Ventricular Repolarization is Associated with Cognitive Function, but Not with Cognitive Decline and Brain Magnetic Resonance Imaging (MRI) Measurements in Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Design
2.3. Study Participants
2.4. Data Collection
2.4.1. ECG Measurements
2.4.2. Cognitive Function Measurements
2.4.3. MRI Measurements
2.4.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cognitive Function Analyses
3.3. Brain MRI Measurements
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Y.T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef]
- Coppola, L.; Mastrolorenzo, L.; Coppola, A.; De Biase, M.; Adamo, G.; Forte, R.; Fiorente, F.; Orlando, R.; Caturano, M.; Cioffi, A.; et al. QT dispersion in mild cognitive impairment: A possible tool for predicting the risk of progression to dementia? Int. J. Geriatr. Psychiatry 2013, 28, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.P.; Mendes de Leon, C.F.; Prineas, R.J.; Bienias, J.L.; Evans, D.A. Relation of cardiac ventricular repolarization and global cognitive performance in a community population. Am. J. Cardiol. 2010, 106, 1169–1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madhavan, M.; Graff-Radford, J.; Piccini, J.P.; Gersh, B.J. Cognitive dysfunction in atrial fibrillation. Nat. Rev. Cardiol. 2018, 15, 744–756. [Google Scholar] [CrossRef]
- Van der Velpen, I.F.; Yancy, C.W.; Sorond, F.A.; Sabayan, B. Impaired Cardiac Function and Cognitive Brain Aging. Can. J. Cardiol. 2017, 33, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Brickman, A.M.; Spitznagel, M.B.; Griffith, E.Y.; Narkhede, A.; Raz, N.; Cohen, R.; Sweet, L.H.; Hughes, J.; Rosneck, J.; et al. Independent and interactive effects of blood pressure and cardiac function on brain volume and white matter hyperintensities in heart failure. J. Am. Soc. Hypertens. JASH 2013, 7, 336–343. [Google Scholar] [CrossRef]
- Breteler, M.M. Vascular risk factors for Alzheimer’s disease: An epidemiologic perspective. Neurobiol. Aging 2000, 21, 153–160. [Google Scholar] [CrossRef]
- De la Torre, J.C. Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004, 3, 184–190. [Google Scholar] [CrossRef]
- De la Torre, J.C. Vascular risk factor detection and control may prevent Alzheimer’s disease. Ageing Res. Rev. 2010, 9, 218–225. [Google Scholar] [CrossRef]
- Mahinrad, S.; Jukema, J.W.; van Heemst, D.; Macfarlane, P.W.; Clark, E.N.; de Craen, A.J.; Sabayan, B. 10-Second heart rate variability and cognitive function in old age. Neurology 2016, 86, 1120–1127. [Google Scholar] [CrossRef]
