Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum
Abstract
1. Introduction
2. Experimental Section
3. Results
3.1. General Data
3.2. Skin and Vascular Uptake of 18F-NaF
3.3. Vascular Calcium Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uitto, J.; Li, Q. Vascular Mineralization in Pseudoxanthoma Elasticum: Etidronate to the Rescue? J. Am. Coll. Cardiol. 2018, 71, 1127–1129. [Google Scholar] [CrossRef]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef] [PubMed]
- Kauffenstein, G.; Yegutkin, G.G.; Khiati, S.; Pomozi, V.; Le Saux, O.; Leftheriotis, G.; Lenaers, G.; Henrion, D.; Martin, L. Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2018, 138, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Pfendner, E.G.; Vanakker, O.M.; Terry, S.F.; Vourthis, S.; McAndrew, P.E.; McClain, M.R.; Fratta, S.; Marais, A.S.; Hariri, S.; Coucke, P.J.; et al. Mutation detection in the ABCC6 gene and genotype-phenotype analysis in a large international case series affected by pseudoxanthoma elasticum. J. Med. Genet. 2007, 44, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Blum, R.R.; Singer, G.K.; Stern, D.K.; Emanuel, P.O.; Fuchs, W.; Phelps, R.G.; Terry, S.F.; Lebwohl, M.G. A randomized controlled trial of oral phosphate binders in the treatment of pseudoxanthoma elasticum. J. Am. Acad. Dermatol. 2011, 65, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Küçükosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; van de Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef]
- Kavukcuoglu, N.B.; Li, Q.; Pleshko, N.; Uitto, J. Connective tissue mineralization in Abcc6-/- mice, a model for pseudoxanthoma elasticum. Matrix Biol. 2012, 31, 246–252. [Google Scholar] [CrossRef][Green Version]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef]
- Oudkerk, S.F.; de Jong, P.A.; Blomberg, B.A.; Scholtens, A.M.; Mali, W.P.T.M.; Spiering, W. Whole-Body Visualization of Ectopic Bone Formation of Arteries and Skin in Pseudoxanthoma Elasticum. JACC Cardiovasc. Imaging 2016, 9, 755–756. [Google Scholar] [CrossRef]
- Mention, P.-J.; Lacoeuille, F.; Leftheriotis, G.; Martin, L.; Omarjee, L. 18F-Flurodeoxyglucose and 18F-Sodium Fluoride Positron Emission Tomography/Computed Tomography Imaging of Arterial and Cutaneous Alterations in Pseudoxanthoma Elasticum. Circ. Cardiovasc. Imaging 2018, 11, e007060. [Google Scholar] [CrossRef]
- Kranenburg, G.; de Jong, P.A.; Bartstra, J.W.; Lagerweij, S.J.; Lam, M.G.; Ossewaarde-van Norel, J.; Risseeuw, S.; van Leeuwen, R.; Imhof, S.M.; Verhaar, H.J.; et al. Etidronate for Prevention of Ectopic Mineralization in Patients with Pseudoxanthoma Elasticum. J. Am. Coll. Cardiol. 2018, 71, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.B.; van Dijk, M.R.; de Jong, P.T.V.M. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med. Genet. Part A 2010, 152, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.C.; Fowkes, F.G.R. The Edinburgh Claudication Questionnaire—An Improved Version of the Who Rose Questionnaire for Use in Epidemiologic Surveys. J. Clin. Epidemiol. 1992, 45, 1101–1109. [Google Scholar] [PubMed]
- European Pharmacopoeia Commission; EDQM; Council of Europe. Sodium fluoride (18F) injection. In European Pharmacopoeia; European Directorate for the Quality of Medicines, Ed.; EDQM: Strasbourg, France, 2014; pp. 1079–1080. [Google Scholar]
- Lamare, F. Standardization in PET/CT Imaging Using EARL FDG-PET/CT Accreditation. Available online: http://earl.eanm.org/html/img/pool/Article_EARL_Accreditation_Dr_Lamare.pdf (accessed on 5 May 2020).
