Abstract
In this report, updated information and future perspectives about the use of saliva as a sample for laboratory analysis of the Covid-19 are highlighted. Saliva can be used for the direct detection of the SARS-CoV-2 virus, the quantification of the specific immunoglobulins produced against it, and for the evaluation of the non-specific, innate immune response of the patient. Moreover, a deeper knowledge of potential changes in the saliva proteome in this disease may allow the identification of new diagnostic and prognostic biomarkers, or even help our understanding of the mechanisms associated with the disease. With the development of appropriate sample collection and processing methods and the use of adequate assays, saliva can provide useful clinical information about the disease and could be potentially included in guidelines for sample collection for the diagnosis, disease management, and control of Covid-19.
1. Introduction
A coronavirus, designated by the World Health Organization as 2019 novel Coronavirus (2019-nCoV) and by the Coronavirus Study Group of the International Committee on Taxonomy of Viruses as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spreading worldwide, producing the coronavirus disease 2019 (Covid-19). Although its mechanism of infection is still not known, it has affinity for and replicates in cells located in the lower airways. The most characteristic clinical sign is the production of a respiratory distress syndrome that can range from mild malaise to death, having worse prognosis in elderly patients with comorbidities [1]. In addition, one of the symptoms, referred by many patients, is the loss of sensory acuity, including smell and taste [2]. On the 18th of April, the number of cases worldwide was 2,261,034 with 154,726 deaths.
Saliva has been increasingly used over the last few decades for evaluating human health. It is an integrated mixture of secretions of the different salivary glands, desquamated oral epithelial cells, gingival crevicular fluid, and different microorganisms [3]. It also contains a large number of proteins such as immunoglobulins, mucins, and enzymes, as well as metabolites, hormones, and electrolytes. This composition allows the detection of pathogens in saliva and also the quantification of biomarkers that can provide information about the immunological, inflammatory, endocrine, and metabolic status of the individual. In some cases, its value to detect physiological changes is similar or superior to serum, such as for the detection of acute stress by alpha-amylase or cortisol [4]. Overall, saliva appears to be a fluid of enormous potential in health assessment, especially due to the clinical information that it can provide and the non-invasive nature of its collection, which can be performed by individuals without particular training and with no major requirements in terms of equipment or facilities.
Currently, naso and oropharyngeal swabs are the two main recommended upper respiratory tract specimen types for Covid-19 diagnostic testing, although the use of saliva for the diagnosis of the disease has been recently suggested [5,6]. Furthermore, in recent days, a test for assessing the RNA in saliva samples was approved by the US Food and Drug Administration [7].
The objective of this report is to provide updated information about the use of saliva as a sample for laboratory analysis in Covid-19 investigations. For this purpose, the advantages of the use of this biofluid are described and the scientific evidence is presented, which supports the concept that the analysis of saliva in patients with Covid-19 could allow for the detection of the virus and antibodies produced against it, as well as the assessment of the non-specific, innate immune response. Finally, the possibility of the use of saliva for finding new biomarkers in this disease and some general recommendations that could contribute to a more appropriate use of this sample will be highlighted.
2. Advantages of the Use of Saliva
The use of naso and oropharyngeal swabs have several limitations, such as the discomfort for the patient and the need for the intervention of a healthcare worker in a disease with a high risk of nosocomial transmission [8]. These collection systems can also induce coughing and sneezing, generating aerosol, which can transmit the virus. In addition, in cases of thrombocytopenia or any other coagulation disorder, this procedure can cause bleeding. These drawbacks can limit the use of swabs, especially in serial monitoring or mass test programs. Sputum has been also proposed as a non-invasive lower respiratory tract specimen, but 72% of Covid-19 patients were not able to produce it for collection [9]. The difficulty of obtaining sputum also has been described in SARS-CoV, a virus with many similarities with the Covid-2019, especially at early stages of infection, when no cough or only dry cough is present [10]. The use of saliva could improve these drawbacks as it has the following advantages:
- -
- It can be collected by the patient, even at home, minimizing the exposure of health care workers to nosocomial infections. This also reduces the need for health care personnel and waiting times for sample collection, resulting in easier crowd control regulations in clinical settings and thus avoiding further virus transmission.
