Diagnostic Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Gastric Mesenchymal Tumors
Abstract
1. Introduction
2. Methods
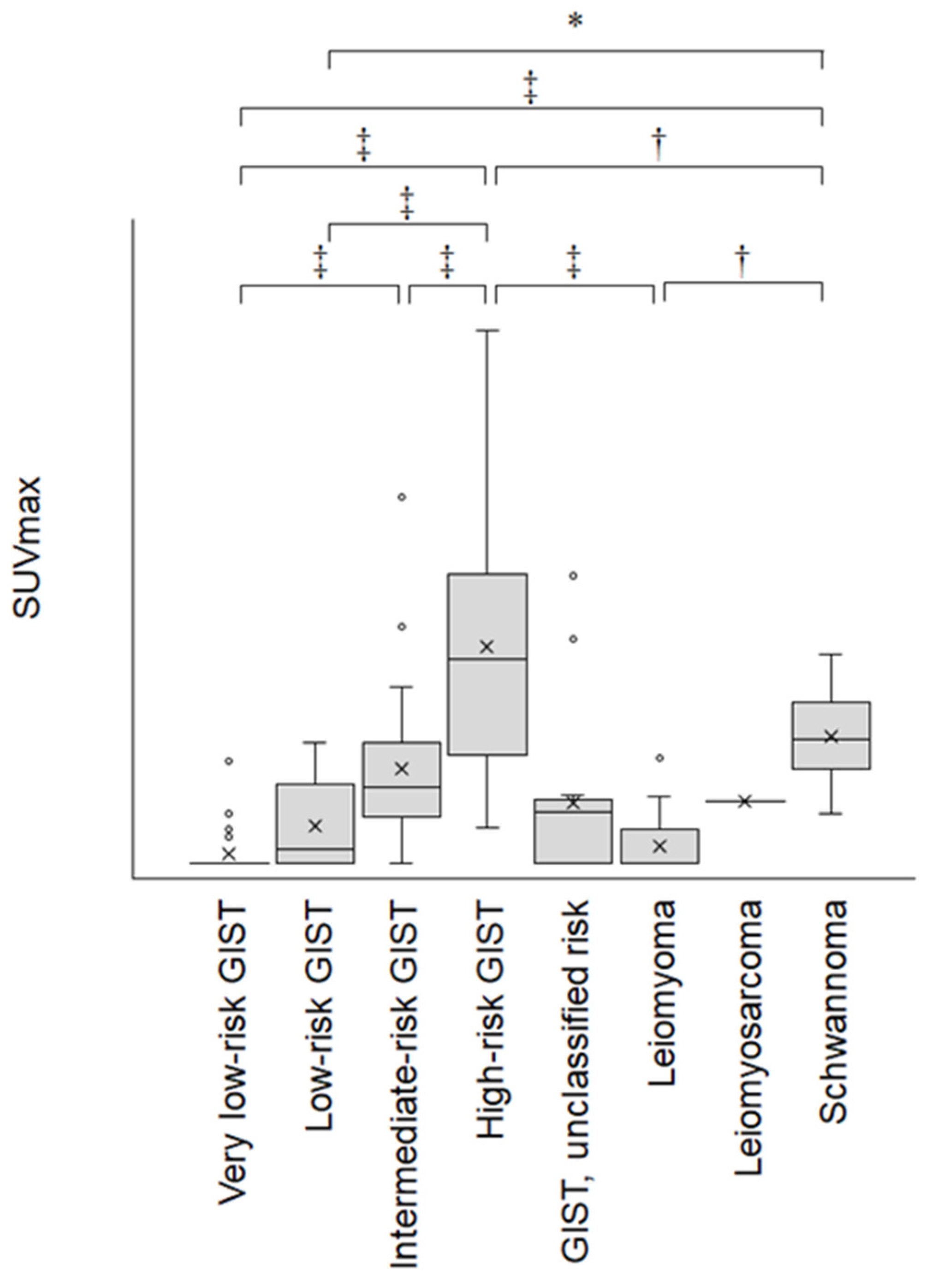
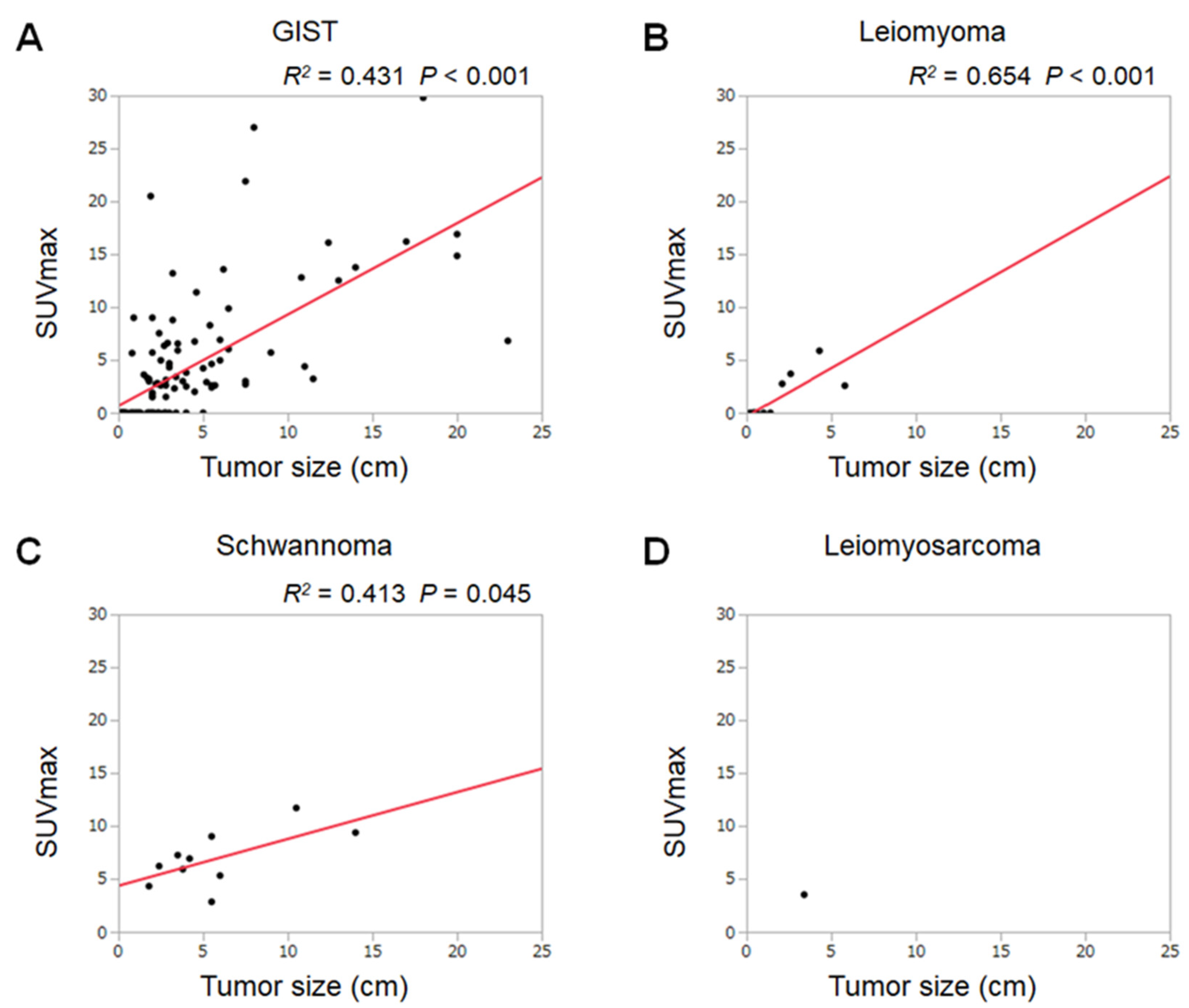
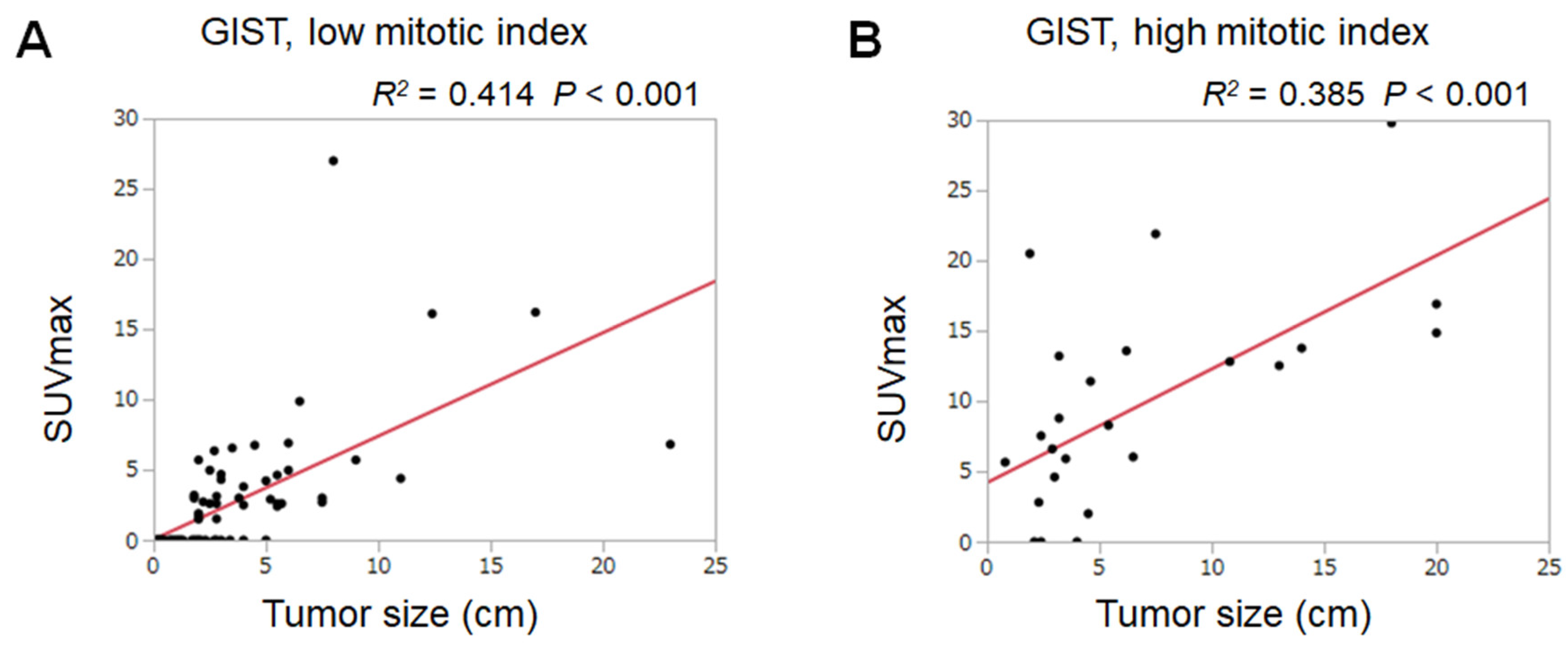
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.S.; Hsu, H.S.; Tsai, C.H.; Li, W.Y.; Huang, M.H. Gastric schwannoma. J. Chin. Med. Assoc. 2004, 67, 583–586. [Google Scholar] [PubMed]
- Tokumoto, N.; Tanabe, K.; Misumi, T.; Fujikuni, N.; Suzuki, T.; Ohdan, H. The usefulness of preoperative 18FDG positron-emission tomography and computed tomography for predicting the malignant potential of gastrointestinal stromal tumors. Dig. Surg. 2014, 31, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.W. Performance of F-18 FDG PET/CT for predicting malignant potential of gastrointestinal stromal tumors: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2018, 33, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Cai, L.; Hou, X.; Shi, H.; Hou, J. Gastric schwannoma mimicking malignant gastrointestinal stromal tumor and misdiagnosed by (18)F-FDG PET/CT. Hell. J. Nucl. Med. 2015, 18, 74–76. [Google Scholar] [PubMed]
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Otomi, Y.; Otsuka, H.; Morita, N.; Terazawa, K.; Furutani, K.; Harada, M.; Nishitani, H. Relationship between FDG uptake and the pathological risk category in gastrointestinal stromal tumors. J. Med. Investig. 2010, 57, 270–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.W.; Cho, C.H.; Jeong, D.S.; Chae, H.D. Role of F-fluoro-2-deoxyglucose positron emission tomography in gastric GIST: Predicting malignant potential pre-operatively. J. Gastric Cancer 