Relationships among Retinal Nonperfusion, Neovascularization, and Vascular Endothelial Growth Factor Levels in Quiescent Proliferative Diabetic Retinopathy
Abstract
1. Introduction
2. Methods
2.1. Patients
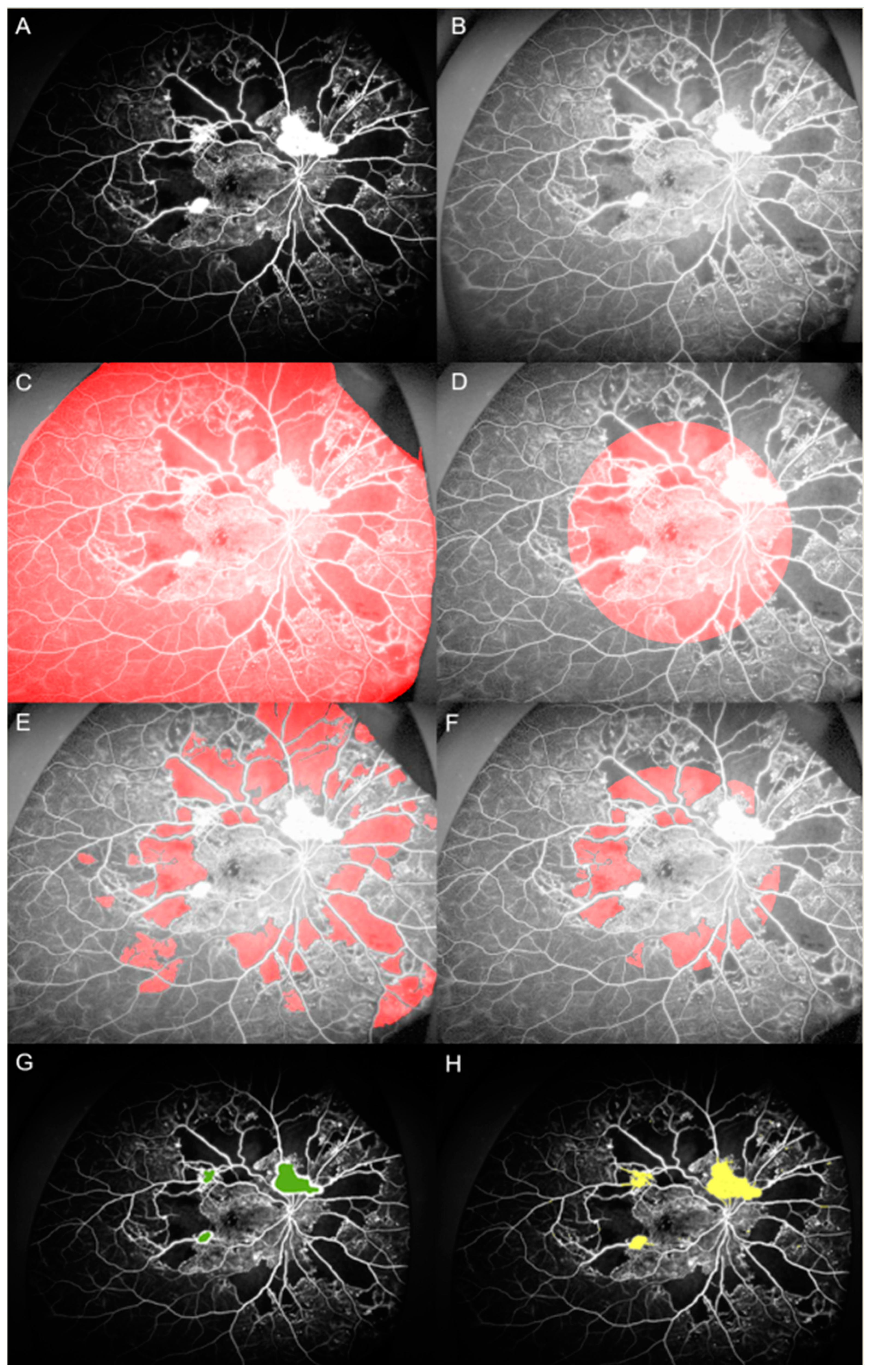
2.2. Image Analysis
2.3. Cytokine Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Features
3.2. Nonperfusion Areas, Neovascularization Areas, and Numbers
3.3. Relationships Among Nonperfusion Areas, Neovascularization Areas and Numbers, and VEGF
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic retinopathy. Lancet 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Grauslund, J.; Green, A.; Sjolie, A.K. Blindness in a 25-year follow-up of a population-based cohort of Danish type 1 diabetic patients. Ophthalmology 2009, 116, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kobayashi, Y.; Muraoka, K. Midperipheral Fundus Involvement in Diabetic Retinopathy. Ophthalmology 1981, 88, 601–612. [Google Scholar] [CrossRef]
- Silva, P.S.; Dela Cruz, A.J.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.D.; Aiello, L.M.; Sun, J.K.; Aiello, L.P. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Bresnick, G.H.; Palta, M. Predicting progression to severe proliferative diabetic retinopathy. Arch. Ophthalmol. 1987, 105, 810–814. [Google Scholar] [CrossRef]
- Klein, R.; Lee, K.E.; Gangnon, R.E.; Klein, B.E. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology 2010, 117, 63–70. [Google Scholar] [CrossRef]
- Fong, D.S.; Aiello, L.P.; Ferris, F.L., III; Klein, R. Diabetic retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef]
- Merin, S.; Ber, I.; Ivry, M. Retinal ischemia (capillary nonperfusion) and retinal neovascularization in patients with diabetic retinopathy. Ophthalmology 1978, 177, 140–145. [Google Scholar] [CrossRef]
- Oliver, S.C.; Schwartz, S.D. Peripheral vessel leakage (PVL): A new angiographic finding in diabetic retinopathy identified with ultra wide-field fluorescein angiography. Semin. Ophthalmol. 2010, 25, 27–33. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Soliman, A.Z.; Aiello, L.M.; Aiello, L.P. Peripheral lesions identified by mydriatic ultrawide field imaging: Distribution and potential impact on diabetic retinopathy severity. Ophthalmology 2013, 120, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.M.; Aaker, G.D.; Parlitsis, G.; Cho, M.; D’Amico, D.J.; Kiss, S. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina 2012, 32, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.L.; Ashir, A.; Nguyen, B.J.; Nudleman, E. Quantification of Retinal Nonperfusion Associated With Posterior Segment Neovascularization in Diabetic Retinopathy Using Ultra-Widefield Fluorescein Angiography. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.; Ramu, J.; Chan, E.W.; Bainbridge, J.W.; Hykin, P.G.; Talks, S.J.; Sivaprasad, S. Retinal Nonperfusion Characteristics on Ultra-Widefield Angiography in Eyes With Severe Nonproliferative Diabetic Retinopathy and Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.R.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Non-perfusion and Neovascularization on Ultra-Wide Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am J Ophthalmol 2019. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Adamis, A.P.; Shima, D.T.; D’Amore, P.A.; Moulton, R.S.; O’Reilly, M.S.; Folkman, J.; Dvorak, H.F.; Brown, L.F.; Berse, B.; et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994, 145, 574–584. [Google Scholar] [CrossRef]
- Witmer, A.N.; Vrensen, G.F.; Van Noorden, C.J.; Schlingemann, R.O. Vascular endothelial growth factors and angiogenesis in eye disease. Prog. Retin. Eye Res. 2003, 22, 1–29. [Google Scholar] [CrossRef]
- Aiello, L.P.; Avery, R.L.; Arrigg, P.G.; Keyt, B.A.; Jampel, H.D.; Shah, S.T.; Pasquale, L.R.; Thieme, H.; Iwamoto, M.A.; Park, J.E.; et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994, 331, 1480–1487. [Google Scholar] [CrossRef]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Tolentino, M.J.; Miller, J.W.; Gragoudas, E.S.; Jakobiec, F.A.; Flynn, E.; Chatzistefanou, K.; Ferrara, N.; Adamis, A.P. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 1996, 103, 1820–1828. [Google Scholar] [CrossRef]
- Ishida, S.; Usui, T.; Yamashiro, K.; Kaji, Y.; Amano, S.; Ogura, Y.; Hida, T.; Oguchi, Y.; Ambati, J.; Miller, J.W.; et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J. Exp. Med. 2003, 198, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Xin, X.; Jee, K.; Babapoor-Farrokhran, S.; Kashiwabuchi, F.; Ma, T.; Bhutto, I.; Hassan, S.J.; Daoud, Y.; Baranano, D.; et al. VEGF secreted by hypoxic Muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes 2013, 62, 3863–3873. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, M.J.; McLeod, D.S.; Taomoto, M.; Otsuji, T.; Adamis, A.P.; Lutty, G.A. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am. J. Ophthalmol. 2002, 133, 373–385. [Google Scholar] [CrossRef]
- Gao, G.; Li, Y.; Zhang, D.; Gee, S.; Crosson, C.; Ma, J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001, 489, 270–276. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991, 98, 823–833. [Google Scholar] [CrossRef]