- -
- It is easily accepted by the patients since it is non-painful and non-stressful. Therefore, it can be used for serial samplings and in large scale or epidemiological studies, being especially advantageous in certain populations, such as children [11].
- -
- It is easy, fast, and cheap to collect, allowing widespread testing.
In the particular case of SARS-CoV-2, saliva could be used for evaluating different aspects of the disease, as shown in Figure 1, the evidence for which will be described in the following sections.
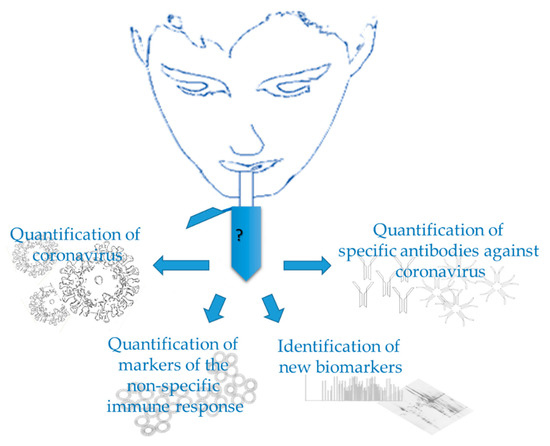
Figure 1.
Use of saliva in Covid-19.
4. Quantification of Specific Antibodies against Virus in Saliva
Another field where saliva may have a major potential for Covid-19 is in screening for immunity. There is scientific evidence that specific antibodies against infectious diseases can be detected in saliva [24]. Salivary IgG and IgM concentrations are much lower than in serum, although it has been suggested that they are in a similar proportion in relation to total protein in saliva as in serum [25]. In the particular case of IgG, plasma and saliva IgG profiles are highly similar for a large number of antigens [26]. It has been hypothesized that both salivary IgG and IgM are derived from blood, whereas IgA is mainly produced by the salivary glands [24].
The presence of IgM and IgG in serum against components of the virus, such as 2019-nCovid nucleoprotein (NP) and spike protein receptor binding domain (RBD), has been described in Covid-19 patients 10 days or later after symptom onset [12]. Although, to the author’s knowledge, there are presently no studies regarding antibodies in saliva in Covid-19. In animal species, there is evidence that saliva can be used for the assessment of antibody responses to coronavirus infections. For example, saliva IgG and IgA can be used to track levels of immunity against PEDV disease over time [20]. In addition, saliva has been used in several viral diseases of humans for the detection of specific immunoglobulins to pathogens such as hepatitis A, B, and C, human immunodeficiency virus, and rubella virus [24,27,28].
The detection of antibodies in saliva could be potentially used for the control of Covid-19 as occurred with other infectious diseases. For example, for infection with rubella, oral fluid testing has been offered by the Public Health Laboratory Service in the UK for cases notified to the Office for National Statistics (ONS). In this program, saliva samples are collected between two and six weeks after the onset of symptoms and tested for virus specific IgM. The assays used have been shown to be greater than 90% sensitive and specific [25]. It could be expected that saliva will have similar value in Covid-19 diagnosis and monitoring. This is particularly interesting for two main reasons: (1) as long as there is no effective treatment or vaccine and person-to-person transmission has to be minimized, the possibility of identifying immune individuals will be invaluable in defining who and how particular individuals can be relieved from confinement and limited social contact; (2) there is a lot to establish and understand about immunity dynamics during the disease, and how long it remains after the individual has recovered [29].
5. Quantification of Markers of the Non-Specific Immune Response in Saliva
In patients with Covid-19, serum concentrations of acute phase proteins (APPs), such as C-reactive protein (CRP) and ferritin, are increased in the cases that develop more severe disease. Their concentrations have been shown to correlate with the severity of the process [30,31]. In inflammatory conditions, APPs can increase before the appearance of clinical signs, being very early biomarkers [32]. In addition, increases in serum of several interleukins (IL), such as IL-6 and IL-10, have been described in Covid-19 patients [33], and these cytokines are known to be mediators of the APPs response.
