Plasma Hyperosmolality Prolongs QTc Interval and Increases Risk for Atrial Fibrillation in Traumatic Brain Injury Patients
Abstract
1. Introduction
2. Methods
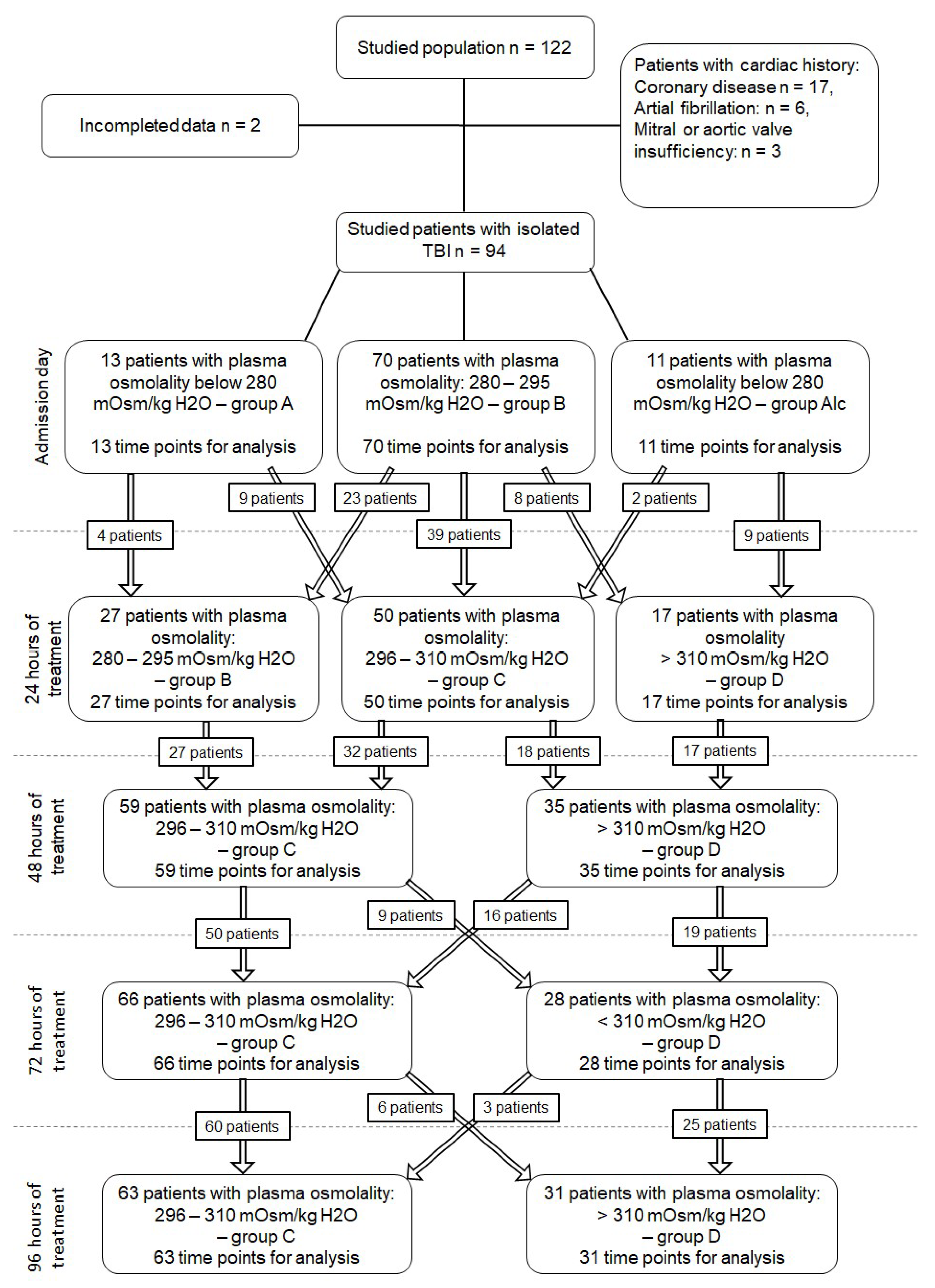
2.1. Patient Selection
2.2. Patient Monitoring and Management
2.3. Plasma Osmolality, Osmolar Gap, and Time Points
2.4. Statistical Analysis
3. Results
3.1. Plasma Osmolality, Blood Sodium Concentrations, and Osmolar Gap
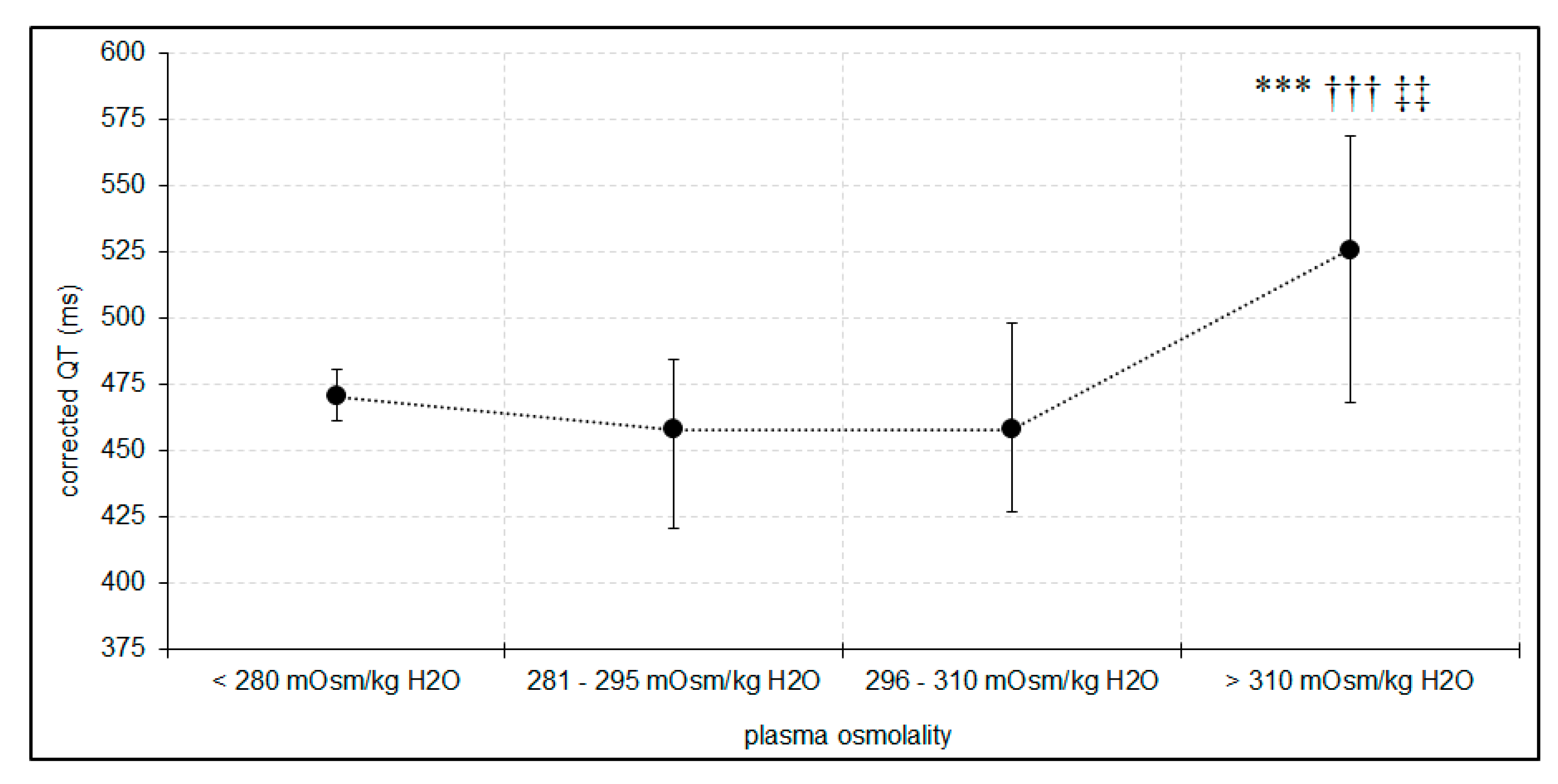
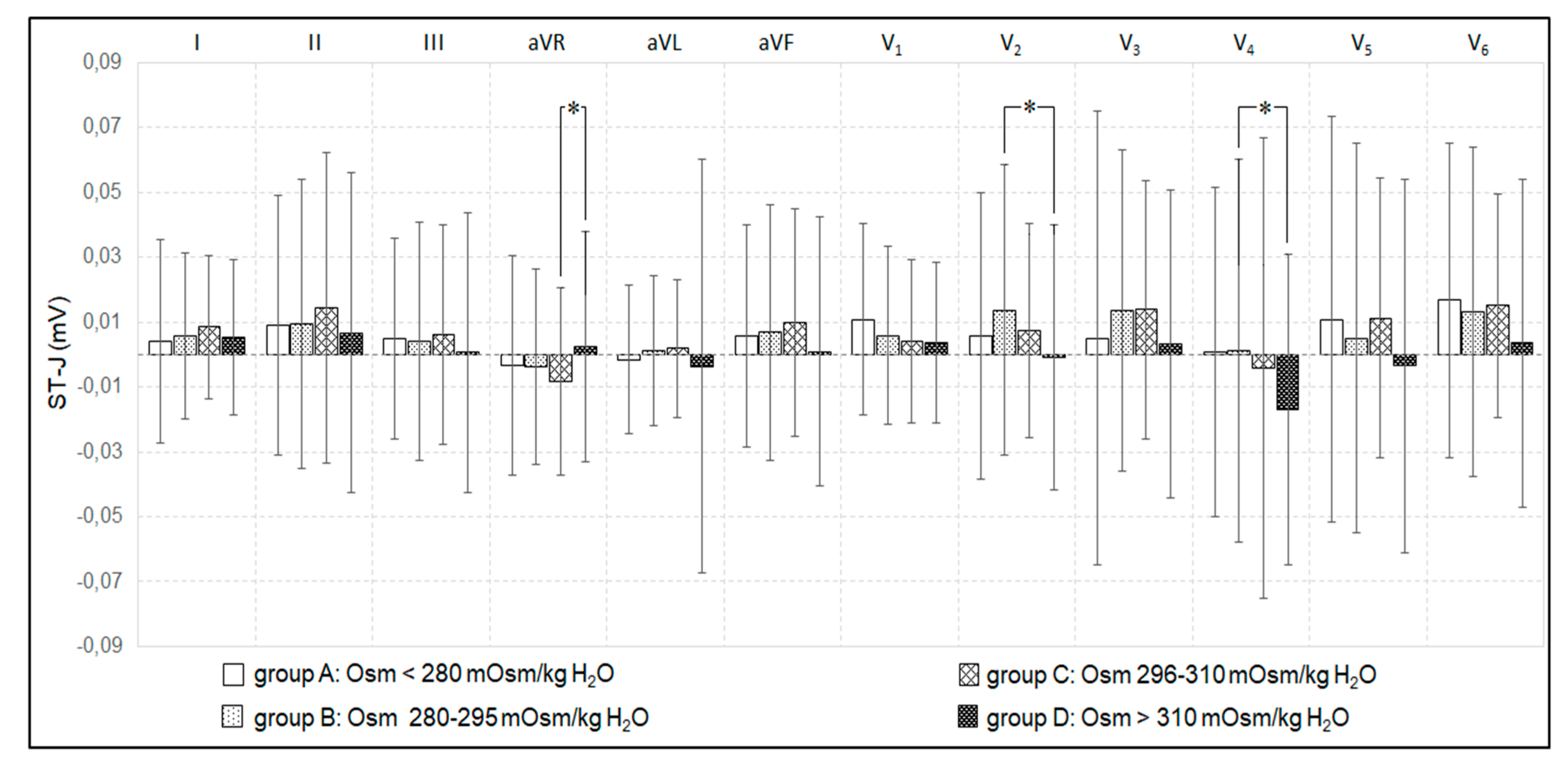
3.2. Plasma Osmolality, Osmolar Gap, and Electrocardiographic Disorders
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raslan, A.; Bhardwaj, A. Medical management of cerebral edema. Neurosurg Focus 2007, 22, 4–6. [Google Scholar] [CrossRef]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef]
- Sweeney, T.E.; Beuchat, C.A. Limitation of methods of osmometry: Measuring the osmolality of biological fluids. Am. J. Physiol. 1993, 264, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Morales, J.E.; Cariappa, R.; Parvin, C.A.; Scott, M.G.; Diringer, M.N. Osmole gap in neurologic-neurosurgical intensive care unitL its normal value, canculation, and relationship with mannitol serum concentrations. Crit. Care Med. 2004, 32, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.A.; Cadnapaphornchai, P. The serum osmole gap. J. Crit. Care 1994, 9, 185–197. [Google Scholar] [CrossRef]
- Persson, P.B. Contrast-induced nephropathy. Eur. Radiol. 2005, 15, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Garrard, A.; Sollee, D.R.; Butterfield, R.C.; Johannsen, L.; Wood, A.; Bertholf, R.L. Validation of a pre-existing formula to calculate the contribution of ethanol to the osmolar gap. Clin. Toxicol. 2012, 50, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Purssell, R.A.; Pudek, M.; Brubacher, J.; Abu-Laban, R.B. Derivation and validation of a formula to calculate the contribution of ethanol to the osmolal gap. Ann. Emerg. Med. 2001, 38, 653–659. [Google Scholar] [CrossRef]