- Cardillo Piccolino, F.; Zingirian, M.; Mosci, C. Classification of proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Rasta, S.H.; Nikfarjam, S.; Javadzadeh, A. Detection of retinal capillary nonperfusion in fundus fluorescein angiogram of diabetic retinopathy. Bioimpacts BI 2015, 5, 183–190. [Google Scholar] [CrossRef]
- Rafael, C.G.; Richard, E.W. Digital Image Processing, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Niki, T.; Muraoka, K.; Shimizu, K. Distribution of capillary nonperfusion in early-stage diabetic retinopathy. Ophthalmology 1984, 91, 1431–1439. [Google Scholar] [CrossRef]
- Machalinska, A.; Mozolewska-Piotrowska, K.; Czepita, M.; Spoz, W.; Dzieciolowska, M.; Kubasik-Kladna, K.; Szmatloch, K.; Lubinski, W.; Safranow, K.; Pius-Sadowska, E. Aqueous levels of VEGF correlate with retinal non-perfusion areas in patients with diabetic macular edema and macular edema secondary to central retinal vein occlusion. Klinika Oczna 2016, 117, 225–229. [Google Scholar]
- Kwon, S.H.; Shin, J.P.; Kim, I.T.; Park, D.H. Aqueous Levels of Angiopoietin-like 4 and Semaphorin 3E Correlate with Nonperfusion Area and Macular Volume in Diabetic Retinopathy. Ophthalmology 2015, 122, 968–975. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Exploring the various aspects of the pathological role of vascular endothelial growth factor (VEGF) in diabetic retinopathy. Pharmacol. Res. 2015, 99, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Wykoff, C.C.; Shapiro, H.; Rubio, R.G.; Ehrlich, J.S. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology 2014, 121, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Elman, M.J.; Aiello, L.P.; Beck, R.W.; Bressler, N.M.; Bressler, S.B.; Edwards, A.R.; Ferris, F.L., 3rd; Friedman, S.M.; Glassman, A.R.; Miller, K.M.; et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010, 117, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.S.; Domalpally, A.; Hopkins, J.J.; Wong, P.; Ehrlich, J.S. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch. Ophthalmol. 2012, 130, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Korobelnik, J.F.; Do, D.V.; Schmidt-Erfurth, U.; Boyer, D.S.; Holz, F.G.; Heier, J.S.; Midena, E.; Kaiser, P.K.; Terasaki, H.; Marcus, D.M.; et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014, 121, 2247–2254. [Google Scholar] [CrossRef]
- Silva, P.S.; Sun, J.K. Proliferative Diabetic Retinopathy. In Retina, 5th ed.; Ryan, S.J., Ed.; Saunders/Elsevier: London, UK; New York, NY, USA, 2013; p. 334. [Google Scholar]
- Couturier, A.; Rey, P.-A.; Erginay, A.; Lavia, C.; Bonnin, S.; Dupas, B.; Gaudric, A.; Tadayoni, R. Widefield OCT-Angiography and Fluorescein Angiography Assessments of Nonperfusion in Diabetic Retinopathy and Edema Treated with Anti-Vascular Endothelial Growth Factor. Ophthalmology 2019, 126, 1685–1694. [Google Scholar] [CrossRef]
