Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population: Patients and Controls
2.2. Cell Preparation and Activations
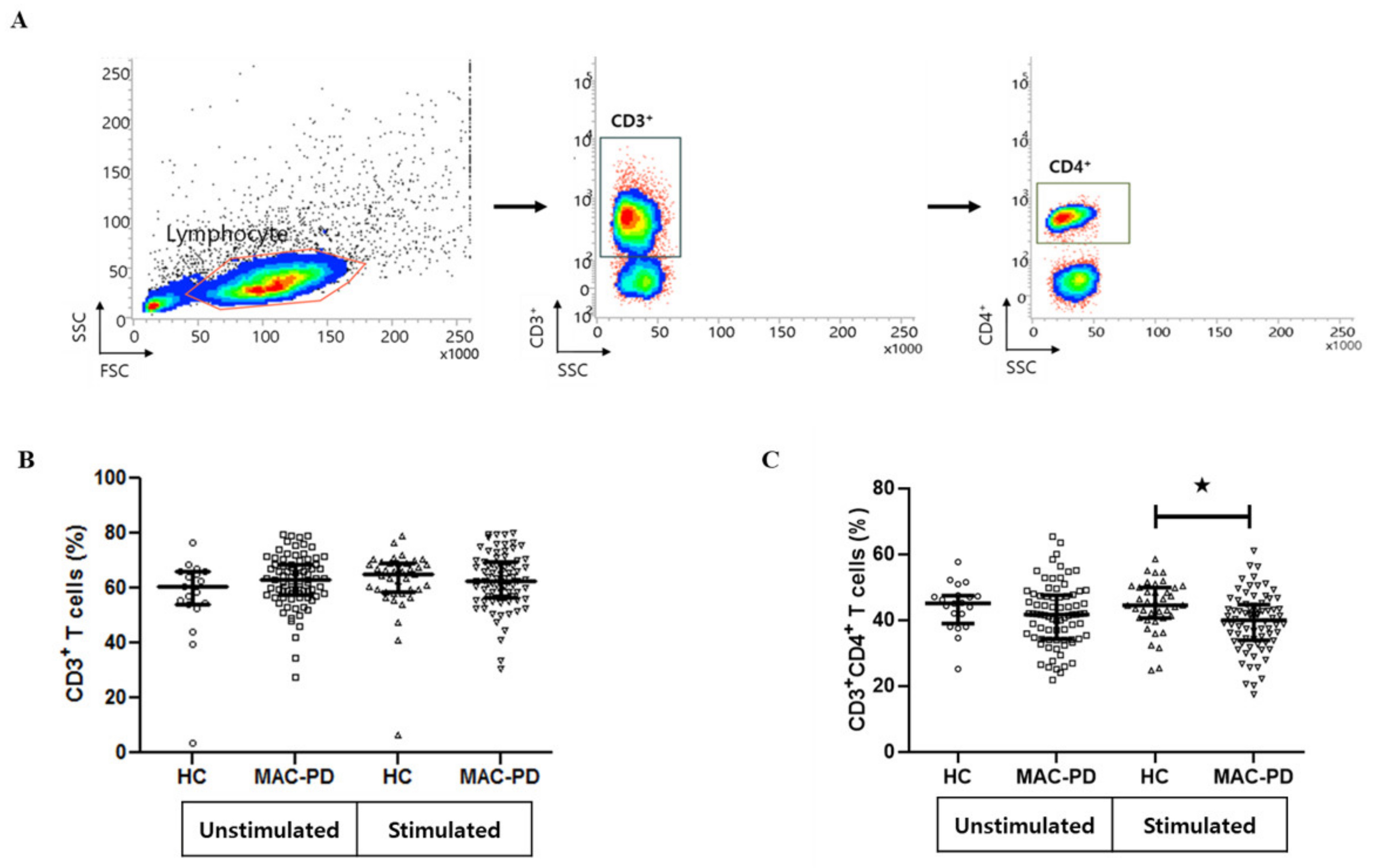
2.3. Flow Cytometric Analysis
2.4. Quantification of Cytokines
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Frequencies of Circulating CD4+ T Cell Subpopulations in MAC-PD Patients
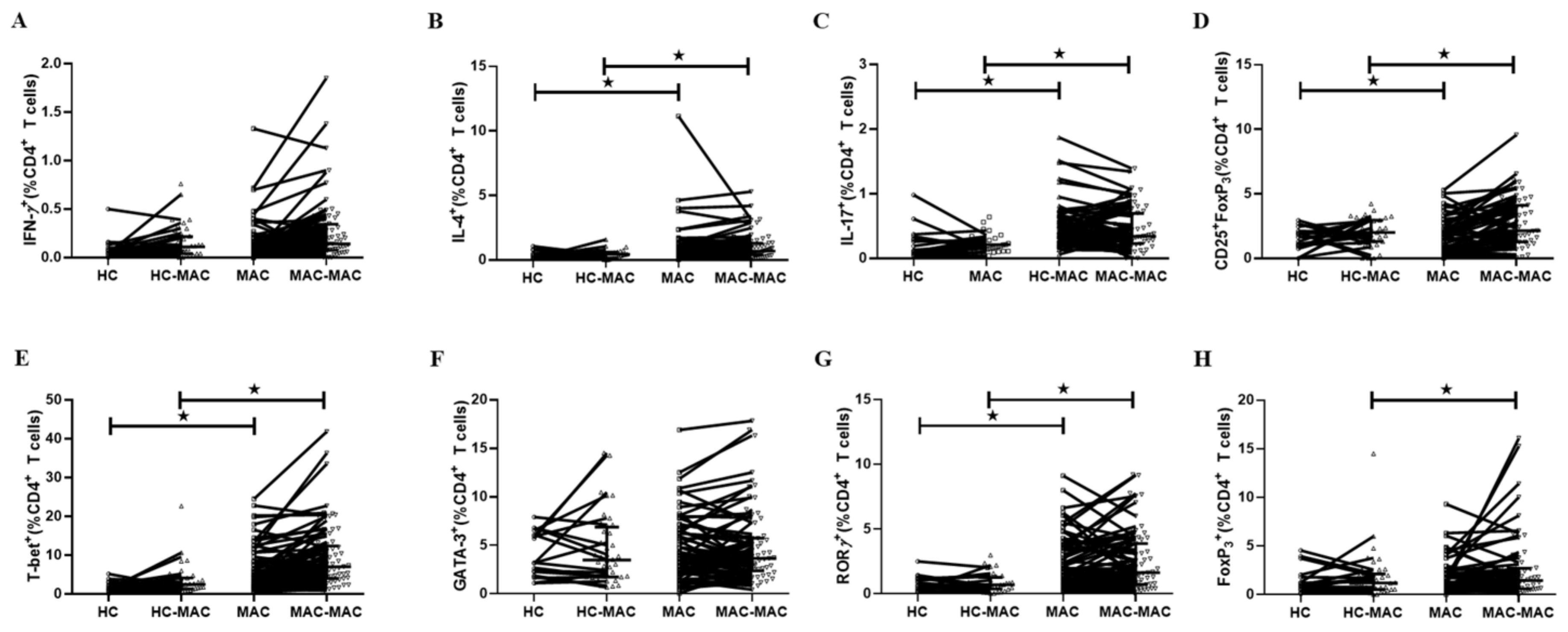
3.3. T Cell Cytokine Production in MAC-PD
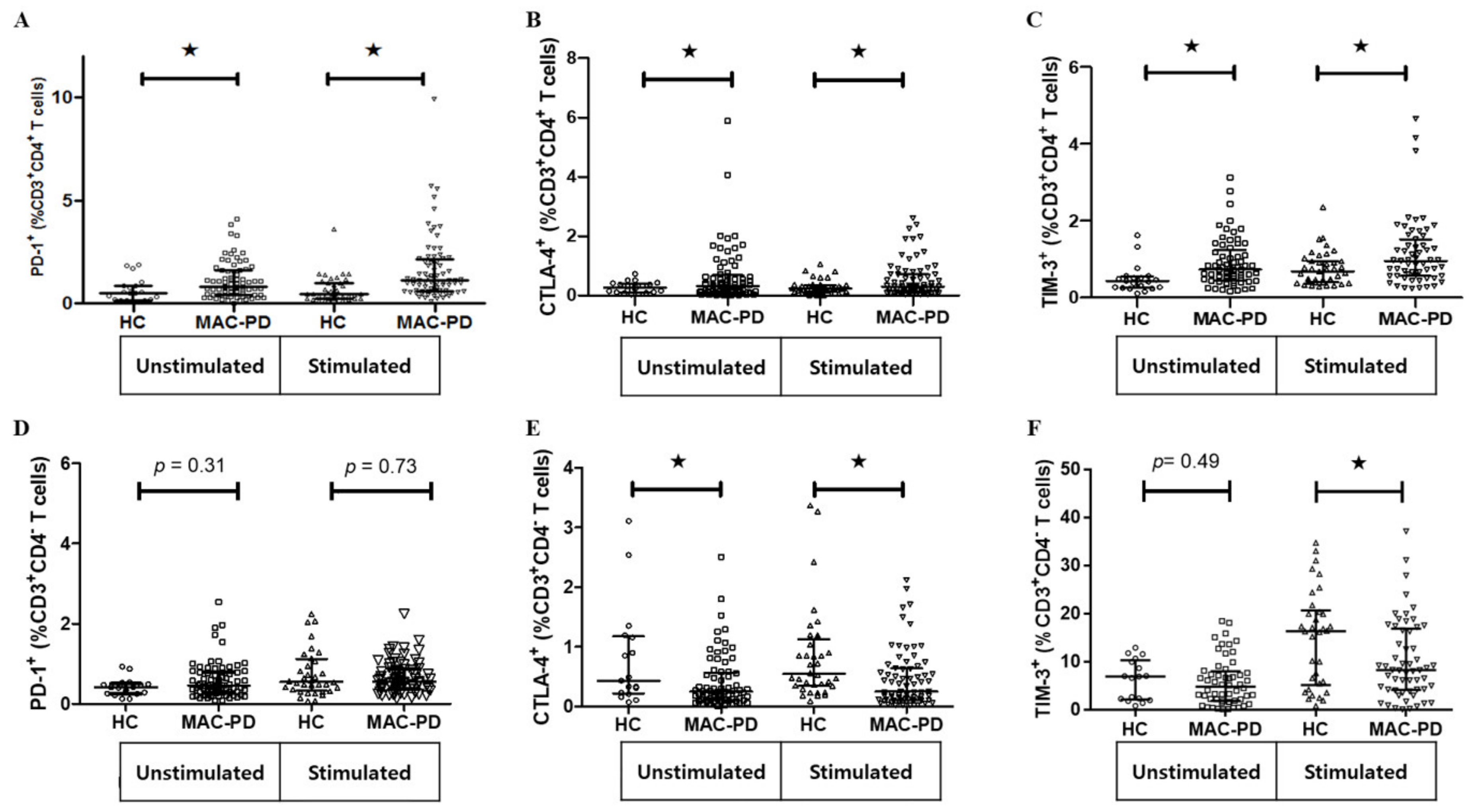
3.4. Expression of Multiple Inhibitory Receptors in Circulating CD4+ T Cells in MAC-PD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef]
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Henkle, E.; Aksamit, T.; Barker, A.; Daley, C.L.; Griffith, D.; Leitman, P.; Leitman, A.; Malanga, E.; Marras, T.K.; Olivier, K.N.; et al. Patient-centered research priorities for pulmonary nontuberculous mycobacteria (NTM) infection. An NTM research consortium workshop report. Ann. Am. Thorac. Soc. 2016, 13, S379–S384. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.R.; Knight, V.; Chan, E.D. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin. Chest Med. 2015, 36, 1–11. [Google Scholar] [CrossRef]
- Caruso, A.M.; Serbina, N.; Klein, E.; Triebold, K.; Bloom, B.R.; Flynn, J.L. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 1999, 162, 5407–5416. [Google Scholar] [PubMed]
- Mogues, T.; Goodrich, M.E.; Ryan, L.; LaCourse, R.; North, R.J. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001, 193, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Serbina, N.V.; Lazarevic, V.; Flynn, J.L. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J. Immunol. 2001, 167, 6991–7000. [Google Scholar] [CrossRef]
- Sundrud, M.S.; Nolan, M.A. Synergistic and combinatorial control of T cell activation and differentiation by transcription factors. Curr. Opin. Immunol. 2010, 22, 286–292. [Google Scholar] [CrossRef]