- Whittington, J.E.; La’ulu, S.L.; Hunsaker, J.J.; Roberts, W.L. The osmolal gap: What has changed? Clin. Chem. 2010, 56, 1353–1355. [Google Scholar] [CrossRef]
- Kraut, J.A. Diagnosis of toxic alcohols: Limitations of present methods. Clin. Toxicol. 2015, 53, 589–595. [Google Scholar] [CrossRef]
- Nomani, A.Z.; Nabi, Z.; Rashid, H.; Janjua, J.; Nomani, H.; Majeed, A.; Chaudry, S.R.; Mazhar, A.S. Osmotic nephrosis with mannitol. Ren. Fail. 2014, 36, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Lei, R.; Yang, S.K.; Luo, M.; Cheng, W.; Xiao, Y.Q.; Li, X.W.; Guo, J.; Duan, S.B. Comparative effect of iso-osmolar versus low-osmolar contrast media on the incidence of contrast-induced acute kidney injury in diabetic patients: A systematic review and meta-analysis. Cancer Imaging 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Yücel, O.; Ege, M.R.; Zorlu, A.; Yücel, H.; Günes, H.; Ekmekci, A.; Yilmaz, M.B. Plasma osmolality predicts mortality in patients with heart failure with reduced ejection fraction. Kardiol. Pol. 2017, 75, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Figuero, S.B.; Jimenez-Mena, M.; Marcos, O.J.; Lopez, A.C.; Santos, S.F.; de la Cal Segura, T.; Costes, M.; Fernandez, M.S.; Gomez, J.L.Z. Dehydration and serum hyperosmolarity as a new predictors of mortality after acute coronary syndrome. Intern. J. Cardiol. 2014, 172, 472–474. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.I.; Morell, M.; Mariangelo, J.I.E.; Petroff, M.V. Hyperosmotic stress promotes endoplasmic reticulum stress-dependent apoptosis in adult rat cardiac myocytes. Apoptosis 2019, 24, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Du, K. Hyperosmolarity may be also associated with worsen outcomes in patients with heart failure. Intern. J. Cardiol. 2017, 229, 53. [Google Scholar] [CrossRef] [PubMed]