- Tonello, M.; Costa, R.A.; Almeida, F.P.; Barbosa, J.C.; Scott, I.U.; Jorge, R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol. 2008, 86, 385–389. [Google Scholar] [CrossRef]
- Figueira, J.; Fletcher, E.; Massin, P.; Silva, R.; Bandello, F.; Midena, E.; Varano, M.; Sivaprasad, S.; Eleftheriadis, H.; Menon, G.; et al. Ranibizumab Plus Panretinal Photocoagulation versus Panretinal Photocoagulation Alone for High-Risk Proliferative Diabetic Retinopathy (PROTEUS Study). Ophthalmology 2018, 125, 691–700. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef]
- Nicholson, L.; Crosby-Nwaobi, R.; Vasconcelos, J.C.; Prevost, A.T.; Ramu, J.; Riddell, A.; Bainbridge, J.W.; Hykin, P.G.; Sivaprasad, S. Mechanistic Evaluation of Panretinal Photocoagulation Versus Aflibercept in Proliferative Diabetic Retinopathy: CLARITY Substudy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4277–4284. [Google Scholar] [CrossRef]
- Noma, H.; Funatsu, H.; Mimura, T.; Harino, S.; Hori, S. Aqueous humor levels of vasoactive molecules correlate with vitreous levels and macular edema in central retinal vein occlusion. Eur. J. Ophthalmol. 2010, 20, 402–409. [Google Scholar] [CrossRef] [PubMed]

| Demographic Features | Mean ± SD | Range |
|---|---|---|
| Age (years) | 54.04 ± 9.33 | 36–76 |
| Gender (male/female) | 23/24 (49) | |
| Hypertension (n) (%) | 37 (79) | |
| DM duration (year) | 14.98 ± 8.83 | 1–50 |
| BP systolic (mmHg) | 141.43 ± 21.36 | 115–183 |
| BP diastolic (mmHg) | 78.57 ± 8.73 | 60–97 |
| BCVA (LogMAR) | 0.23 ± 0.34 | 0–1.70 |
| Central macular thickness (um) | 281.77 ± 41.22 | 203–367 |
| Glucose (mg/dL) | 226.88 ± 102.5 | 88–415 |
| BUN (mg/dL) | 15.36 ± 5.11 | 8.7–34.3 |
| Cr (mg/dL) | 0.95 ± 0.53 | 0.46–2.84 |
| HbA1c (%) | 8.6 ± 2.25 | 5.5–13.6 |
| Angiographic Parameters | Mean ± SD | Range |
|---|---|---|
| Nonperfusion area, total (DA) | 73.49 ± 61.63 | 0.18–225.96 |
| Nonperfusion area, posterior pole (DA) | 4.69 ± 5.42 | 0–25.19 |
| Nonperfusion area, periphery (DA) | 68.8 ± 59.35 | 0–218.41 |
| Number of neovascularization | 4.59 ± 4.3 | 1–18.5 |
| Neovascularization area (DA) | 2.91 ± 4.06 | 0.05–16.5 |
| NVD (n) (%) | 23 (49) |
| NP Area, Total (DA) | NP Area, Posterior Pole (DA) | NP Area, Periphery (DA) | NV Area (DA) | Number of NV | NVD | ||
|---|---|---|---|---|---|---|---|
| NP area, total (DA) | Pearson’s Correlation | 0.457 ** | 0.997 ** | 0.436 ** | 0.476 ** | 0.243 | |
| p-value | 0.001 | 0.000 | 0.002 | 0.001 | 0.100 | ||
| NP area, posterior pole (DA) | Pearson’s Correlation | 0.457 ** | 0.383 ** | 0.310 * | 0.043 | 0.317 * | |
