrossRef]
- Grünberg, J.; Lindenblatt, D.; Dorrer, H.; Cohrs, S.; Zhernosekov, K.; Köster, U.; Türler, A.; Fischer, E.; Schibli, R. Anti-L1CAM radioimmunotherapy is more effective with the radiolanthanide terbium-161 compared to lutetium-177 in an ovarian cancer model. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1907–1915. [Google Scholar] [CrossRef]
- Hoefnagel, C.A.; Rutgers, M.; Buitenhuis, C.K.M.; Smets, L.A.; De Kraker, J.; Meli, M.; Carrel, F.; Amstutz, H.; Schubiger, P.A.; Novak-Hofer, I. A comparison of targetting of neuroblastoma with mIBG and anti L1-CAM antibody mAb chCE7: Therapeutic efficacy in a neuroblastoma xenograft model and imaging of neuroblastoma patients. Eur. J. Nucl. Med. 2001, 28, 359–368. [Google Scholar] [CrossRef]
- Song, I.H.; Jeong, M.S.; Hong, H.J.; Shin, J., II; Park, Y.S.; Woo, S.K.; Moon, B.S.; Kim, K., II; Lee, Y.J.; Kang, J.H.; et al. Development of a theranostic convergence bioradiopharmaceutical for immuno-PET based radioimmunotherapy of L1CAM in cholangiocarcinoma model. Clin. Cancer Res. 2019, 25, 6148–6159. [Google Scholar] [CrossRef]
- Lindenblatt, D.; Fischer, E.; Cohrs, S.; Schibli, R.; Grünberg, J. Paclitaxel improved anti-L1CAM lutetium-177 radioimmunotherapy in an ovarian cancer xenograft model. EJNMMI Res. 2014, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Lindenblatt, D.; Terraneo, N.; Pellegrini, G.; Cohrs, S.; Spycher, P.R.; Vukovic, D.; Béhé, M.; Schibli, R.; Grünberg, J. Combination of lutetium-177 labelled anti-L1CAM antibody chCE7 with the clinically relevant protein kinase inhibitor MK1775: A novel combination against human ovarian carcinoma. BMC Cancer 2018, 18, 922. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; DiGiusto, D.L.; Slovak, M.; Wright, C.; Naranjo, A.; Wagner, J.; Meechoovet, H.B.; Bautista, C.; Chang, W.C.; Ostberg, J.R.; et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 2007, 15, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Brown, C.E.; Ostberg, J.R.; Priceman, S.J.; Chang, W.C.; Weng, L.; Lin, P.; Wakabayashi, M.T.; Jensen, M.C.; Forman, S.J. L1 cell adhesion molecule-specific chimeric antigen receptor-redirected Human T cells exhibit specific and efficient antitumor activity against human ovarian cancer in mice. PLoS ONE 2016, 11, e0146885. [Google Scholar] [CrossRef] [PubMed]
- Andersch, L.; Radke, J.; Klaus, A.; Schwiebert, S.; Winkler, A.; Schumann, E.; Grunewald, L.; Zirngibl, F.; Flemmig, C.; Jensen, M.C.; et al. CD171- and GD2-specific CAR-T cells potently target retinoblastoma cells in preclinical in vitro testing. BMC Cancer 2019, 19, 895. [Google Scholar] [CrossRef] [PubMed]
- Grage-Griebenow, E.; Jerg, E.; Gorys, A.; Wicklein, D.; Wesch, D.; Freitag-Wolf, S.; Goebel, L.; Vogel, I.; Becker, T.; Ebsen, M.; et al. L1CAM promotes enrichment of immunosuppressive T cells in human pancreatic cancer correlating with malignant progression. Mol. Oncol. 2014, 8, 982–997. [Google Scholar] [CrossRef]
- Aikawa, Y. Rabex-5 protein regulates the endocytic trafficking pathway of ubiquitinated neural cell adhesion molecule L1. J. Biol. Chem. 2012, 287, 32312–32323. [Google Scholar] [CrossRef]
- Panicker, A.K.; Buhusi, M.; Erickson, A.; Maness, P.F. Endocytosis of β1 integrins is an early event in migration promoted by the cell adhesion molecule L1. Exp. Cell Res. 2006, 312, 299–307. [Google Scholar] [CrossRef]
- Grünberg, J.; Novak-Hofer, I.; Honer, M.; Zimmermann, K.; Knogler, K.; Bläuenstein, P.; Ametamey, S.; Maecke, H.R.; Schubiger, P.A. In vivo evaluation of177Lu- and 67/64Cu-labeled recombinant fragments of antibody chCE7 for radioimmunotherapy and PET imaging of L1-CAM-positive tumors. Clin. Cancer Res. 2005, 11, 5112–5120. [Google Scholar] [CrossRef]
- Zimmermann, K.; Grünberg, J.; Honer, M.; Ametamey, S.; Schubiger, P.A.; Novak-Hofer, I. Targeting of renal carcinoma with 67/64Cu-labeled anti-L1-CAM antibody chCE7: Selection of copper ligands and PET imaging. Nucl. Med. Biol. 2003, 30, 417–427. [Google Scholar] [CrossRef]
