Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Inclusion and Exclusion Criteria
2.4. Data Collection and Extraction
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Polyzos, S.A.; Kountouras, J. Helicobacter pylori infection and nonalcoholic fatty liver disease: Time for large clinical trials evaluating eradication therapy. Helicobacter 2019, 24, e12588. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Kotronis, G.; Gialamprinou, D.; Kountouras, J.; Katsinelos, P. Non-alcoholic fatty liver disease: An update with special focus on the role of gut microbiota. Metabolism 2017, 71, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Doulberis, M.; Polyzos, S.A.; Papaefthymiou, A.; Katsinelos, P.; Kiosses, C.; Kountouras, J. Treatment of nonalcoholic fatty liver disease: From adult trials to perspectives in the management of children and adolescents. Expert Opin. Pharmacother. 2020, 21, 247–275. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Anastasiadis, S.; Doulberis, M.; Katsinelos, P. Nonalcoholic fatty liver disease: Is it time for combination treatment and a diabetes-like approach? Hepatology 2018, 68, 389. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mantzoros, C.S. Helicobacter pylori infection and nonalcoholic fatty liver disease: Are the four meta-analyses favoring an intriguing association pointing to the right direction? Metabolism 2019, 96, iii–v. [Google Scholar] [CrossRef]
- Kountouras, J.; Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Touloumtzi, M.; Elisabeth, V.; Kapetanakis, N.; Liatsos, C.; Gavalas, E.; Katsinelos, P. Helicobacter pylori infection and gastrointestinal tract cancer biology: Considering a double-edged sword reflection. Cell. Mol. Life Sci. 2019, 76, 2487–2488. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Hunt, R. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Doulberis, M.; Kotronis, G.; Thomann, R.; Polyzos, S.A.; Boziki, M.; Gialamprinou, D.; Kountouras, J. Impact of Helicobacter pylori on Alzheimer’s disease: What do we know so far? Helicobacter 2018, 23, e12454. [Google Scholar] [CrossRef]
- He, C.; Cheng, D.; Wang, H.; Wu, K.; Zhu, Y.; Lu, N. Helicobacter pylori infection aggravates diet-induced nonalcoholic fatty liver in mice. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 360–367. [Google Scholar] [CrossRef]
- Kim, T.J.; Sinn, D.H.; Min, Y.W.; Son, H.J.; Chang, Y.; Baek, S.-Y.; Ahn, S.H.; Lee, H.; Ryu, S. A cohort study on Helicobacter pylori infection associated with nonalcoholic fatty liver disease. J. Gastroenterol. 2017, 29, 559–1210. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, H.J.; Kim, D.; Ahmed, A. Association between cagA negative Helicobacter pylori status and nonalcoholic fatty liver disease among adults in the United States. PLoS ONE 2018, 13, e0202325. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, C.; Wang, X.; Zhang, F.; Zhang, Z.; Ma, P.; Feng, S. Helicobacter pylori infection may increase the severity of nonalcoholic fatty liver disease via promoting liver function damage, glycometabolism, lipid metabolism, inflammatory reaction and metabolic syndrome. Eur. J. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Abo-Amer, Y.E.