Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective
Abstract
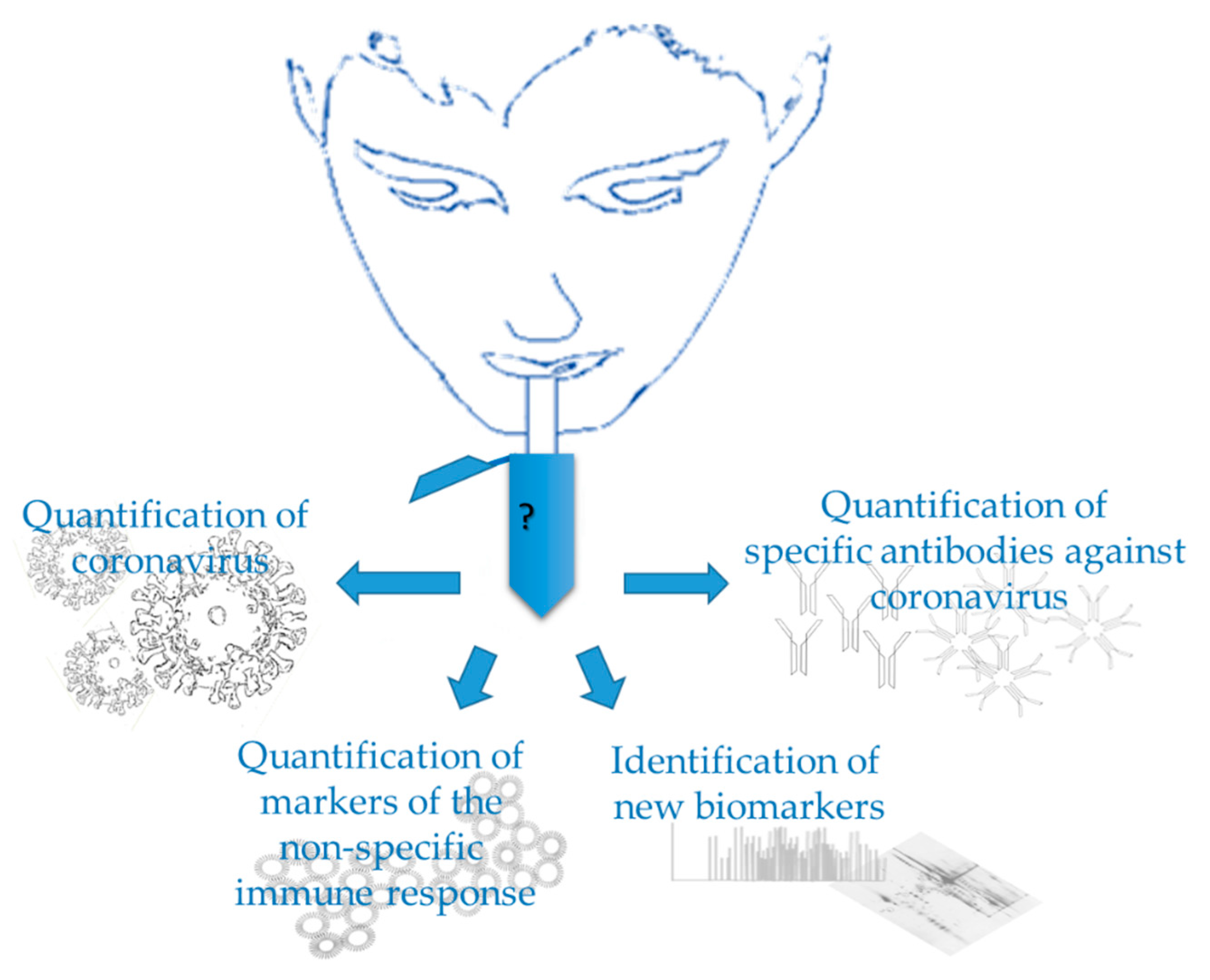
1. Introduction
2. Advantages of the Use of Saliva
- -
- It can be collected by the patient, even at home, minimizing the exposure of health care workers to nosocomial infections. This also reduces the need for health care personnel and waiting times for sample collection, resulting in easier crowd control regulations in clinical settings and thus avoiding further virus transmission.