- Mahinrad, S.; Vriend, A.E.; Jukema, J.W.; van Heemst, D.; Sattar, N.; Blauw, G.J.; Macfarlane, P.W.; Clark, E.N.; de Craen, A.J.M.; Sabayan, B. Left Ventricular Hypertrophy and Cognitive Decline in Old Age. J. Alzheimer’s Dis. JAD 2017, 58, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Moazzami, K.; Ostovaneh, M.R.; Ambale Venkatesh, B.; Habibi, M.; Yoneyama, K.; Wu, C.; Liu, K.; Pimenta, I.; Fitzpatrick, A.; Shea, S.; et al. Left Ventricular Hypertrophy and Remodeling and Risk of Cognitive Impairment and Dementia: MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension 2018, 71, 429–436. [Google Scholar] [CrossRef]
- Obara, T.; Nagai, K.; Shibata, S.; Hirasawa, A.; Koshiba, H.; Hasegawa, H.; Ebihara, T.; Kozaki, K. Relationship between the severity of cerebral white matter hyperintensities and sympathetic nervous activity in older adults. Geriatr. Gerontol. Int. 2018, 18, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Akoudad, S.; Wolters, F.J.; Viswanathan, A.; de Bruijn, R.F.; van der Lugt, A.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A.; Vernooij, M.W. Association of Cerebral Microbleeds with Cognitive Decline and Dementia. JAMA Neurol. 2016, 73, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.O.; Duarte, M.G.F.; Pelichek, A.; Pfrimer, K.; Ferriolli, E.; Moriguti, J.C.; Lima, N.K.C. Cardiovascular risk factors and inflammatory activity among centenarians with and without dementia. Aging Clin. Exp. Res. 2017, 29, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Irimata, K.E.; Dugger, B.N.; Wilson, J.R. Impact of the Presence of Select Cardiovascular Risk Factors on Cognitive Changes among Dementia Subtypes. Curr. Alzheimer Res. 2018, 15, 1032–1044. [Google Scholar] [CrossRef]
- Becker, D.E. Fundamentals of electrocardiography interpretation. Anesth. Prog. 2006, 53, 53–63, quiz 4. [Google Scholar]
- Mahinrad, S.; Ferguson, I.; Macfarlane, P.W.; Clark, E.N.; Stott, D.J.; Ford, I.; Mooijaart, S.P.; Trompet, S.; van Heemst, D.; Jukema, J.W.; et al. Spatial QRS-T Angle and Cognitive Decline in Older Subjects. J. Alzheimer’s Dis. JAD 2019, 67, 279–289. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet (Lond. Engl.) 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Cobbe, S.M.; Bollen, E.L.; Buckley, B.M.; Ford, I.; Jukema, J.W.; Hyland, M.; Gaw, A.; et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am. J. Cardiol. 1999, 84, 1192–1197. [Google Scholar] [CrossRef]