- Marmor, A.; Prober, A. Effects of contrast material osmolality on the electrocardiogram during intrauterine injection. Investig. Radiol. 1993, 28, 439–441. [Google Scholar] [CrossRef]
- Chai, C.M.; Almen, T.; Baath, L.; Besjakov, J.; Towart, R. Signs in vector-electrocardiography (VECG) predicting the fibrillatory propensity of lodixanol and mannitol solutions after injection into the left coronary artery of pigs. Acad. Radiol. 2007, 14, 162–177. [Google Scholar] [CrossRef]
- Zhang, N.; Gong, M.; Tse, G.; Zhang, Z.; Meng, L.; Yan, B.P.; Zhang, L.; Wu, G.; Xia, Y.; Xin-Yan, G.; et al. Prolonged corrected QT interval in predicting atrial fibrillation: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2018, 41, 321–327. [Google Scholar] [CrossRef]
- Voulgari, C.; Pagoni, S.; Tesfaye, S.; Tentolouris, N. The spatial QRS-T angle: Implications in clinical practice. Curr. Cardiol. Rev. 2013, 9, 197–210. [Google Scholar] [CrossRef]
- Niemeijer, M.N.; van den Berg, M.E.; Deckers, J.W.; Franco, O.H.; Hofman, A.; Kors, J.A.; Stricker, B.H.; Rijnbeek, P.R.; Eijgelsheim, M. Consistency of heart rate-QTc prolongation consistency and sudden cardiac death: The Rotterdam Study. Heart Rhythm 2015, 12, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Kochanek, M.; Pfister, R. Life-threatening cardiac arrhthmias due to drug-induced QT prolongation: A retrospective study over 6 years from a medical intensive care unit. Med. Klin. Intensivmed. Notfmed. 2016, 111, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Wośko, J.; Brzozowska, H.; Rola, R.; Trojanowski, T.; Bialy, M.; Rzecki, Z.; Schlegel, T.T.; Jaroszynski, A. Association between spatial QRS-T angle, QTc interval and intra-abdominal pressure in patients with traumatic brain injury. J. Electrocardiol. 