2011, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Shimada, M.; Kurita, N.; Sato, H.; Iwata, T.; Morimoto, S.; Miyatani, T.; Kashihara, H.; Takasu, C.; Matsumoto, N. Efficacy of PET-CT for predicting the malignant potential of gastrointestinal stromal tumors. Surg. Today 2013, 43, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Park, C.K.; Park, M.; Kim, W.K.; Cho, A.; Kim, H. Clinicopathologic features and molecular characteristics of glucose metabolism contributing to 18F-fluorodeoxyglucose uptake in gastrointestinal stromal tumors. PLoS ONE 2015, 10, e0141413. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, Y.; Aihara, R.; Nakabayashi, T.; Mochiki, E.; Asao, T.; Kuwano, H.; Oriuchi, N.; Endo, K. 18F-fluorodeoxyglucose positron emission tomography: Useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J. Surg. 2005, 29, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Niwa, Y.; Matsuura, T.; Miyahara, R.; Ohashi, A.; Maeda, O.; Ando, T.; Ohmiya, N.; Itoh, A.; Hirooka, Y.; et al. Gastric GIST malignancy evaluated by 18FDG-PET as compared with EUS-FNA and endoscopic biopsy. Scand. J. Gastroenterol. 2007, 42, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Vanhove, K.; Thomeer, M.; Derveaux, E.; Shkedy, Z.; Owokotomo, O.E.; Adriaensens, P.; Mesotten, L. Correlations between the metabolic profile and 18F-FDG-positron emission tomography-computed tomography parameters reveal the complexity of the metabolic reprogramming within lung cancer patients. Sci. Rep. 2019, 9, 16212. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.; Kim, S.Y.; Yun, M.J.; Kim, H.I. Potential utility of FDG PET-CT as a non-invasive tool for monitoring local immune responses. J. Gastric Cancer 2017, 17, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, U.; Yamaguchi, U.; Seki, K.; Terauchi, T.; Arai, Y.; Hasegawa, T. Glut-1 expression and enhanced glucose metabolism are associated with tumour grade in bone and soft tissue sarcomas: A prospective evaluation by [18F] fluorodeoxyglucose positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kaneta, T.; Takahashi, S.; Fukuda, H.; Arisaka, Y.; Oriuchi, N.; Hayashi, T.; Fujii, H.; Terauchi, T.; Tateishi, U.; Kubota, K.; et al. Clinical significance of performing 18F-FDG PET on patients with gastrointestinal stromal tumors: A summary of a Japanese multicenter study. Ann. Nucl. Med. 2009, 23, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, D.; Koide, N.; Hiraga, R. Gastric schwannoma exhibiting increased fluorodeoxyglucose uptake. Gastric Cancer 2009, 12, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.W.; Sanches, A.; Squire, O.D.; Macapinlac, H.A.; Larson, S.M.; Erdi, Y.E. Gastric schwannomas revisited: Has precise preoperative diagnosis become feasible? Gastric Cancer 2013, 16, 318–323. [Google Scholar]
- Ohno, T.; Ogata, K.; Kogure, N.; Ando, H.; Aihara, R.; Mochiki, E.; Zai, H.; Sano, A.; Kato, T.; Sakurai, S.; et al. Gastric schwannomas show an obviously increased fluorodeoxyglucose uptake in positron emission tomography: Report of two cases. Surg. Today 2011, 41, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.W.; Sanches, A.; Squire, O.D.; Macapinlac, H.A.; Larson, S.M.; Erdi, Y.E. Standardized uptake value in pediatric patients: An investigation to determine the optimum measurement parameter. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.; Erdi, Y.E.; Chao, K.; Gonen, M.; Larson, S.M.; Yeung, H.W. Clinical implications of different image reconstruction parameters for interpretation of whole-body PET studies in cancer patients. J. Nucl. Med. 2004, 45, 559–566. [Google Scholar] [PubMed]