- Zhu, J.; Yamane, H.; Paul, W.E. Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 2010, 28, 445–489. [Google Scholar] [CrossRef]
- Chan, E.D.; Bai, X.; Kartalija, M.; Orme, I.M.; Ordway, D.J. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am. J. Respir. Cell Mol. Biol. 2010, 43, 387–393. [Google Scholar] [CrossRef]
- Lim, A.; Allison, C.; Price, P.; Waterer, G. Susceptibility to pulmonary disease due to Mycobacterium avium-intracellulare complex may reflect low IL-17 and high IL-10 responses rather than Th1 deficiency. Clin. Immunol. 2010, 137, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Mayer-Barber, K.D.; Sher, A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011, 4, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koh, W.J.; Kim, Y.H.; Jeong, B.H.; Park, H.Y.; Jeon, K.; Kim, J.S.; Cho, S.N.; Shin, S.J. Importance of reciprocal balance of T cell immunity in Mycobacterium abscessus complex lung disease. PLoS ONE 2014, 9, e109941. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, M.; Ishii, Y.; Yageta, Y.; Ohtsuka, S.; Ano, S.; Matsuno, Y.; Morishima, Y.; Yoh, K.; Takahashi, S.; Ogawa, K.; et al. Role of Th1/Th17 balance regulated by T-bet in a mouse model of Mycobacterium avium complex disease. J. Immunol. 2014, 192, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; van Ingen, J.; Ten Oever, J.; Merkus, P.J.; Ferwerda, G.; Netea, M.G.; Magis-Escurra, C.; Reijers, M.H.; van de Veerdonk, F.L. Deficient interleukin-17 production in response to Mycobacterium abscessus in cystic fibrosis. Eur. Respir. J. 2016, 47, 990–993. [Google Scholar] [CrossRef]
- Allie, N.; Alexopoulou, L.; Quesniaux, V.J.; Fick, L.; Kranidioti, K.; Kollias, G.; Ryffel, B.; Jacobs, M. Protective role of membrane tumour necrosis factor in the host’s resistance to mycobacterial infection. Immunology 2008, 125, 522–534. [Google Scholar] [CrossRef]