- Macfarlane, P.W.D.B.; Clark, E. The University of Glasgow (Uni-G) ECG analysis program. In Proceedings of the Computers in Cardiology, Lyon, France, 25–28 September 2005; pp. 451–454. [Google Scholar]
- Hodges, M.; Salerno, D.; Erlien, D.; Bazett’s, Q.T. correction reviewed. Evidence that a linear correction for heart rate is better. J. Am. Coll Cardiol. 1983, 1, 694. [Google Scholar]
- Houx, P.J.; Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Ford, I.; Bollen, E.L.; Buckley, B.; Stott, D.J.; Jukema, W.; Hyland, M.; et al. Testing cognitive function in elderly populations: The PROSPER study. PROspective Study of Pravastatin in the Elderly at Risk. J. Neurol. Neurosurg. Psychiatry 2002, 73, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sabayan, B.; van der Grond, J.; Westendorp, R.G.; van Buchem, M.A.; de Craen, A.J. Accelerated progression of white matter hyperintensities and subsequent risk of mortality: A 12-year follow-up study. Neurobiol. Aging 2015, 36, 2130–2135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stenfors, C.U.; Hanson, L.M.; Theorell, T.; Osika, W.S. Executive Cognitive Functioning and Cardiovascular Autonomic Regulation in a Population-Based Sample of Working Adults. Front. Psychol. 2016, 7, 1536. [Google Scholar] [CrossRef]
- Niemeijer, M.N.; van den Berg, M.E.; Eijgelsheim, M.; van Herpen, G.; Stricker, B.H.; Kors, J.A.; Rijnbeek, P.R. Short-term QT variability markers for the prediction of ventricular arrhythmias and sudden cardiac death: A systematic review. Heart 2014, 100, 1831–1836. [Google Scholar] [CrossRef]
- Vandeput, S. Nonlinear heart rate variability in a healthy population: Influence of age. In Proceedings of the Computers in Cardiology, Bologna, Italy, 14–17 September 2008; pp. 53–56. [Google Scholar]
- Piccirillo, G.; Cacciafesta, M.; Lionetti, M.; Nocco, M.; Di Giuseppe, V.; Moise, A.; Naso, C.; Marigliano, V. Influence of age, the autonomic nervous system and anxiety on QT-interval variability. Clin. Sci. (Lond) 2001, 101, 429–438. [Google Scholar] [CrossRef]
- Koschke, M.; Boettger, M.K.; Schulz, S.; Berger, S.; Terhaar, J.; Voss, A.; Yeragani, V.K.; Bar, K.J. Autonomy of autonomic dysfunction in major depression. Psychosom. Med. 2009, 71, 852–860. [Google Scholar] [CrossRef]