| N | |
|---|---|
| Sex | |
| Male | 90 |
| Female | 52 |
| Mean age (range), years | 68.2 (15–89) |
| Histology | |
| GIST | 118 |
| Very low risk | 42 |
| Low risk | 26 |
| Intermediate risk | 22 |
| High risk | 20 |
| Unclassified * | 8 |
| Leiomyoma | 15 |
| Schwannoma | 10 |
| Leiomyosarcoma | 1 |
| Methods for pathological diagnosis | |
| Surgical resection | 103 |
| EUS-FNA | 31 |
| Endoscopic forceps biopsy | 9 |
| ESD | 1 |
| FDG-PET was performed for | |
| Gastric subepithelial lesions | 77 |
| Other diseases | 65 |
| Cancer screening | 2 |
| Mean tumor size (range), cm | 3.7 (0.1–23.0) |
| Mean follow-up period (range), years | 3.6 (0.0–11.3) |
| Outcome | |
| Alive | 114 |
| Died of GIST | 4 |
| Died of other diseases | 24 |
| P Value | |
|---|---|
| High-risk GIST vs. very low-risk GIST | <0.0001 |
| High-risk GIST vs. leiomyoma | <0.0001 |
| High-risk GIST vs. low-risk GIST | <0.0001 |
| High-risk GIST vs. leiomyosarcoma | 0.3126 |
| High-risk GIST vs. intermediate-risk GIST | <0.0001 |
| Schwannoma vs. very low-risk GIST | 0.0001 |
| Schwannoma vs. leiomyoma | 0.0051 |
| High-risk GIST vs. schwannoma | 0.0061 |
| Schwannoma vs. low-risk GIST | 0.0217 |
| Intermediate-risk GIST vs. very low-risk GIST | 0.0007 |
| Intermediate-risk GIST vs. leiomyoma | 0.0515 |
| Schwannoma vs. leiomyosarcoma | 0.9898 |
| Leiomyosarcoma vs. very low-risk GIST | 0.9940 |
| Intermediate-risk GIST vs. low-risk GIST | 0.2011 |
| Leiomyosarcoma vs. leiomyoma | 0.9982 |