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.J.A.; Lindner, O.; Sciagra, R.; Agostini, D.; Übleis, C.; Gimelli, A.; Hacker, M. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.F.; Myers, K.S.; Bansilal, S.; Machac, J.; Pinto, C.A.; Tong, C.; Rafique, A.; Hargeaves, R.; Farkouh, M.; Fuster, V.; et al. Atherosclerosis Inflammation Imaging with 18F-FDG PET: Carotid, Iliac, and Femoral Uptake Reproducibility, Quantification Methods, and Recommendations. J. Nucl. Med. 2008, 49, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Chow, M.W.L.; Joshi, N.V.; Williams, M.C.; Jones, C.; Fletcher, A.M.; Richardson, H.; White, A.; McKillop, G.; van Beek, E.J.R.; et al. Coronary Arterial 18F-Sodium Fluoride Uptake. J. Am. Coll. Cardiol. 2012, 59, 1539–1548. [Google Scholar] [CrossRef]
- Germain, D.P. Pseudoxanthoma elasticum. Orphanet J. Rare Dis. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Kranenburg, G.; de Jong, P.A.; Mali, W.P.; Attrach, M.; Visseren, F.L.J.; Spiering, W. Prevalence and severity of arterial calcifications in pseudoxanthoma elasticum (PXE) compared to hospital controls. Novel insights into the vascular phenotype of PXE. Atherosclerosis 2017, 256, 7–14. [Google Scholar] [CrossRef]
- Gheduzzi, D.; Sammarco, R.; Quaglino, D.; Bercovitch, L.; Terry, S.; Taylor, W.; Ronchetti, I.P. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastruct. Pathol. 2003, 27, 375–384. [Google Scholar] [CrossRef]
- Lefthériotis, G.; Omarjee, L.; Le Saux, O.; Henrion, D.; Abraham, P.; Prunier, F.; Willoteaux, S.; Martin, L. The vascular phenotype in Pseudoxanthoma elasticum and related disorders: Contribution of a genetic disease to the understanding of vascular calcification. Front. Genet. 2013, 4, 4. [Google Scholar] [CrossRef]