- -
- It is easily accepted by the patients since it is non-painful and non-stressful. Therefore, it can be used for serial samplings and in large scale or epidemiological studies, being especially advantageous in certain populations, such as children [11].
- -
- It is easy, fast, and cheap to collect, allowing widespread testing.
3. Quantification of Coronavirus in Saliva
3.1. Evidence for SARS-CoV-2 in Saliva
- -
- The group of Dr. To published two reports using saliva collected by asking the patient to cough out saliva from their throat into a sterile container and adding a viral transport medium to the sample [12,13]. These samples, in addition to salivary gland secretions, contained material from the posterior oropharynx, that could have come from respiratory secretions swept up from the tracheal–bronchial tree, and also secretions coming down from the nasopharynx.In these reports, the RNA of the virus was identified in the saliva of 20 out of 23 patients who had previously been confirmed as infected by the detection of SARS-CoV-2 RNA in their nasopharyngeal or sputum specimens, giving an overall diagnostic sensitivity of 87%. In addition, saliva tested negative in 33 patients from whom nasopharyngeal specimens were negative for SARS-Cov-2. The detection of the virus was by a real-time reverse transcription-quantitative polymerase chain reaction (rRT-PCR) and the range of values was from 9.9 × 102 copies/mL to 1.2 × 108 copies/mL.
- -
- Azzi et al. [14] collected saliva through passive drool. In cases of patients who were undergoing endotracheal intubation and mechanical ventilation, the collection was performed intraorally by a physician with the use of a pipette. These specimens possibly also contained respiratory secretions. In these conditions, SARS-CoV-2 was detected in all saliva samples collected from a group of 25 patients with severe to very severe disease, who were diagnosed by detection of the virus in pharyngeal or bronchoalveolar swabs. An rRT-PCR that detected a trend in viral load without quantification of the viral copies per millimeter, was used for virus detection.In this study, two patients who were monitored showed positive salivary results on the same days that their pharyngeal or bronchoalveolar swabs were negative. This raises the possibility that individuals can be contagious through their saliva even when pharyngeal swabs are negative. This could be a point in favor of the use of saliva for the virus detection and would be in line with the description of salivary glands as potential reservoirs for Covid-19 in asymptomatic but infected people [15].
- -
- Han et al. [16] collected saliva from a 27-day-old neonate diagnosed with Covid-19 and reported values in the range of 105 copies/mL that were similar to the values obtained with pharyngeal swabs but lower than those from bronchoalveolar swabs.
3.2. Evidence from the Previous SARS-CoV Epidemic
3.3. Evidence from Other Coronavirus and Virus in General
4. Quantification of Specific Antibodies against Virus in Saliva
5. Quantification of Markers of the Non-Specific Immune Response in Saliva
6. Saliva as a Source for New Disease Biomarkers and/or Understanding of Pathways Involved in Disease
7. Recommendations and Points to Improve for an Optimal Use of Saliva as Sample
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabutti, G.; d’Anchera, E.; Sandri, F.; Savio, M.; Stefanati, A. Coronavirus: Update Related to the Current Outbreak of COVID-19. Infect. Dis. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Martinez-Subiela, S.; Lopez-Jornet, P.; Lamy, E. (Eds.) Saliva in Health and Disease. The Present and Future of A Unique Sample for Diagnosis; Springer Nature: Cham, Switzerland, 2020; ISBN 9783030376802. [Google Scholar]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Silva, R.; Jardim, A.C.G.; Siqueira, W.L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 2020, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Asiri, F.Y.I.; Al Wadaani, H. Human Saliva: Non-Invasive Fluid for Detecting Novel Coronavirus (2019-nCoV). Int. J. Environ. Res. Public Health 2020, 17, 2225. [Google Scholar] [CrossRef] [PubMed]
- First Saliva Test for COVID-19 Approved for Emergency Use by FDA|The Scientist Magazine®. Available online: https://www.the-scientist.com/news-opinion/first-saliva-test-for-covid-19-approved-for-emergency-use-by-fda-67416 (accessed on 13 May 2020).