- Hoos, A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef]
- Kaufmann, D.E.; Kavanagh, D.G.; Pereyra, F.; Zaunders, J.J.; Mackey, E.W.; Miura, T.; Palmer, S.; Brockman, M.; Rathod, A.; Piechocka-Trocha, A.; et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007, 8, 1246–1254. [Google Scholar] [CrossRef]
- Bengsch, B.; Seigel, B.; Ruhl, M.; Timm, J.; Kuntz, M.; Blum, H.E.; Pircher, H.; Thimme, R. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010, 6, e1000947. [Google Scholar] [CrossRef]
- Raziorrouh, B.; Heeg, M.; Kurktschiev, P.; Schraut, W.; Zachoval, R.; Wendtner, C.; Wachtler, M.; Spannagl, M.; Denk, G.; Ulsenheimer, A.; et al. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS ONE 2014, 9, e105703. [Google Scholar] [CrossRef]
- Kaufmann, D.E.; Walker, B.D. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 2009, 182, 5891–5897. [Google Scholar] [CrossRef] [PubMed]
- Jurado, J.O.; Alvarez, I.B.; Pasquinelli, V.; Martinez, G.J.; Quiroga, M.F.; Abbate, E.; Musella, R.M.; Chuluyan, H.E.; Garcia, V.E. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J. Immunol. 2008, 181, 116–125. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.W.; Berry, M.P.; Graham, C.M.; Bloch, S.A.; Oni, T.; Wilkinson, K.A.; Wilkinson, R.J.; Kon, O.M.; Banchereau, J.; Chaussabel, D.; et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur. J. Immunol. 2011, 41, 1941–1947. [Google Scholar] [CrossRef] [PubMed]
- Lutzky, V.P.; Ratnatunga, C.N.; Smith, D.J.; Kupz, A.; Doolan, D.L.; Reid, D.W.; Thomson, R.M.; Bell, S.C.; Miles, J.J. Anomalies in T cell function are associated with Individuals at risk of Mycobacterium abscessus complex infection. Front. Immunol. 2018, 9, 1319. [Google Scholar] [CrossRef]
- Shu, C.C.; Wang, J.Y.; Wu, M.F.; Wu, C.T.; Lai, H.C.; Lee, L.N.; Chiang, B.L.; Yu, C.J. Attenuation of lymphocyte immune responses during Mycobacterium avium complex-induced lung disease due to increasing expression of programmed death-1 on lymphocytes. Sci. Rep. 2017, 7, 42004. [Google Scholar] [CrossRef]