- Kamenskiy, A.; Poulson, W.; Sim, S.; Reilly, A.; Luo, J.; MacTaggart, J. Prevalence of Calcification in Human Femoropopliteal Arteries and its Association with Demographics, Risk Factors, and Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e48–e57. [Google Scholar] [CrossRef] [PubMed]
- Ishiwata, Y.; Kaneta, T.; Nawata, S.; Hino-Shishikura, A.; Yoshida, K.; Inoue, T. Quantification of temporal changes in calcium score in active atherosclerotic plaque in major vessels by18F-sodium fluoride PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, I.; Elgazzar, A.H.; Sarikaya, A.; Alfeeli, M. Normal bone and soft tissue distribution of fluorine-18-sodium fluoride and artifacts on18F-NaF PET/CT bone scan: A pictorial review. Nucl. Med. Commun. 2017, 38, 810–819. [Google Scholar] [CrossRef] [PubMed]
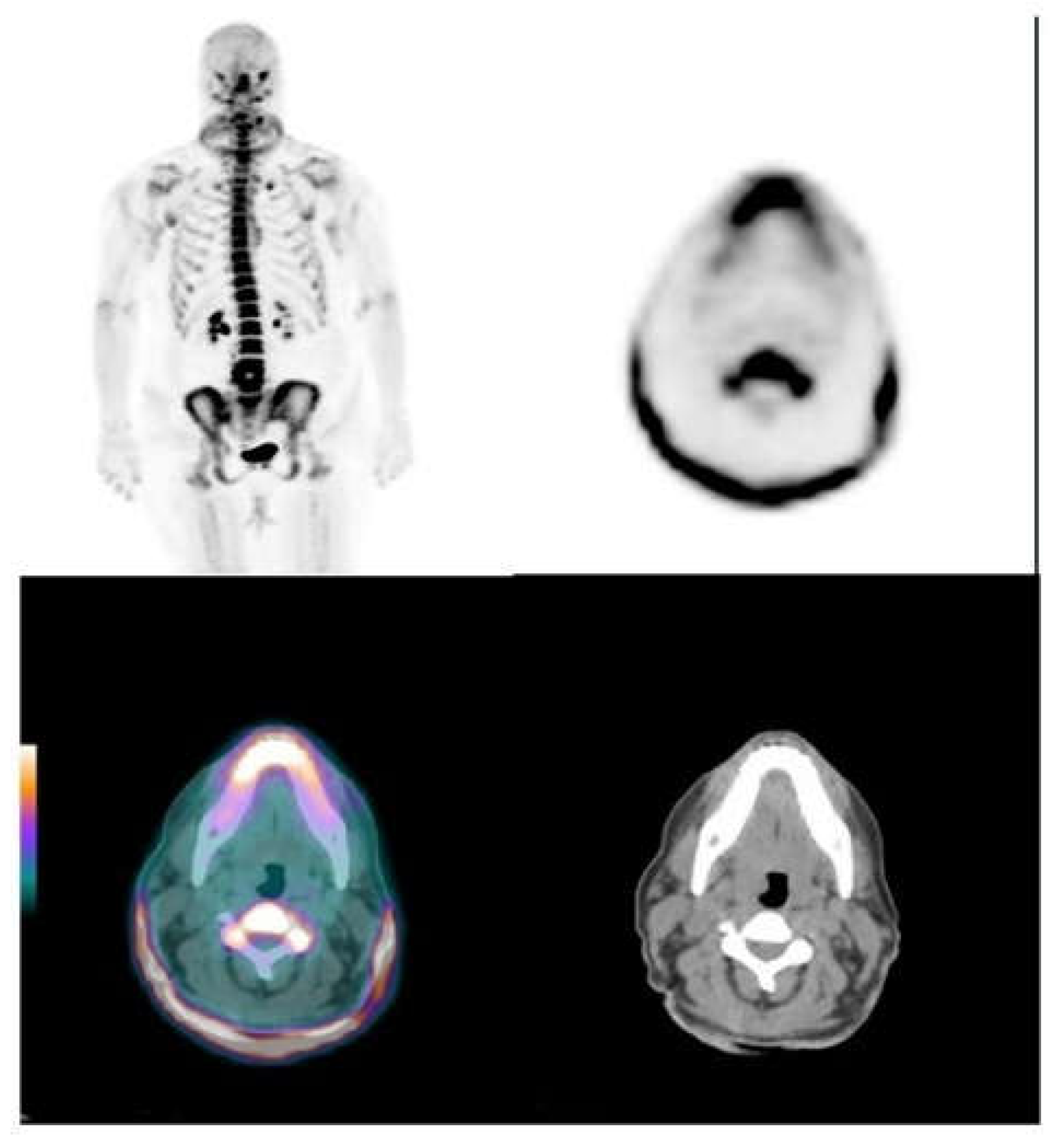
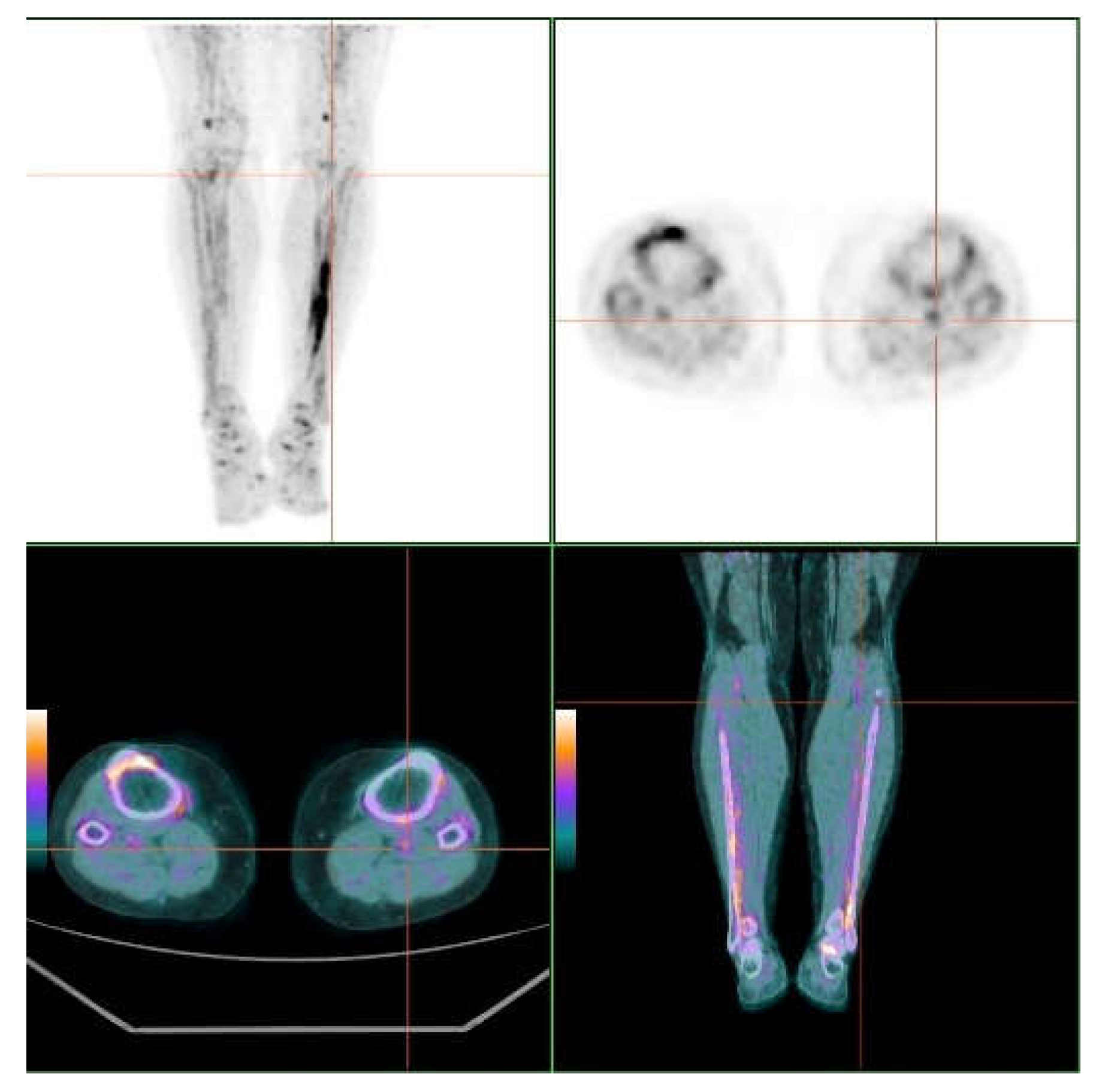
| Territory | TBRmax | TBRmean |
|---|---|---|
| Lumbar skin SUV | 0.54 (0.47–0.81) | 0.39 (0.31–0.54) |
| Neck | ||
| Right | 5.91 (3.68–13.39) | 4.14 (2.41–9.43) |
| Left | 6.67 (3.32–13.4) | 3.53 (2.20–8.96) |
| Axillae | ||
| Right | 5.89 (3.47–9.93) | 3.67 (2.30–6.11) |
| Left | 5.00 (3.13–9.97) | 3.19 (2.00–3,35) |
| Elbow | ||
| Right | 2.32 (1.08–4.45) | 1.72 (0.97–3.35) |
| Left | 1.86 (0.99–5.35) | 1.44 (0.70–3.67) |
| Groin | ||
| Right | 4.09 (1.42–6.14) | 2.93 (1.15–4.04) |
| Left | 2.08 (1.47–5.69) | 1.54 (1.09–4.10) |
| Popliteal | ||
| Right | 3.24 (2.52–4.93) | 2.16 (1.56–3.51) |
| Left | 3.21 (2.19–4.89) | 2.10 (1.56–3.51) |
| AVERAGE | 4.22 (2.44–7.64) | 2.79 (1.71–5.32) |
| PHENODEX | |||
|---|---|---|---|
| < p50 | > p50 | Sig | |
| Av Vasc TBRmax | 2.03 (1.80–275) | 2.60 (2.24–2.84) | NS |