- To, K.K.W.; Yip, C.C.Y.; Lai, C.Y.W.; Wong, C.K.H.; Ho, D.T.Y.; Pang, P.K.P.; Ng, A.C.K.; Leung, K.H.; Poon, R.W.S.; Chan, K.H.; et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: A diagnostic validity study. Clin. Microbiol. Infect. 2019, 25, 372–378. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, S.Y.; Liu, I.J.; Chen, Y.C.; Chen, H.L.; Yang, C.F.; Chen, P.J.; Yeh, S.H.; Kao, C.L.; Huang, L.M.; et al. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg. Infect. Dis. 2004, 10, 1213–1219. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martinez-Lozano, N.; Rios, R.; Marcilla de Teruel, M.C.; Garaulet, M.; Cerón, J.J. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- To, K.; Tsang, O.; Chik-Yan Yip, C.; Chan, K.; Wu, C.; Chan, J.; Leung, W.; Chik, T.; Choi, C.; Kandamby, D.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva | Clinical Infectious Diseases|Oxford Academic. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Dent. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Seong, M.; Heo, E.; Park, J.; Kim, N.; Shin, S.; Cho, S.; Park, S.; Choi, E. Sequential Analysis of Viral Load in a Neonate and Her Mother Infected With SARS-CoV-2-PubMed. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Drosten, C.; Chiu, L.L.; Panning, M.; Leong, H.N.; Preiser, W.; Tam, J.S.; Günther, S.; Kramme, S.; Emmerich, P.; Ng, W.L.; et al. Evaluation of Advanced Reverse Transcription-PCR Assays and an Alternative PCR Target Region for Detection of Severe Acute Respiratory Syndrome-Associated Coronavirus. J. Clin. Microbiol. 2004, 42, 2043–2047. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef]
- To, K.K.; Lu, L.; Yip, C.C.; Poon, R.W.; Fung, A.M.; Cheng, A.; Lui, D.H.; Ho, D.T.; Hung, I.F.; Chan, K.H.; et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg. Microbes Infect. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Bjustrom-Kraft, J.; Woodard, K.; Giménez-Lirola, L.; Rotolo, M.; Wang, C.; Sun, Y.; Lasley, P.; Zhang, J.; Baum, D.; Gauger, P.; et al. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet. Res. 2016, 12, 99. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Nietfeld, J.C.; Bai, J.; Peddireddi, L.; Breazeale, B.; Anderson, J.; Kerrigan, M.A.; An, B.; Oberst, R.D.; Crawford, K.; et al. Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of Porcine epidemic diarrhea virus following inoculation of 4-week-old feeder pigs. J. Vet. Diagn. Investig. 2016, 28, 671–678. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Khan, E.; Mali, M.; Latif, M. Human saliva can be a diagnostic tool for Zika virus detection. J. Infect. Public Health 2019, 12, 601–604. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Shimamura, M.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; Tolan, R.W.; Novak, Z.; et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N. Engl. J. Med. 2011, 364, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.V.; Perry, K.R.; Mortimer, P.P. Sensitive assays for viral antibodies in saliva: An alternative to tests on serum. Lancet 1987, 330, 72–75. [Google Scholar] [CrossRef]
- McKie, A.; Vyse, A.; Maple, C. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect. Dis. 2002, 2, 18–24. [Google Scholar] [CrossRef]
- Hettegger, P.; Huber, J.; Paßecker, K.; Soldo, R.; Kegler, U.; Nöhammer, C.; Weinhäusel, A. High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PLoS ONE 2019, 14, e0218456. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.P.; Parry, J.V. Detection of antibody to HIV in saliva: A brief review. Clin. Diagn. Virol. 1994, 2, 231–243. [Google Scholar] [CrossRef]
- González, V.; Martró, E.; Folch, C.; Esteve, A.; Matas, L.; Montoliu, A.; Grífols, J.R.; Bolao, F.; Tural, C.; Muga, R.; et al. Detection of hepatitis C virus antibodies in oral fluid specimens for prevalence studies. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 121–126. [Google Scholar] [CrossRef]