In humans and in animal models, a high correlation in CRP between serum and saliva has been demonstrated [11,34]. Additionally, other APPs such as ferritin, haptoglobin, serum amyloid A, different interleukins, and other analytes related to the immune response, such as adenosine deaminase (ADA), can be measured in saliva [11,35,36].
These analytes can potentially be used as salivary biomarkers to assess the severity of the process and also to predict the development of more severe cases in Covid-19.
6. Saliva as a Source for New Disease Biomarkers and/or Understanding of Pathways Involved in Disease
The use of proteomic techniques allows the identification of multiple proteins in saliva [37]. By comparing these proteins between healthy individuals and those with disease, it is possible to assess the differences, which can result from changes in the circulating levels of proteins and/or from changes in the salivary gland secretion, associated with a disease. These differences are valuable to understand the pathophysiological processes associated with the disease, and identify biomarkers that may allow an early and easy diagnosis, and have been studied in other viral diseases [38].
In Covid-19, proteomic studies could allow for the discovery of new biomarkers for the disease and help to elucidate the loss of smell and taste by some patients [39]. It is known that the saliva proteome is related to taste sensitivity [40]. In this context, potential changes in the saliva proteome in this disease may be associated with the reduced taste perception.
7. Recommendations and Points to Improve for an Optimal Use of Saliva as Sample
Although saliva is a sample easy to collect, general recommendations should be followed for its use, some of which are particularly focused in the application to this disease, as shown in Table 1.

Table 1.
Recommendations for the use of saliva as sample in Covid-19.
1. The use of a standardized method of collection.
Different methods can be used for saliva collection, such as passive drool, different absorbent materials, or stimulation with citrate. These methods may have, in some cases, interference with selected analytes. In the particular case of the Covid-19, two aspects should be addressed regarding the method of collection:
-The possible differences between saliva obtained by passive drool or absorbent materials and by clearing the throat should be evaluated. In addition, some patients might not be able to clear the throat effectively to cough out saliva from deep in the throat and, therefore, this could decrease test sensitivity with this collection method, which should be explored. Although there are no studies comparing the use of saliva with throat wash in Covid-19, in the previous SARS-CoV epidemic similar results were obtained when throat wash with 10 mL of saline and saliva were compared [10].
-Instead of direct spitting, the use of a straw or any device that avoids the creation and expansion of drops should be encouraged. The saliva of infected patients can contain viruses that may allow airborne transmission and also by oral droplets and, therefore, should be handled with care [14]. In SARS-CoV, it has been hypothesized that very small particles of the virus can be spread in the air [10]. Therefore, special care should be taken during all the processes of sample collection, management, and analysis to eliminate this potential risk of transmission via contact with saliva droplets or aerosol.
Taking these facts into consideration, preliminary tests should ideally be made to establish the optimal method of collection, which may be different depending on the analyte to be measured. For example, in Covid-19, it would be of interest to evaluate if the saliva obtained by clearing the throat could be more convenient for the detection of the virus, whereas the whole saliva obtained directly by drool could be more suitable for the quantification of antibodies or acute phase proteins.
In addition, ideally, the salivary flow should be controlled by establishing a fixed amount of time during which saliva is collected (e.g., one minute). It is worthwhile to indicate that the report of results should be standardized, since in some cases, such as for selected interleukins or alpha-amylase, the way of expressing the values can influence their interpretation [4]. Furthermore, the need for saliva sample centrifugation and the effect of this centrifugation on the analyte of interest should be assessed. Avoiding centrifugation can speed the sample processing, allowing the use of rapid point of care tests; however, centrifugation helps to eliminate components that can interfere with subsequent assays.
2. Use of appropriate conditions for sample preservation.
It is known that the degradation of saliva components can occur under certain storage conditions [41]. As such, it is important to know the best storage conditions for samples that allow the maximum preservation of the analytes to be analyzed. Nevertheless, while such data are lacking, it is recommended to keep samples refrigerated until arrival at the lab to be analyzed and use −80 °C temperatures if the samples are stored.