| Av Vasc TBRmean | 1.73 (1.62–2.32) | 2.16 (1.74–2.37) | NS |
| Av Skin TBRmax | 4.38 (2.29–9.30) | 4.07 (2.92-–6.38) | NS |
| Av Skin TBRmean | 2.64 (1.52–6.23) | 2.94 (2.05–4.63) | NS |
| Av calcium score | 1.50 (0.25–41) | 301 (159–541) | p < 0.05 |
| Territory | Number | Calcium Score | Calcium Volume | Calcium Mass |
|---|---|---|---|---|
| Carotid | 7 | |||
| Right | 0 (0–33,6) | 0 (0–29,0) | 0 (0–18,4) | |
| Left | 0 (0–18.4) | 0 (0–20,7) | 0 (0–3,4) | |
| Ascending aorta | 3 | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Aortic arch | 10 | 9 (0–54) | 24 (0–75) | 2.9 (0–12) |
| Descending aorta | 6 | 0 (0–168) | 0 (0–186) | 0 (0–33) |
| Abdominal aorta | 7 | 38 (0–447) | 36 (0–409) | 7.8 (0–105) |
| Iliac | 8 | |||
| Right | 0 (0–517) | 0 (0–436) | 0 (0–107) | |
| Left | 0 (0–279) | 0 (0–236) | 0 (0–62) | |
| Femoral | 10 | |||
| Right | 101 (0–684) | 137 (0–680) | 22 (0–143) | |
| Left | 82 (0–732) | 70 (0–693) | 14 (0–136) | |
| Popliteal | 12 | |||
| Right | 20 (0–493) | 43 (0.775) | 6 (0–116) | |
| Left | 21 (0–489) | 40 (0–745) | 5 (0–112) |
| Territory | TBRmax | TBRmean |
|---|---|---|
| SUV/cava | 0.88 (0.72–0.98) | 0.67 (0.48–0.76) |
| Carotid | ||
| Right | 1,74 (1.43–2.19) | 1,58 (1.27–1.97) |
| Left | 1.83 (1.53–2.35) | 1.64 (1.29-1.96) |
| Aorta | ||
| Ascending | 1.60 (1.41–1.89) | 1.26 (1.15–1.50) |
| Arch | 2.16 (1.72–2.34) | 1.69 (1.40–1.87) |
| Descending | 2.79 (2.19–3.22) | 2.21 (1.78–2.85) |
| Abdominal | 3.88 (3.36–4.64) | 3.31 (2.79–4.04) |
| Iliac | ||
| Right | 2.11 (1.54–2.92) | 1.72 (1.04–2.30) |
| Left | 1.93 (1.40–2.40) | 1.59 (1.20–2.01) |
| Femoral | ||
| Right | 2.83 (2.44–3.34) | 2.23 (1.84–2.86) |
| Left | 2.88 (2.26–3.70) | 2.34 (1.88–2.66) |