-E.; Sabal, A.; Ahmed, R.; Hasan, N.F.E.; Refaie, R.; Mostafa, S.M.; Mohamed, A.A.; Khalil, M.; Elagawy, W.; Abd-Elsalam, S. Relationship between helicobacter pylori infection and nonalcoholic fatty liver disease (Nafld) in a developing country: A cross-sectional study. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Valladares, S.; López-Cano, C.; Gutiérrez, L.; Ciudin, A.; Fort, J.M.; Reñé, J.M.; Matias-Guiu, X.; De Torres, I.; Bueno, M.; et al. The Role of Morbid Obesity in the Promotion of Metabolic Disruptions and Non-Alcoholic Steatohepatitis by Helicobacter Pylori. PLoS ONE. 2016, 11, e0166741. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-J.; Hao, N.-B.; Liu, J.-J.; Li, X.; Wang, R.-L. Correlation between Helicobacter pylori Infection and Metabolic Abnormality in General Population: A Cross-Sectional Study. Gastroenterol. Res. Pract. 2018, 2018, 7410801. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifard, M.; Saremi, Z.; Rastgoo, M.; Akbari, E. Relevance between Helicobacter pylori Infection and Non-Alcoholic Fatty Liver Disease in Birjand, Iran. J. Med. Life 2019, 12, 168–172. [Google Scholar]
- Erim, T.; Cruz-Correa, M.R.; Szomstein, S.; Velis, E.; Rosenthal, R. Prevalence of Helicobacter pylori seropositivity among patients undergoing bariatric surgery: A preliminary study. World J. Surg. 2008, 32, 2021–2025. [Google Scholar] [CrossRef]
- Okushin, K.; Takahashi, Y.; Yamamichi, N.; Shimamoto, T.; Enooku, K.; Fujinaga, H.; Tsutsumi, T.; Shintani, Y.; Sakaguchi, Y.; Ono, S.; et al. Helicobacter pylori infection is not associated with fatty liver disease including non-alcoholic fatty liver disease: A large-scale cross-sectional study in Japan. BMC Gastroenterol. 2015, 15, 25. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Cai, J.-T.; Song, Z.-Y.; Tong, Y.-L.; Wang, J.-H. The associations among Helicobacter pylori infection, white blood cell count and nonalcoholic fatty liver disease in a large Chinese population. Medicine 2018, 97, e13271. [Google Scholar] [CrossRef]
- Fan, N.; Peng, L.; Xia, Z.; Zhang, L.; Wang, Y.; Peng, Y. Helicobacter pylori Infection Is Not Associated with Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study in China. Front. Microbiol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, L.; Qin, Y.; Li, G. Correlation between Helicobacter pylori infection and polymorphism of adiponectin gene promoter -11391G/A, superoxide dismutase gene in nonalcoholic fatty liver disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2016, 41, 359–366. [Google Scholar] [PubMed]
- Baeg, M.K.; Yoon, S.K.; Ko, S.-H.; Noh, Y.-S.; Lee, I.-S.; Choi, M.-G. Helicobacter pylori infection is not associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Cai, O.; Huang, Z.; Li, M.; Zhang, C.; Xi, F.; Tan, S. Association between Helicobacter pylori Infection and Nonalcoholic Fatty Liver Disease: A Single-Center Clinical Study. Gastroenterol. Res. Pract. 2018, 2018, 8040262. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razik, A.; Mousa, N.; Shabana, W.; Refaey, M.; Elhelaly, R.; Elzehery, R.; Abdelsalam, M.; Elgamal, A.; Nassar, M.R.; Abu El-Soud, A.; et al. Helicobacter pylori and non-alcoholic fatty liver disease: A new enigma? Helicobacter 2018, 23, e12537. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Z.; Filik, L.; Ergül, B.; Sarikaya, M.; Akbal, E. Association between Helicobacter pylori and liver-to-spleen ratio: A randomized-controlled single-blind study. Eur. J. Gastroenterol. Hepatol. 2013, 25, 107–110. [Google Scholar] [CrossRef]