3. Use of appropriate assays.
Ideally, the assays used should be sensitive enough for detecting low amounts of the analyte that can appear in saliva. In this line, highly sensitive immunoassays, such as those based on time-resolved fluorescence or similar technologies, would be recommended for immunoglobulins and acute phase proteins quantification [25]. In the case of qRT-PCR, it would be of importance to select an appropriate housekeeping gene and explore if the virus detected in saliva is cell-free or cell-associated. Overall, assays should be fully validated in order to avoid analytical errors due to assay technical limitations.
4. Increase the knowledge base on clinical applications.
Further studies should be made to evaluate and refine the interpretation and clinical application in Covid-19 of the different analytes that can be measured in saliva. These studies should be focused on their use in different clinical aspects such as in diagnosis, evaluation of disease severity, and in monitoring the response to treatment and predict relapses.
As an example, studies on the kinetics of antibodies in saliva compared to serum could be undertaken. In some diseases, a delay in the appearance of antibodies in saliva compared to serum exists and should be taken in consideration for an appropriate interpretation of the test [42].
8. Conclusions
Saliva can have potential applications in the context of Covid-19 by direct detection of the virus, quantification of the specific immunoglobulins produced against it, and for the evaluation of the non-specific, innate immune response of the patient. Moreover, a deeper knowledge about potential changes in the saliva proteome may allow for the identification of new diagnostic biomarkers or help to understand the mechanisms associated with the disease. With the development of appropriate sample collection and processing methods and the use of adequate assays, saliva can provide useful clinical information about the disease and could be potentially included in guidelines for sample collection for the diagnosis, disease management, and control of Covid-19.
Author Contributions
Conceptualization, J.J.C., E.L., and A.T.; writing—original draft preparation, J.J.C., E.L., P.D.E., F.C.-S., S.M.-S., P.L.-J., and A.T.; writing—reviewing and editing, J.J.C., E.L., P.D.E., F.C.-S., S.M.-S., P.L.-J., and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Program for Research Groups of Excellence of the Seneca Foundation, Murcia, Spain (grant 19894/GERM/15) and “Ministerio de Economía y Competitividad”, Spain, through a post-doctoral fellowship “Ramón y Cajal” to A.T. Funding was additionally provided by the FCT–Portuguese Science Foundation, through UID/AGR/00115/2019 and research contract CEECIND/04397/2017 to E.L.
Acknowledgments
The support of the Garcia Cugat Foundation to research activities in the field of saliva analysis of Interlab-UMU is acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gabutti, G.; d’Anchera, E.; Sandri, F.; Savio, M.; Stefanati, A. Coronavirus: Update Related to the Current Outbreak of COVID-19. Infect. Dis. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Martinez-Subiela, S.; Lopez-Jornet, P.; Lamy, E. (Eds.) Saliva in Health and Disease. The Present and Future of A Unique Sample for Diagnosis; Springer Nature: Cham, Switzerland, 2020; ISBN 9783030376802. [Google Scholar]
- Contreras-Aguilar, M.D.; Escribano, D.; Martínez-Subiela, S.; Martínez-Miró, S.; Rubio, M.; Tvarijonaviciute, A.; Tecles, F.; Cerón, J.J. Influence of the way of reporting alpha-amylase values in saliva in different naturalistic situations: A pilot study. PLoS ONE 2017, 12, e0180100. [Google Scholar] [CrossRef] [PubMed]
- Sabino-Silva, R.; Jardim, A.C.G.; Siqueira, W.L. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin. Oral Investig. 2020, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Asiri, F.Y.I.; Al Wadaani, H. Human Saliva: Non-Invasive Fluid for Detecting Novel Coronavirus (2019-nCoV). Int. J. Environ. Res. Public Health 2020, 17, 2225. [Google Scholar] [CrossRef] [PubMed]
- First Saliva Test for COVID-19 Approved for Emergency Use by FDA|The Scientist Magazine®. Available online: https://www.the-scientist.com/news-opinion/first-saliva-test-for-covid-19-approved-for-emergency-use-by-fda-67416 (accessed on 13 May 2020).