- Flodgren, G. Immunity after SARS-CoV-2 Infection. Rapid Review 2020; Norwegian Institute of Public Health: Oslo, Norway, 2020; ISBN 978-82-8406-081-1. [Google Scholar]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Peng, Y.D.; Meng, K.; Guan, H.Q.; Leng, L.; Zhu, R.R.; Wang, B.Y.; He, M.A.; Cheng, L.X.; Huang, K.; Zeng, Q.T. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, E004. [Google Scholar]
- Cerón, J.J.; Martinez-Subiela, S.; Ohno, K.; Caldin, M. A seven-point plan for acute phase protein interpretation in companion animals. Vet. J. 2008, 177, 6. [Google Scholar] [CrossRef]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Relationships among Lymphocyte Subsets, Cytokines, and the Pulmonary Inflammation Index in Coronavirus (COVID-19) Infected Patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef]
- Parra, M.D.; Tecles, F.; Subiela, S.M.; Cerón, J.J. C-Reactive Protein Measurement in Canine Saliva. J. Vet. Diagn. Investig. 2005, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2019, 23, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J. Acute phase proteins, saliva and education in laboratory science: An update and some reflections. BMC Vet. Res. 2019, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Franco-Martínez, L.; Rubio, C.P.; Contreras-Aguilar, M.D. Methodology Assays for the Salivary Biomarkers’ Identification and Measurement. In Saliva in Health and Disease; Tvarijonaviciute, A., Martinez-Subiela, S., Lopez-Jornet, P., Lamy, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 67–95. [Google Scholar]
- Katsani, K.R.; Sakellari, D. Saliva proteomics updates in biomedicine. J. Biol. Res. 2019, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Gautier, J.-F.; Ravussin, Y. A New Symptom of COVID-19: Loss of Taste and Smell. Obesity 2020, 28, 848. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Torregrossa, A.-M.; Castelo, P.M.; Capela e Silva, F. Saliva in Ingestive Behavior Research: Association with Oral Sensory Perception and Food Intake. In Saliva in Health and Disease; Tvarijonaviciute, A., Martinez-Subiela, S., Lopez-Jornet, P., Lamy, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 23–48. [Google Scholar]
- Barranco, T.; Rubio, C.P.; Tvarijonaviciute, A.; Rubio, M.; Damia, E.; Lamy, E.; Cugat, R.; Cerón, J.J.; Tecles, F.; Escribano, D. Changes of salivary biomarkers under different storage conditions: Effects of temperature and length of storage. Biochem. Med. 2019, 29, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Cantos-Barreda, A.; Escribano, D.; Egui, A.; Thomas, M.C.; López, M.C.; Tecles, F.; Bernal, L.J.; Cerón, J.J.; Martínez-Subiela, S. One-year follow-up of anti-Leishmania antibody concentrations in serum and saliva from experimentally infected dogs. Int. J. Parasitol. 2019, 49, 893–900. [Google Scholar] [CrossRef] [PubMed]
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceron, J.J.; Lamy, E.; Martinez-Subiela, S.; Lopez-Jornet, P.; Capela-Silva, F.; Eckersall, P.D.; Tvarijonaviciute, A. Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective. J. Clin. Med. 2020, 9, 1491. https://doi.org/10.3390/jcm9051491
Ceron JJ, Lamy E, Martinez-Subiela S, Lopez-Jornet P, Capela-Silva F, Eckersall PD, Tvarijonaviciute A. Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective. Journal of Clinical Medicine. 2020; 9(5):1491. https://doi.org/10.3390/jcm9051491
Chicago/Turabian StyleCeron, Jose J., Elsa Lamy, Silvia Martinez-Subiela, Pia Lopez-Jornet, Fernando Capela-Silva, Peter David Eckersall, and Asta Tvarijonaviciute. 2020. "Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective" Journal of Clinical Medicine 9, no. 5: 1491. https://doi.org/10.3390/jcm9051491
APA StyleCeron, J. J., Lamy, E., Martinez-Subiela, S., Lopez-Jornet, P., Capela-Silva, F., Eckersall, P. D., & Tvarijonaviciute, A. (2020). Use of Saliva for Diagnosis and Monitoring the SARS-CoV-2: A General Perspective. Journal of Clinical Medicine, 9(5), 1491. https://doi.org/10.3390/jcm9051491