- Chen, C.-X.; Mao, Y.-S.; Foster, P.; Zhu, Z.-W.; Du, J.; Guo, C.-Y. Possible association between Helicobacter pylori infection and nonalcoholic fatty liver disease. Appl. Physiol. Nutr. Metab. 2017, 42, 295–301. [Google Scholar] [CrossRef]
- Kim, T.J.; Lee, H.; Kang, M.; Kim, J.E.; Choi, Y.-H.; Min, Y.W.; Min, B.-H.; Lee, J.H.; Son, H.J.; Rhee, P.-L.; et al. Helicobacter pylori is associated with dyslipidemia but not with other risk factors of cardiovascular disease. Sci. Rep. 2016, 6, 38015. [Google Scholar] [CrossRef]
- Kountouras, J.; Mylopoulos, N.; Chatzopoulos, D.; Zavos, C.; Boura, P.; Konstas, A.G.P.; Venizelos, J. Eradication of Helicobacter pylori may be beneficial in the management of chronic open-angle glaucoma. Arch. Intern. Med. 2002, 162, 1237–1244. [Google Scholar] [CrossRef]
- Bedossa, P.; FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Darma, A.; Nugroho, B.S.T.; Yoanna, V.; Sulistyani, I.; Athiyyah, A.F.; Ranuh, R.G.; Sudarmo, S.M. Comparison of Helicobacter pylori stool antigen, salivary IgG, serum IgG, and serum IgM as diagnostic markers of H. pylori infection in children. Iran. J. Microbiol. 2019, 11, 206–211. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, X.; Xia, C.; Liu, H.; Yan, H.; Wang, G.; Wu, Z. Association between Helicobacter pylori infection and non-alcoholic fatty liver disease in North Chinese: A cross-sectional study. Sci. Rep. 2019, 9, 4874. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Nascimbeni, F.; Bedossa, P.; Fedchuk, L.; Pais, R.; Charlotte, F.; Lebray, P.; Poynard, T.; Ratziu, V. Clinical validation of the FLIP algorithm and the SAF score in patients with non-alcoholic fatty liver disease. J. Hepatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2005, 28, S37–S42. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Polyzos, S.; Slavakis, A.; Koumerkeridis, G.; Katsinelos, P.; Kountouras, J. Noninvasive Liver Fibrosis Tests in Patients with Nonalcoholic Fatty Liver Disease: An External Validation Cohort. Horm. Metab. Res. 2019, 51, 134–140. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Papatheodorou, A.; Patsiaoura, K.; Katsiki, E.; Zafeiriadou, E.; Zavos, C.; Anastasiadou, K.; Terpos, E. Helicobacter pylori infection in patients with nonalcoholic fatty liver disease. Metabolism 2013, 62, 121–126. [Google Scholar] [CrossRef]
- Sumida, Y.; Kanemasa, K.; Imai, S.; Mori, K.; Tanaka, S.; Shimokobe, H.; Kitamura, Y.; Fukumoto, K.; Kakutani, A.; Ohno, T.; et al. Helicobacter pylori infection might have a potential role in hepatocyte ballooning in nonalcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 996–1004. [Google Scholar] [CrossRef]
- Mantovani, A.; Turino, T.; Altomari, A.; Lonardo, A.; Zoppini, G.; Valenti, L.; Tilg, H.; Byrne, C.D.; Targher, G. Association between Helicobacter pylori infection and risk of nonalcoholic fatty liver disease: An updated meta-analysis. Metabolism 2019, 96, 56–65. [Google Scholar] [CrossRef]
- Ning, L.; Liu, R.; Lou, X.; Du, H.; Chen, W.; Zhang, F.; Li, S.; Chen, X.; Xu, G. Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A systemic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 735–742. [Google Scholar] [CrossRef]