- Koh, W.J.; Moon, S.M.; Kim, S.Y.; Woo, M.A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.S.; et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur. Respir. J. 2017, 50, 16. [Google Scholar] [CrossRef]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Yamane, H.; Zhu, J.; Paul, W.E. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J. Exp. Med. 2005, 202, 793–804. [Google Scholar] [CrossRef]
- Kumar, P. IFN gamma-producing CD4(+) T lymphocytes: The double-edged swords in tuberculosis. Clin. Transl. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Vankayalapati, R.; Wizel, B.; Samten, B.; Griffith, D.E.; Shams, H.; Galland, M.R.; Von Reyn, C.F.; Girard, W.M.; Wallace, R.J., Jr.; Barnes, P.F. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J. Infect. Dis. 2001, 183, 478–484. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kim, E.J.; Lee, S.H.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Koh, W.J. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung 2007, 185, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lyadova, I.V.; Panteleev, A.V. Th1 and Th17 Cells in tuberculosis: Protection, pathology, and biomarkers. Mediat. Inflamm. 2015, 2015, 854507. [Google Scholar] [CrossRef] [PubMed]
- Jasenosky, L.D.; Scriba, T.J.; Hanekom, W.A.; Goldfeld, A.E. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol. Rev. 2015, 264, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G. Immunity by equilibrium. Nat. Rev. Immunol. 2016, 16, 524–532. [Google Scholar] [CrossRef]
- Pfeffer, P.E.; Hopkins, S.; Cropley, I.; Lowe, D.M.; Lipman, M. An association between pulmonary Mycobacterium avium-intracellulare complex infections and biomarkers of Th2-type inflammation. Respir. Res. 2017, 18, 93. [Google Scholar] [CrossRef]