- Eshhar, Z.; Waks, T.; Gross, G.; Schindler, D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA 1993, 90, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.T.; Carpenter, R.O.; Maryalice, S.S.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Zander, H.; Rawnaq, T.; von Wedemeyer, M.; Tachezy, M.; Kunkel, M.; Wolters, G.; Bockhorn, M.; Schachner, M.; Izbicki, J.R.; Kaifi, J. Circulating levels of cell adhesion molecule l1 as a prognostic marker in gastrointestinal stromal tumor patients. BMC Cancer 2011, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, R.; Krause, M.; Mayer, S.; Peukert, N.; Suttkus, A.; Müller, W.C.; Lacher, M.; Meixensberger, J.; Nestler, U. Increased L1CAM (CD171) levels are associated with glioblastoma and metastatic brain tumors. Medicine 2018, 97, e12396. [Google Scholar] [CrossRef] [PubMed]
| Interactors | L1CAM Motif Involved | Type of Interaction | References |
|---|---|---|---|
| L1CAM | Ig1-6, FN2-3 | cis, trans | [11,15,16,17] |
| NCAM | Ig5 | cis | [18,19] |
| Neurocan | Ig1 | ND | [20] |
| Integrins | Ig6, FN3 | cis | [17,21,22] |
| Laminin | Ig6* | trans | [23] |
| Neuropilin | Ig1 | cis, trans | [10,24] |
| Ankyrin | Cytoplasmic domain | [25,26] | |
| FGFR | FN3, Ig1, Ig2 | cis, trans | [27] |
| EGFR | FN5, Ig3* | cis, trans | [28] |
| Cancer Type | Prognostic Value | Clinico-Pathological Parameters Correlating with L1CAM | References |
|---|---|---|---|
| Endometrial cancer | Negative (OS, DFS) | High grade, lymph node metastasis, tumor relapse | [55,56,57,58,59,60] |
| Ovarian cancer | Negative (OS, DFS) | Low tumor resectability, lymph node metastasis, chemoresistance, | [61,62,63,64] |
| Melanoma | Negative (DFS) | Metastasis | [65] |
| Breast cancer | Negative (DFS) | Larger tumor size, lymph node involvement, higher histologic grade, advanced TNM stage | [66] |
| Gastric cancer | Negative (OS, DFS) | Distant metastasis | [67] |
| Colon cancer | Negative (OS) | Advanced cancer stage, distant metastasis and tumor recurrence | [68] |
| Pancreatic cancer | Negative (OS) | Lymph node involvement, vascular invasion, perineural invasion and higher degree of pain | [69] |
| Non-small cell lung cancer | Negative (PFS) | None | [70] |
| Kidney cancer | Negative (OS) | ND | [71] |
| Cancer Type | Therapeutic Strategy | Antibody Clone | Effect | References |
|---|---|---|---|---|
| Ovarian cancer | Antibody alone | chCE7, L1-11A | ↓ Proliferation ↓ Migration | [121] |
| Ovarian cancer | Antibody alone | CE7 | ↓ Proliferation | [62] |
| Ovarian cancer | Antibody alone | L1-9.3 | ↓ Tumor growth ↑ Survival | [122] |
| Melanoma Pancreatic cancer | Antibody alone | L1-9.3 | ↓ Tumor growth EMT induction | [123] |
| Cholangiocarcinoma | Antibody alone | cA10-A3 | ↓ Tumor growth | [124] |
| Pancreatic cancer | Antibody + gemcitabine | L1-14.10, L1-9.3 | ↓ Tumor growth ↑ Apoptosis | [125] |
| Ovarian cancer | Antibody + paclitaxel | L1-14.10, L1-9.3 | ↓ Tumor growth ↑ Apoptosis | [125] |
| Cholangiocarcinoma | Antibody + gemcitabine | Ab417 | ↓ Tumor growth | [126] |
| Cholangiocarcinoma | Antibody + cisplatin | Ab417 | ↓ Tumor growth | [126] |
| Ovarian cancer | Radioimmunoconjugate | 177Lu-DOTA-chCE7 | ↑ Survival | [127] |
| Ovarian cancer | Radioimmunoconjugate | 161Tb-chCE7 | ↓ Tumor growth | [128] |
| Neuroblastoma | Radioimmunoconjugate | 131I-chCE7 | ↓ Tumor growth | [129] |
| Cholangiocarcinoma | Radioimmunoconjugate | 177Lu-NOTA-cA10-A3 | ↓ Tumor growth ↑ Apoptosis ↓ Proliferation | [130] |
| Ovarian cancer | Radioimmunoconjugate + paclitaxel | 177Lu-DOTA-chCE7 | ↑ Survival Tumor growth delay | [131] |
| Ovarian cancer | Radioimmunoconjugate + protein kinase inhibitor | 177Lu-DOTA-chCE7 | ↓ Proliferation ↓ Tumor growth | [132] |
| Neuroblastoma | CAR-T cell | CE7R | Various responses | [133] |
| Ovarian cancer | CAR-T cell | CE7R | ↓ Tumor growth ↓ Ascites | [134] |
| Retinoblastoma | CAR-T cell | CE7R | Cytotoxicity | [135] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, M.; Cavallaro, U. Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells. J. Clin. Med. 2020, 9, 1502. https://doi.org/10.3390/jcm9051502
Giordano M, Cavallaro U. Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells. Journal of Clinical Medicine. 2020; 9(5):1502. https://doi.org/10.3390/jcm9051502
Chicago/Turabian StyleGiordano, Marco, and Ugo Cavallaro. 2020. "Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells" Journal of Clinical Medicine 9, no. 5: 1502. https://doi.org/10.3390/jcm9051502
APA StyleGiordano, M., & Cavallaro, U. (2020). Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells. Journal of Clinical Medicine, 9(5), 1502. https://doi.org/10.3390/jcm9051502