- Zhou, B.-G.; Yang, H.-J.; Xu, W.; Wang, K.; Guo, P.; Ai, Y.-W. Association between Helicobacter pylori infection and nonalcoholic fatty liver disease: A systematic review and meta-analysis of observational studies. Helicobacter 2019, 24, e12576. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Panjawatanan, P.; Manatsathit, W.; Jaruvongvanich, V.; Ungprasert, P. Helicobacter pylori and Risk of Nonalcoholic Fatty Liver Disease. J. Clin. Gastroenterol. 2018, 52, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Q.; He, Y.; Shi, W.; Xu, Q.; Yuan, Q.; Lin, Q.; Li, B.; Ye, L.; Min, Y.; et al. Association between Helicobacter pylori infection and nonalcoholic fatty liver: A meta-analysis. Medicine 2019, 98, e17781. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Deretzi, G. The association between Helicobacter pylori infection and insulin resistance: A systematic review. Helicobacter 2011, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Upala, S.; Jaruvongvanich, V.; Riangwiwat, T.; Jaruvongvanich, S.; Sanguankeo, A. Association between Helicobacter pylori infection and metabolic syndrome: A systematic review and meta-analysis. J. Dig. Dis. 2016, 17, 433–440. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J. Novel Advances in the Association Between Helicobacter pylori Infection, Metabolic Syndrome, and Related Morbidity. Helicobacter 2015, 20, 405–409. [Google Scholar] [CrossRef]
- Sabaté, J.-M.; Jouët, P.; Harnois, F.; Mechler, C.; Msika, S.; Grossin, M.; Coffin, B. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: A contributor to severe hepatic steatosis. Obes. Surg. 2008, 18, 371–377. [Google Scholar] [CrossRef]

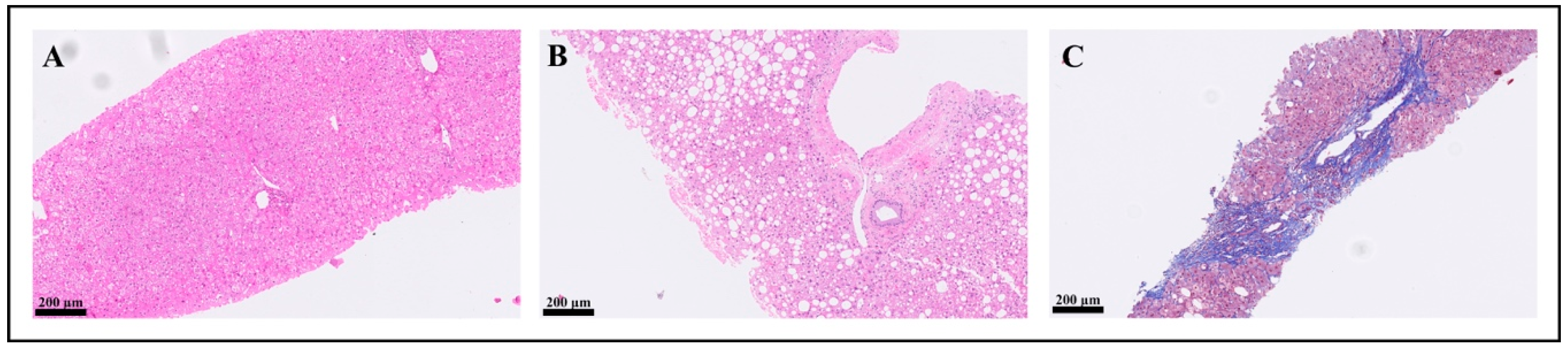
| Hp(−) N = 49 (76.6%) | Hp(+) N = 15 (23.4%) | p-Value * | |
|---|---|---|---|
| Women [N (%)] | 37 (75.5) | 10 (66.7) | 0.52 |
| Age (years) | 46.7 ± 13.3 | 49.1 ± 12.4 | 0.51 |
| BMI (kg/m2) | 45.5 ± 10.2 | 42.6 ± 5.9 | 0.70 |
| AST (U/L) | 24.5 ± 9.0 | 42.7 ± 18.9 | 0.001 |
| ALT (U/L) | 28.6 ± 20.5 | 51.6 ± 24.5 | <0.001 |
| GGT (U/L) | 35.7 ± 43.9 | 86.3 ± 60.2 | <0.001 |
| Bilirubin (μmol/L) | 7.8 ± 5.2 | 12.2 ± 8.3 | 0.013 |
| Triglycerides (mmol/L) | 1.48 ± 1.58 | 2.02 ± 0.98 | 0.003 |
| Total cholesterol (mmol/L) | 4.39 ± 1.14 | 4.79 ± 0.46 | 0.10 |
| HDL-C (mmol/L) | 1.29 ± 0.37 | 1.23 ± 0.46 | 0.55 |
| LDL-C (mmol/L) | 2.48 ± 0.85 | 2.72 ± 0.64 | 0.20 |
| HbA1c (%) | 5.7 ± 0.6 | 6.0 ± 1.3 | 0.33 |
| HOMA-IR | 4.3 ± 3.9 | 10.1 ± 6.8 | <0.001 |
| Prediabetes/Diabetes [N (%)] | 24 (49.0) | 9 (60.0) | 0.46 |
| Arterial hypertension [N (%)] | 18 (36.7) | 10 (66.7) | 0.041 |
| FLIP [N (%)] | <0.001 | ||
| No NAFLD | 9 (18.4) | 0 (0.0) | |
| NAFL | 27 (55.1) | 2 (13.3) | |
| NASH | 13 (26.5) | 13 (86.7) | |
| Severe NAFLD [N (%)] | 6 (12.2) | 7 (46.7) | 0.008 |
| NAS [N (%)] | 0.001 | ||
| No NAFLD | 8 (16.3) | 0 (0.0) | |
| NAFL | 24 (49.0) | 1 (6.6) | |
| Borderline NASH | 10 (20.4) | 7 (46.7) | |
| Definite NASH | 7 (14.3) | 7 (46.7) | |
| Steatosis [N (%)] | 0.027 | ||
| Grade 0 | 9 (18.4) | 0 (0.0) | |
| Grade 1 | 22 (44.9) | 3 (20.0) | |
| Grade 2 | 11 (22.4) | 7 (46.7) | |
| Grade 3 | 7 (14.3) | 5 (33.3) | |
| Ballooning [N (%)] | <0.001 | ||