2018, 51, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Silvani, A.; Calandra-Buonaura, G.; Dampney, R.A.; Cortelli, P. Brain-heart interactions: Physiology and clinical implications. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Hrishi, P.A.; Lionel, R.K.; Prathapadas, U. Head rules over the heart: Cardiac manifestations of cerebral disorders. Indian J. Crit. Care Med. 2019, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.L.; Schlegel, T.T. When deriving the spatial QRS-T angle from 12-lead electrocardiogram, which transform in more Frank: Regression or inverse Dower? J. Electrocardiol. 2010, 43, 302–309. [Google Scholar] [CrossRef]
- Edenbrandt, L.; Pahlm, O. Vectorcardiogram synthesis from 12-lead ECG: Superiority of the inverse Dover matrix. J. Electrocardiol. 1988, 21, 361–367. [Google Scholar] [CrossRef]
- Shawkat, H.; Westwood, M.M.; Mortimer, A. Mannitol: A review of its clinical uses. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 82–85. [Google Scholar] [CrossRef]
- Lang, F. Osmotic diuresis. Ren. Physiol. 1987, 10, 160–173. [Google Scholar] [CrossRef]
- Howarth, F.C.; Qureshi, M.A.; White, E. Effects of hyperosmotic shrinking on ventricular myocyte shortening and intracellular Ca+2 in streptozotocin-induced diabetic rats. Pflugers. Arch. Eur. J. Physiol. 2002, 444, 446–451. [Google Scholar] [CrossRef]
- Tatlisu, M.A.; Kaya, A.; Keskin, M.; Uzman, O.; Borklu, E.B.; Cinier, G.; Hayiroglu, M.I.; Tatlisu, K.; Eren, M. Can we use plasma hyperosmolality as a predictor of mortality for ST-segment elevation myocardial infarction? Coron. Artery Dis. 2017, 28, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C. Diagnosis and clinical management of long-QT syndrome. Curr. Opin. Cardiol. 