- Ivanov, S.; Bozinovski, S.; Bossios, A.; Valadi, H.; Vlahos, R.; Malmhall, C.; Sjostrand, M.; Kolls, J.K.; Anderson, G.P.; Linden, A. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am. J. Respir. Cell Mol. Biol. 2007, 36, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Wu, U.I.; Olivier, K.N.; Kuhns, D.B.; Fink, D.L.; Sampaio, E.P.; Zelazny, A.M.; Shallom, S.J.; Marciano, B.E.; Lionakis, M.S.; Holland, S.M. Patients with Idiopathic Pulmonary Nontuberculous Mycobacterial Disease Have Normal Th1/Th2 Cytokine Responses but Diminished Th17 Cytokine and Enhanced Granulocyte-Macrophage Colony-Stimulating Factor Production. Open Forum Infect. Dis. 2019, 6, ofz484. [Google Scholar] [CrossRef]
- Belkaid, Y.; Rouse, B.T. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005, 6, 353–360. [Google Scholar] [CrossRef]
- Pinheiro, R.O.; de Oliveira, E.B.; Dos Santos, G.; Sperandio da Silva, G.M.; de Andrade Silva, B.J.; Teles, R.M.; Milagres, A.; Sarno, E.N.; Dalcolmo, M.P.; Sampaio, E.P. Different immunosuppressive mechanisms in multi-drug-resistant tuberculosis and non-tuberculous mycobacteria patients. Clin. Exp. Immunol. 2013, 171, 210–219. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dey, A.B.; Mohan, A.; Mitra, D.K. Programmed death-1 receptor suppresses gamma-IFN producing NKT cells in human tuberculosis. Tuberculosis (Edinb) 2014, 94, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Lord, G.M.; Dardalhon, V.; Lee, D.H.; Sabatos-Peyton, C.A.; Glimcher, L.H.; Kuchroo, V.K. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 2010, 40, 859–866. [Google Scholar] [CrossRef]
- Crawford, A.; Angelosanto, J.M.; Kao, C.; Doering, T.A.; Odorizzi, P.M.; Barnett, B.E.; Wherry, E.J. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 2014, 40, 289–302. [Google Scholar] [CrossRef]