| p-value | 0.001 | 0.008 | 0.034 | 0.773 | 0.030 | ||
| NP area, periphery (DA) | Pearson’s Correlation | 0.997 ** | 0.383 ** | 0.425 ** | 0.490 ** | 0.223 | |
| p-value | 0.000 | 0.008 | 0.003 | 0.000 | 0.131 | ||
| NV area (DA) | Pearson’s Correlation | 0.436 ** | 0.310 * | 0.425 ** | 0.615 ** | 0.629 ** | |
| p-value | 0.002 | 0.034 | 0.003 | 0.000 | 0.000 | ||
| Number of NV | Pearson’s Correlation | 0.476 ** | 0.043 | 0.490 ** | 0.615 ** | 0.295 * | |
| p-value | 0.001 | 0.773 | 0.000 | 0.000 | 0.044 | ||
| NVD | Chi-square | 0.243 | 0.317 * | 0.223 | 0.629 ** | 0.295 * | |
| p-value | 0.100 | 0.030 | 0.131 | 0.000 | 0.044 |
| Angiographic Parameters | NP Area, Total (DA) | NP Area, Posterior Pole (DA) | NP Area, Periphery (DA) | NV Area (DA) | Number of NV | NVD | |
|---|---|---|---|---|---|---|---|
| VEGF (pg/mL) | Pearson’s Correlation | 0.575 ** | 0.422 ** | 0.558 ** | 0.362 * | 0.210 | 0.382 ** |
| Sig. (2-tailed) | 0.000 | 0.003 | 0.000 | 0.012 | 0.156 | 0.008 | |
| Clinical Factors | VEGF (pg/mL) | |
|---|---|---|
| Pearson’s Correlation | p-Value | |
| Age | −0.136 | 0.361 |
| Gender | −0.215 | 0.146 |
| HBP | 0.055 | 0.714 |
| BP, systolic | 0.095 | 0.550 |
| BP, diastolic | 0.256 | 0.102 |
| DM duration | −0.221 | 0.160 |
| BCVA | 0.156 | 0.296 |
| CMT | 0.115 | 0.441 |
| Glucose | −0.054 | 0.739 |
| BUN | 0.048 | 0.764 |
| Cr | −0.187 | 0.247 |
| HbA1c | 0.048 | 0.769 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ra, H.; Park, J.H.; Baek, J.U.; Baek, J. Relationships among Retinal Nonperfusion, Neovascularization, and Vascular Endothelial Growth Factor Levels in Quiescent Proliferative Diabetic Retinopathy. J. Clin. Med. 2020, 9, 1462. https://doi.org/10.3390/jcm9051462
Ra H, Park JH, Baek JU, Baek J. Relationships among Retinal Nonperfusion, Neovascularization, and Vascular Endothelial Growth Factor Levels in Quiescent Proliferative Diabetic Retinopathy. Journal of Clinical Medicine. 2020; 9(5):1462. https://doi.org/10.3390/jcm9051462
Chicago/Turabian StyleRa, Ho, Jae Hyun Park, Jin Uk Baek, and Jiwon Baek. 2020. "Relationships among Retinal Nonperfusion, Neovascularization, and Vascular Endothelial Growth Factor Levels in Quiescent Proliferative Diabetic Retinopathy" Journal of Clinical Medicine 9, no. 5: 1462. https://doi.org/10.3390/jcm9051462
APA StyleRa, H., Park, J. H., Baek, J. U., & Baek, J. (2020). Relationships among Retinal Nonperfusion, Neovascularization, and Vascular Endothelial Growth Factor Levels in Quiescent Proliferative Diabetic Retinopathy. Journal of Clinical Medicine, 9(5), 1462. https://doi.org/10.3390/jcm9051462