2018, 33, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Raheja, H.; Namana, V.; Chopra, K.; Sinha, A.; Gupta, S.S.; Kamholz, S.; Moskovits, N.; Shani, J.; Hollander, G. Electrocardiogram changes with acute alcohol intoxication: A systematic review. Open Cardiovasc. Med. J. 2018, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, D.; Jurisch, D.; Neef, M.; Hagendorff, A. Alcohol and arrhythmias. Herz 2016, 41, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Oe, K.; Mori, K.; Otsuji, M.; Konno, T.; Fujino, N.; Yamagishi, M. Takotsubo cardiomyopathy with marked ST-segment elevation and electrical alternans complicated with hyperglycemic hyperosmolar state. Int. Heart J. 2008, 49, 629–635. [Google Scholar] [CrossRef][Green Version]
- Finsterer, J.; Wahbi, K. CNS disease triggering Takotsubo stress cardiomyopathy. Int. J. Cardiol. 2014, 177, 322–329. [Google Scholar] [CrossRef]
- Han, J.; Ren, H.Q.; Zhao, Q.B.; Wu, Y.L.; Qiao, Z.Y. Comparison of 3% and 7.5% hypertonic saline in resuscitation after traumatic hypovolemic shock. Shock 2015, 43, 244–249. [Google Scholar] [CrossRef]
- Etchegoyen, C.V.; Keller, G.A.; Mrad, S.; Cheng, S.; Di Girolamo, G. Drug-induced QT interval prolongation in the intensive care unit. Curr. Clin. Pharmacol. 2017, 12, 210–222. [Google Scholar] [CrossRef]
- Staikou, C.; Stamelos, M.; Stavroulakis, E. Impact of anaesthetic drug and adjuvants on ECG merkers of torsadogenicity. Br. J. Anaesth. 2014, 112, 217–230. [Google Scholar] [CrossRef]

| Patients | Male | n = 72 | ||
| Female | n = 22 | |||
| GCS | 4.56 ± 1.52 (min 3, max 8) | |||
| 28-day outcome | Mortality | 23 (24.5%) | ||
| Discharged from ICU | 56 (59.6%) | |||
| Treated longer than 28 days | 15 (15.9%) | |||
| Type of cerebral injury | Subdural/epidural hematoma | 35 (37.2%) | ||
| Intra-cerebral haemorrhage with cerebral edema | 27 (28.7%) | |||
| Traumatic SAH with cerebral edema | 18 (19.1%) | |||
| Isolated cerebral edema | 14 (15%) | |||
| Mortality rate in accordance to type of injury | Subdural/epidural hematoma | 2 (5.7%) | ||
| Intra-cerebral hemorrhage with cerebral edema | 11 (40.7%) | |||