| Schwannoma vs. intermediate-risk GIST | 0.8802 |
| Low-risk GIST vs. very low-risk GIST | 0.6802 |
| Intermediate-risk GIST vs. leiomyosarcoma | 1.0000 |
| Leiomyosarcoma vs. low-risk GIST | 1.0000 |
| Low-risk GIST vs. leiomyoma | 0.9827 |
| Leiomyoma vs. very low-risk GIST | 0.9999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamuro, M.; Miyahara, K.; Sakaguchi, C.; Takenaka, R.; Kobayashi, S.; Mouri, H.; Tanaka, S.; Toyokawa, T.; Tanaka, S.; Nishimura, M.; et al. Diagnostic Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Gastric Mesenchymal Tumors. J. Clin. Med. 2020, 9, 1301. https://doi.org/10.3390/jcm9051301
Iwamuro M, Miyahara K, Sakaguchi C, Takenaka R, Kobayashi S, Mouri H, Tanaka S, Toyokawa T, Tanaka S, Nishimura M, et al. Diagnostic Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Gastric Mesenchymal Tumors. Journal of Clinical Medicine. 2020; 9(5):1301. https://doi.org/10.3390/jcm9051301
Chicago/Turabian StyleIwamuro, Masaya, Koji Miyahara, Chihiro Sakaguchi, Ryuta Takenaka, Sayo Kobayashi, Hirokazu Mouri, Shigetomi Tanaka, Tatsuya Toyokawa, Shouichi Tanaka, Mamoru Nishimura, and et al. 2020. "Diagnostic Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Gastric Mesenchymal Tumors" Journal of Clinical Medicine 9, no. 5: 1301. https://doi.org/10.3390/jcm9051301
APA StyleIwamuro, M., Miyahara, K., Sakaguchi, C., Takenaka, R., Kobayashi, S., Mouri, H., Tanaka, S., Toyokawa, T., Tanaka, S., Nishimura, M., Yamauchi, K., Tanaka, T., & Okada, H. (2020). Diagnostic Role of 18F-Fluorodeoxyglucose Positron Emission Tomography in Gastric Mesenchymal Tumors. Journal of Clinical Medicine, 9(5), 1301. https://doi.org/10.3390/jcm9051301