- Baumert, M.; Lambert, G.W.; Dawood, T.; Lambert, E.A.; Esler, M.D.; McGrane, M.; Barton, D.; Nalivaiko, E. QT interval variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H962–H968. [Google Scholar] [CrossRef]
- Yeragani, V.K.; Tancer, M.; Uhde, T. Heart rate and QT interval variability: Abnormal alpha-2 adrenergic function in patients with panic disorder. Psychiatry Res. 2003, 121, 185–196. [Google Scholar] [CrossRef]
- Kallergis, E.M.; Goudis, C.A.; Simantirakis, E.N.; Kochiadakis, G.E.; Vardas, P.E. Mechanisms, risk factors, and management of acquired long QT syndrome: A comprehensive review. Sci. World J. 2012, 2012, 212178. [Google Scholar] [CrossRef]
- Danese, A.; Federico, A.; Martini, A.; Mantovani, E.; Zucchella, C.; Tagliapietra, M.; Tamburin, S.; Cavallaro, T.; Marafioti, V.; Monaco, S.; et al. QTc Prolongation in Patients with Dementia and Mild Cognitive Impairment: Neuropsychological and Brain Imaging Correlations. J. Alzheimer’s Dis. JAD 2019, 72, 1241–1249. [Google Scholar] [CrossRef]
| Age, y, mean (SD) | 75.2 (3.3) |
| Female, n (%) | 2480 (53.6) |
| Age left school, y, mean (SD) | 15.1 (2.0) |
| Current smoker, n (%) | 1255 (27.1) |
| Cardiovascular risk factors | |
| History of CVD, n (%) | 1996 (43.1) |
| History of stroke or TIA, n (%) | 511 (11.0) |
| History of MI, n (%) | 540 (11.7) |
| Serum cholesterol, mmol/L, mean (SD) | 5.7 (0.9) |
| Body mass index, kg/m2, mean (SD) | 26.8 (4.2) |
| Diabetes mellitus, n (%) | 474 (10.2) |
| Antihypertensive therapy, n (%) | 3425 (74.0) |
| Pravastatin treatment, n (%) | 2309 (50.0) |
| SBP, mmHg, mean (SD) | 154.7 (21.6) |
| DBP, mmHg, mean (SD) | 83.8 (11.4) |
| Electrocardiogram measurements | |
| QT duration, ms, mean (SD) | 413.0 (35.6) |
| QTc duration, ms, mean (SD) | 424.9 (25.5) |
| JT duration, ms, mean (SD) | 319.6 (34.3) |
| JTc duration, ms, mean (SD) | 332.5 (25.0) |
| QRS duration, ms, mean (SD) | 93.4 (11) |
| Cognitive function | |
| Stroop Test, seconds, mean (SD) 1 | 65.6 (26.1) |
| LDCT, digits coded, mean (SD) 2 | 23.3 (7.9) |
| PLTi, pictures remembered mean (SD) 3 | 9.3 (1.9) |
| PLTd, pictures remembered, mean (SD) 3 | 10.1 (2.6) |
| Brain MRI measurements | |
| White matter hypertensities 4 | |
| Total lesion volume, ml, median (IQR) | 1.6 (0.5–5.5) |
| Subcortical, mL/y, median (IQR) | 0.5 (0.1–1.4) |
| Periventricular, mL/y, median (IQR) | 1 (0.3–3.9) |
| Brain atrophy 5 | |
| Intracranial volume, ml, mean (SD) | 1401 (144.5) |
| Parenchymal volume, ml, mean (SD) | 1034.3 (26.1) |