| Traumatic SAH with cerebral edema | 7 (39.9%) | |||
| Isolated cerebral edema | 3 (21.4%) | |||
| Number of patients with 28-day mortality based on peak plasma osmolality range: | Below 280 mOsm/kg H2O | 280–295 mOsm/kg H2O | 296–310 mOsm/kg H2O | Above 310 mOsm/kg H2O |
| 0 | 0 | 6 | 17 * | |
| Dependent Variable | Independent | B | St. Error | β | p |
|---|---|---|---|---|---|
| QTc | Plasma osmolality | 1.55 | 0.05 | 0.35 | 0.0001 |
| Dose of norepinephrine | 32.81 | 0.05 | 0.15 | 0.01 | |
| Model (R = 0.4, R2 = 0.19) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Badenes, R.; Bialy, M.; Iwaniuk, P.; Schlegel, T.T.; Jaroszynski, A. Plasma Hyperosmolality Prolongs QTc Interval and Increases Risk for Atrial Fibrillation in Traumatic Brain Injury Patients. J. Clin. Med. 2020, 9, 1293. https://doi.org/10.3390/jcm9051293
Dabrowski W, Siwicka-Gieroba D, Robba C, Badenes R, Bialy M, Iwaniuk P, Schlegel TT, Jaroszynski A. Plasma Hyperosmolality Prolongs QTc Interval and Increases Risk for Atrial Fibrillation in Traumatic Brain Injury Patients. Journal of Clinical Medicine. 2020; 9(5):1293. https://doi.org/10.3390/jcm9051293
Chicago/Turabian StyleDabrowski, Wojciech, Dorota Siwicka-Gieroba, Chiara Robba, Rafael Badenes, Mateusz Bialy, Paulina Iwaniuk, Todd T Schlegel, and Andrzej Jaroszynski. 2020. "Plasma Hyperosmolality Prolongs QTc Interval and Increases Risk for Atrial Fibrillation in Traumatic Brain Injury Patients" Journal of Clinical Medicine 9, no. 5: 1293. https://doi.org/10.3390/jcm9051293
APA StyleDabrowski, W., Siwicka-Gieroba, D., Robba, C., Badenes, R., Bialy, M., Iwaniuk, P., Schlegel, T. T., & Jaroszynski, A. (2020). Plasma Hyperosmolality Prolongs QTc Interval and Increases Risk for Atrial Fibrillation in Traumatic Brain Injury Patients. Journal of Clinical Medicine, 9(5), 1293. https://doi.org/10.3390/jcm9051293