- To, K.K.W.; Yip, C.C.Y.; Lai, C.Y.W.; Wong, C.K.H.; Ho, D.T.Y.; Pang, P.K.P.; Ng, A.C.K.; Leung, K.H.; Poon, R.W.S.; Chan, K.H.; et al. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: A diagnostic validity study. Clin. Microbiol. Infect. 2019, 25, 372–378. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, W.K.; Chen, S.Y.; Liu, I.J.; Chen, Y.C.; Chen, H.L.; Yang, C.F.; Chen, P.J.; Yeh, S.H.; Kao, C.L.; Huang, L.M.; et al. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg. Infect. Dis. 2004, 10, 1213–1219. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martinez-Lozano, N.; Rios, R.; Marcilla de Teruel, M.C.; Garaulet, M.; Cerón, J.J. Saliva as a non-invasive tool for assessment of metabolic and inflammatory biomarkers in children. Clin. Nutr. 2019. [Google Scholar] [CrossRef]
- To, K.; Tsang, O.; Chik-Yan Yip, C.; Chan, K.; Wu, C.; Chan, J.; Leung, W.; Chik, T.; Choi, C.; Kandamby, D.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva | Clinical Infectious Diseases|Oxford Academic. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef]
- Azzi, L.; Carcano, G.; Gianfagna, F.; Grossi, P.; Gasperina, D.D.; Genoni, A.; Fasano, M.; Sessa, F.; Tettamanti, L.; Carinci, F.; et al. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary Glands: Potential Reservoirs for COVID-19 Asymptomatic Infection. J. Dent. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Seong, M.; Heo, E.; Park, J.; Kim, N.; Shin, S.; Cho, S.; Park, S.; Choi, E. Sequential Analysis of Viral Load in a Neonate and Her Mother Infected With SARS-CoV-2-PubMed. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Drosten, C.; Chiu, L.L.; Panning, M.; Leong, H.N.; Preiser, W.; Tam, J.S.; Günther, S.; Kramme, S.; Emmerich, P.; Ng, W.L.; et al. Evaluation of Advanced Reverse Transcription-PCR Assays and an Alternative PCR Target Region for Detection of Severe Acute Respiratory Syndrome-Associated Coronavirus. J. Clin. Microbiol. 2004, 42, 2043–2047. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef]
- To, K.K.; Lu, L.; Yip, C.C.; Poon, R.W.; Fung, A.M.; Cheng, A.; Lui, D.H.; Ho, D.T.; Hung, I.F.; Chan, K.H.; et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg. Microbes Infect. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Bjustrom-Kraft, J.; Woodard, K.; Giménez-Lirola, L.; Rotolo, M.; Wang, C.; Sun, Y.; Lasley, P.; Zhang, J.; Baum, D.; Gauger, P.; et al. Porcine epidemic diarrhea virus (PEDV) detection and antibody response in commercial growing pigs. BMC Vet. Res. 2016, 12, 99. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Nietfeld, J.C.; Bai, J.; Peddireddi, L.; Breazeale, B.; Anderson, J.; Kerrigan, M.A.; An, B.; Oberst, R.D.; Crawford, K.; et al. Tissue localization, shedding, virus carriage, antibody response, and aerosol transmission of Porcine epidemic diarrhea virus following inoculation of 4-week-old feeder pigs. J. Vet. Diagn. Investig. 2016, 28, 671–678. [Google Scholar] [CrossRef]
- Khurshid, Z.; Zafar, M.; Khan, E.; Mali, M.; Latif, M. Human saliva can be a diagnostic tool for Zika virus detection. J. Infect. Public Health 2019, 12, 601–604. [Google Scholar] [CrossRef]
- Boppana, S.B.; Ross, S.A.; Shimamura, M.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; Tolan, R.W.; Novak, Z.; et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N. Engl. J. Med. 2011, 364, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Parry, J.V.; Perry, K.R.; Mortimer, P.P. Sensitive assays for viral antibodies in saliva: An alternative to tests on serum. Lancet 1987, 330, 72–75. [Google Scholar] [CrossRef]
- McKie, A.; Vyse, A.; Maple, C. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect. Dis. 2002, 2, 18–24. [Google Scholar] [CrossRef]