| Popliteal | ||
| Right | 1.73 (1.15–2.20) | 1.48 (0.98–1.74) |
| Left | 2.00 (1.31–2.60) | 1.52 (1.10–2.21) |
| AVERAGED | 2.53 (2.00–2.78) | 2.00 (1.64–2.32) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Cardo, A.; Lillo, E.; Murcia-Casas, B.; Carrillo-Linares, J.L.; García-Argüello, F.; Sánchez-Sánchez, P.; Rodriguez-Morata, A.; Baquero Aranda, I.; Sánchez-Chaparro, M.Á.; García-Fernández, M.; et al. Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum. J. Clin. Med. 2020, 9, 1393. https://doi.org/10.3390/jcm9051393
Gutierrez-Cardo A, Lillo E, Murcia-Casas B, Carrillo-Linares JL, García-Argüello F, Sánchez-Sánchez P, Rodriguez-Morata A, Baquero Aranda I, Sánchez-Chaparro MÁ, García-Fernández M, et al. Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum. Journal of Clinical Medicine. 2020; 9(5):1393. https://doi.org/10.3390/jcm9051393
Chicago/Turabian StyleGutierrez-Cardo, Antonio, Eugenia Lillo, Belén Murcia-Casas, Juan Luis Carrillo-Linares, Francisco García-Argüello, Purificación Sánchez-Sánchez, Alejandro Rodriguez-Morata, Isabel Baquero Aranda, Miguel Ángel Sánchez-Chaparro, María García-Fernández, and et al. 2020. "Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum" Journal of Clinical Medicine 9, no. 5: 1393. https://doi.org/10.3390/jcm9051393
APA StyleGutierrez-Cardo, A., Lillo, E., Murcia-Casas, B., Carrillo-Linares, J. L., García-Argüello, F., Sánchez-Sánchez, P., Rodriguez-Morata, A., Baquero Aranda, I., Sánchez-Chaparro, M. Á., García-Fernández, M., & Valdivielso, P. (2020). Skin and Arterial Wall Deposits of 18F-NaF and Severity of Disease in Patients with Pseudoxanthoma Elasticum. Journal of Clinical Medicine, 9(5), 1393. https://doi.org/10.3390/jcm9051393