- Ye, B.; Liu, X.; Li, X.; Kong, H.; Tian, L.; Chen, Y. T-cell exhaustion in chronic hepatitis B infection: Current knowledge and clinical significance. Cell Death Dis. 2015, 6, e1694. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, P.; Liang, Y.; Zhou, Y.; Shen, H.; Fan, C.; Moorman, J.P.; Yao, Z.Q.; Jia, Z.; Zhang, Y. T-bet-mediated Tim-3 expression dampens monocyte function during chronic hepatitis C virus infection. Immunology 2017, 150, 301–311. [Google Scholar] [CrossRef]

| MAC-PD | HC | |
|---|---|---|
| (n = 71) | (n = 20) | |
| MAC species | ||
| M. avium | 30 (42.3) | |
| M. intracellulare | 41 (57.7) | |
| Age, years | 60.6 ± 9.6 | 46.3 ± 7.9 |
| Female | 37 (52.1) | 16 (22.5) |
| Radiologic feature | ||
| Nodular bronchiectatic form | 41 (57.7) | |
| Fibro-cavitary form | 30 (42.3) | |
| Comorbidities | ||
| Previous pulmonary tuberculosis | 27 (38.0) | |
| Chronic airway disease | 12 (16.9) | |
| Chronic pulmonary aspergillosis | 2 (2.8) | |
| Idiopathic pulmonary fibrosis | 1 (1.4) | |
| Diabetes | 7 (9.9) | |
| Chronic kidney disease | 1 (1.4) | |
| Chronic liver disease | 2 (2.8) | |
| Rheumatic disease | 2 (2.8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.A.; Ko, Y.; Shin, S.J.; Jhun, B.W. Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease. J. Clin. Med. 2020, 9, 1331. https://doi.org/10.3390/jcm9051331
Han SA, Ko Y, Shin SJ, Jhun BW. Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease. Journal of Clinical Medicine. 2020; 9(5):1331. https://doi.org/10.3390/jcm9051331
Chicago/Turabian StyleHan, Sun Ae, Yousang Ko, Sung Jae Shin, and Byung Woo Jhun. 2020. "Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease" Journal of Clinical Medicine 9, no. 5: 1331. https://doi.org/10.3390/jcm9051331
APA StyleHan, S. A., Ko, Y., Shin, S. J., & Jhun, B. W. (2020). Characteristics of Circulating CD4+ T Cell Subsets in Patients with Mycobacterium avium Complex Pulmonary Disease. Journal of Clinical Medicine, 9(5), 1331. https://doi.org/10.3390/jcm9051331