- Hettegger, P.; Huber, J.; Paßecker, K.; Soldo, R.; Kegler, U.; Nöhammer, C.; Weinhäusel, A. High similarity of IgG antibody profiles in blood and saliva opens opportunities for saliva based serology. PLoS ONE 2019, 14, e0218456. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, P.P.; Parry, J.V. Detection of antibody to HIV in saliva: A brief review. Clin. Diagn. Virol. 1994, 2, 231–243. [Google Scholar] [CrossRef]
- González, V.; Martró, E.; Folch, C.; Esteve, A.; Matas, L.; Montoliu, A.; Grífols, J.R.; Bolao, F.; Tural, C.; Muga, R.; et al. Detection of hepatitis C virus antibodies in oral fluid specimens for prevalence studies. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 121–126. [Google Scholar] [CrossRef]
- Flodgren, G. Immunity after SARS-CoV-2 Infection. Rapid Review 2020; Norwegian Institute of Public Health: Oslo, Norway, 2020; ISBN 978-82-8406-081-1. [Google Scholar]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Peng, Y.D.; Meng, K.; Guan, H.Q.; Leng, L.; Zhu, R.R.; Wang, B.Y.; He, M.A.; Cheng, L.X.; Huang, K.; Zeng, Q.T. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, E004. [Google Scholar]
- Cerón, J.J.; Martinez-Subiela, S.; Ohno, K.; Caldin, M. A seven-point plan for acute phase protein interpretation in companion animals. Vet. J. 2008, 177, 6. [Google Scholar] [CrossRef]
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z.; et al. Relationships among Lymphocyte Subsets, Cytokines, and the Pulmonary Inflammation Index in Coronavirus (COVID-19) Infected Patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef]
- Parra, M.D.; Tecles, F.; Subiela, S.M.; Cerón, J.J. C-Reactive Protein Measurement in Canine Saliva. J. Vet. Diagn. Investig. 2005, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Zamora, C.; Martinez-Subiela, S.; Tecles, F.; Pina, F.; Lopez-Jornet, P. Salivary adiponectin, but not adenosine deaminase, correlates with clinical signs in women with Sjögren’s syndrome: A pilot study. Clin. Oral Investig. 2019, 23, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Cerón, J.J. Acute phase proteins, saliva and education in laboratory science: An update and some reflections. BMC Vet. Res. 2019, 15, 197. [Google Scholar] [CrossRef] [PubMed]
- Franco-Martínez, L.; Rubio, C.P.; Contreras-Aguilar, M.D. Methodology Assays for the Salivary Biomarkers’ Identification and Measurement. In Saliva in Health and Disease; Tvarijonaviciute, A., Martinez-Subiela, S., Lopez-Jornet, P., Lamy, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 67–95. [Google Scholar]
- Katsani, K.R.; Sakellari, D. Saliva proteomics updates in biomedicine. J. Biol. Res. 2019, 26, 17. [Google Scholar] [CrossRef] [PubMed]
- Gautier, J.-F.; Ravussin, Y. A New Symptom of COVID-19: Loss of Taste and Smell. Obesity 2020, 28, 848. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Torregrossa, A.-M.; Castelo, P.M.; Capela e Silva, F. Saliva in Ingestive Behavior Research: Association with Oral Sensory Perception and Food Intake. In Saliva in Health and Disease; Tvarijonaviciute, A., Martinez-Subiela, S., Lopez-Jornet, P., Lamy, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 23–48. [Google Scholar]
- Barranco, T.; Rubio, C.P.; Tvarijonaviciute, A.; Rubio, M.; Damia, E.; Lamy, E.; Cugat, R.; Cerón, J.J.; Tecles, F.; Escribano, D. Changes of salivary biomarkers under different storage conditions: Effects of temperature and length of storage. Biochem. Med. 2019, 29, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Cantos-Barreda, A.; Escribano, D.; Egui, A.; Thomas, M.C.; López, M.C.; Tecles, F.; Bernal, L.J.; Cerón, J.J.; Martínez-Subiela, S. One-year follow-up of anti-Leishmania antibody concentrations in serum and saliva from experimentally infected dogs. Int. J. Parasitol. 2019, 49, 893–900. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).