| 0 | 28 (57.1) | 1 (6.6) | |
| 1 | 20 (40.8) | 10 (66.7) | |
| 2 | 1 (2.0) | 4 (26.7) | |
| Lobular inflammation [N (%)] | 0.003 | ||
| 0 | 30 (61.2) | 2 (13.3) | |
| 1 | 13 (26.5) | 7 (46.7) | |
| 2 | 6 (12.2) | 6 (40.0) | |
| Fibrosis stage [N (%)] | <0.001 | ||
| F0 | 37 (75.5) | 2 (13.3) | |
| F1 | 11 (22.4) | 7 (46.7) | |
| F2/3 | 1 (2.0) | 6 (40.0) |
| Independent Variables | Beta | Exp(Beta) | p-Value | 95% CI for Exp(Beta) |
|---|---|---|---|---|
| Hp-I diagnosis (0: Negative; 1: Positive) | 3.27 | 26.32 | 0.002 | 3.36–206.23 |
| Gender (0: Women; 1: Men) | 1.30 | 3.68 | 0.14 | 0.65–20.73 |
| Hypertension (0: No; 1: Yes) | −1.17 | 0.31 | 0.19 | 0.05–1.80 |
| Hyperglyceridemia (0: No; 1: Yes) | −0.33 | 0.72 | 0.70 | 0.14–3.75 |
| Age (years) | 0.03 | 1.03 | 0.40 | 0.97–1.09 |
| BMI (kg/m2) | 0.05 | 1.06 | 0.15 | 0.98–1.14 |
| HOMA-IR | 0.15 | 1.17 | 0.12 | 0.96–1.41 |
| Independent Variables | Beta | Exp(Beta) | p-Value | 95% CI for Exp(Beta) |
|---|---|---|---|---|
| Hp-I diagnosis (0: Negative; 1: Positive) | 2.37 | 10.73 | 0.018 | 1.50–76.46 |
| Gender (0: Women; 1: Men) | 0.06 | 1.06 | 0.95 | 0.17–6.58 |
| Hypertension (0: No; 1: Yes) | 0.06 | 1.07 | 0.94 | 0.20–5.74 |
| Hyperglyceridemia (0: No; 1: Yes) | 0.44 | 1.56 | 0.62 | 0.27–9.09 |
| Age (years) | 0.09 | 1.10 | 0.028 | 1.01–1.19 |
| BMI (kg/m2) | 0.10 | 1.10 | 0.035 | 1.01–1.21 |
| HOMA-IR | 0.02 | 1.02 | 0.82 | 0.88–1.17 |
| Independent Variables | Beta | Exp(Beta) | p-value | 95% CI for Exp(Beta) |
|---|---|---|---|---|
| Hp-I diagnosis (0: Negative; 1: Positive) | 3.86 | 47.28 | 0.001 | 4.76–469.5 |
| Gender (0: Women; 1: Men) | 1.95 | 7.00 | 0.026 | 1.26–39.02 |
| Hypertension (0: No; 1: Yes) | −0.18 | 0.84 | 0.82 | 0.18–3.90 |
| Hyperglyceridemia (0: No; 1: Yes) | −0.20 | 0.82 | 0.81 | 0.16–4.11 |
| Age (years) | 0.05 | 1.05 | 0.08 | 0.99–1.12 |
| BMI (kg/m2) | −0.05 | 0.95 | 0.37 | 0.86–1.06 |
| HOMA-IR | −0.04 | 0.96 | 0.56 | 0.83–1.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doulberis, M.; Srivastava, S.; Polyzos, S.A.; Kountouras, J.; Papaefthymiou, A.; Klukowska-Rötzler, J.; Blank, A.; Exadaktylos, A.K.; Srivastava, D.S. Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. J. Clin. Med. 2020, 9, 933. https://doi.org/10.3390/jcm9040933
Doulberis M, Srivastava S, Polyzos SA, Kountouras J, Papaefthymiou A, Klukowska-Rötzler J, Blank A, Exadaktylos AK, Srivastava DS. Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Journal of Clinical Medicine. 2020; 9(4):933. https://doi.org/10.3390/jcm9040933
Chicago/Turabian StyleDoulberis, Michael, Simone Srivastava, Stergios A Polyzos, Jannis Kountouras, Apostolis Papaefthymiou, Jolanta Klukowska-Rötzler, Annika Blank, Aristomenis K Exadaktylos, and David S Srivastava. 2020. "Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients" Journal of Clinical Medicine 9, no. 4: 933. https://doi.org/10.3390/jcm9040933
APA StyleDoulberis, M., Srivastava, S., Polyzos, S. A., Kountouras, J., Papaefthymiou, A., Klukowska-Rötzler, J., Blank, A., Exadaktylos, A. K., & Srivastava, D. S. (2020). Active Helicobacter pylori Infection is Independently Associated with Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Journal of Clinical Medicine, 9(4), 933. https://doi.org/10.3390/jcm9040933