| Of % atrophy (ICV-Par/ICV ×100), mean (SD) | 26.1 (3.1) |
| Grey matter volume, mL, median (IQR) 6 | 23.2 (20.6–29.2) |
| Participants with microbleeds, n (%) 7 | 104 (2.2) |
| Participants with deep white matter microbleeds, n (%) | 28 (0.6) |
| QT (per SD) | QTc (per SD) | JT (per SD) | JTc (per SD) | QRS (per SD) | |
|---|---|---|---|---|---|
| Cognitive Test | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) |
| Minimally adjusted 1 | |||||
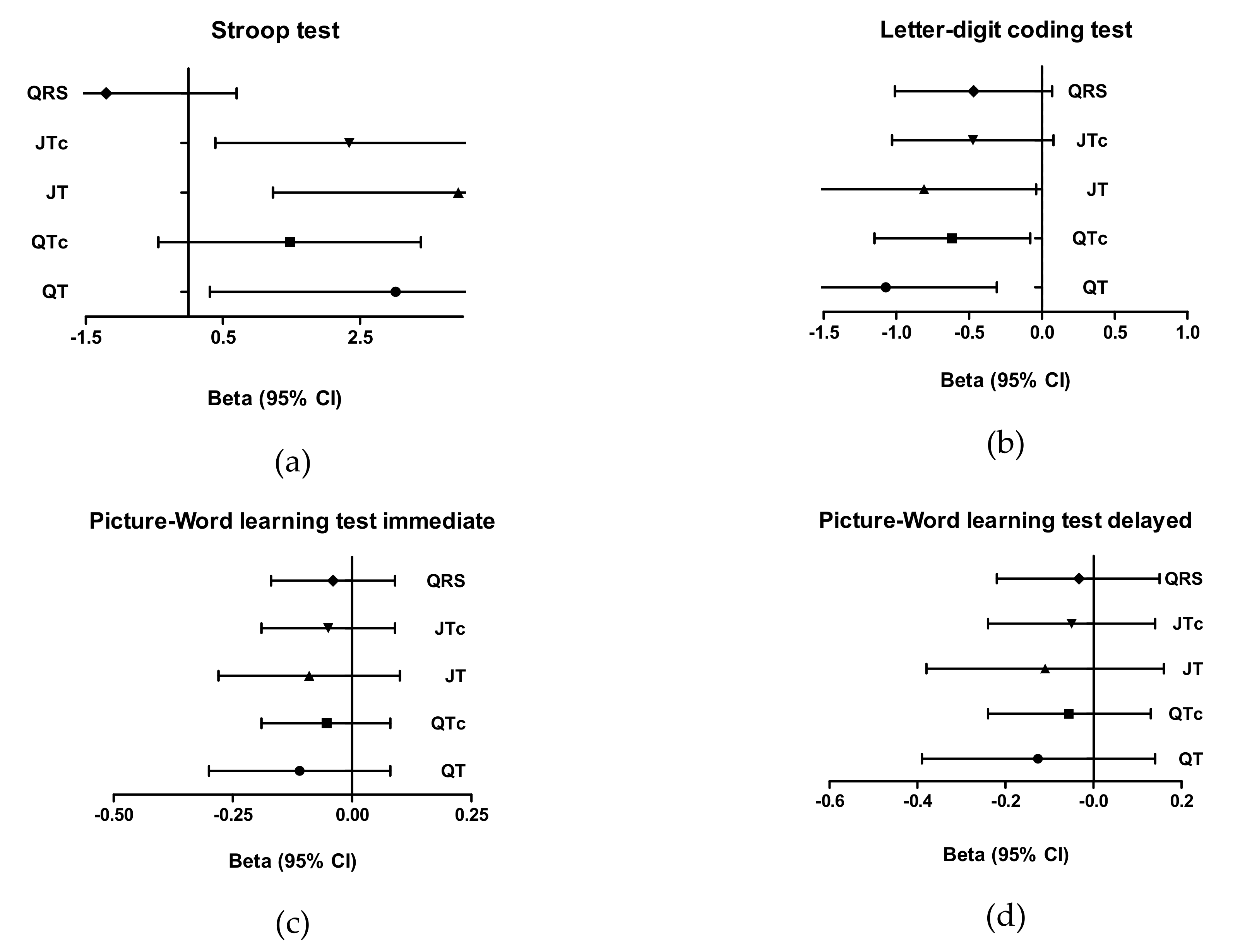
| Stroop, s | 2.60 (1.48; 3.72) | 1.13 (0.36; 1.91) | 2.22 (1.10; 3.34) | 1.00 (0.20; 1.79) | 0.74 (–0.06; 1.53) |
| LDCT, digits coded | –0.97 (–1.31; –0.63) | –0.49 (–0.73; –0.25) | –0.67 (–1.01; –0.34) | –0.34 (–0.57; –0.10) | –0.51 (–0.75; –0.26) |
| PLTi, pictures remembered | –0.14 (–0.22; –0.06) | –0.0 (–0.005; 0.00) | –0.09 (–0.17; –0.01) | –0.04 (–0.10; 0.01) | –0.08 (–0.14; –0.02) |
| PLTd, pictures remembered | –0.17 (–0.28; –0.06) | –0.07 (–0.14; 0.01) | –0.12 (–0.23; 0.00) | –0.04 (–0.12; 0.04) | –0.10 (–0.18; –0.02) |
| Fully adjusted 2 | |||||
| Stroop, s | 3.02 (0.31; 5.73) | 1.48 (–0.44; 3.39) | 3.93 (1.23; 6.64) | 2.34 (0.39; 4.30) | –1.20 (–3.11; 0.70) |
| LDCT, digits coded | –1.07 (–1.84; –0.31) | –0.62 (–1.15; –0.08) | –0.81 (–1.58; –0.04) | –0.47 (–1.03; 0.08) | –0.47 (–1.01; 0.07) |
| PLTi, pictures remembered | –0.11 (–0.30; 0.08) | –0.05 (–0.19; 0.08) | –0.09 (–0.28; 0.10) | –0.05 (–0.19; 0.09) | –0.04 (–0.17; 0.09) |
| PLTd, pictures remembered | –0.13 (–0.39; 0.14) | –0.06 (–0.24; 0.13) | –0.11 (–0.38; 0.16) | –0.05 (–0.24; 0.14) | –0.03 (–0.22; 0.15) |
| QT (per SD) | QTc (per SD) | JT (per SD) | JTc (per SD) | QRS (per SD) | |
|---|---|---|---|---|---|
| Cognitive Test | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) |
| Minimally adjusted 1 | |||||
| Stroop, s | –0.03 (–0.3; 0.24) | –0.02 (–0.29; 0.25) | –0.07 (–0.33; 0.20) | –0.06 (–0.33; 0.20) | 0.10 (–0.17; 0.37) |
| LDCT, digits coded | 0.04 (–0.03; 0.12) | 0.05 (–0.03; 0.12) | 0.05 (–0.03; 0.12) | 0.05 (–0.02; 0.13) | –0.01 (–0.08; 0.06) |
| PLTi, pictures remembered | 0.00 (–0.02; 0.02) | 0.006 (–0.01; 0.03) | 0.00 (–0.02; 0.02) | 0.01 (–0.01; 0.03) | 0.00 (–0.02; 0.02) |
| PLTd, pictures remembered | 0.00 (–0.03; 0.02) | –0.007 (–0.04; 0.02) | 0.00 (–0.03; 0.03) | 0.00 (–0.03; 0.03) | –0.006 (–0.04; 0.02) |
| Fully adjusted 2 | |||||
| Stroop, s | 0.02 (–0.61; 0.65) | –0.13 (–0.77; 0.52) | –0.15 (–0.79; 0.49) | –0.36 (–1.02; 0.29) | 0.44 (–0.18; 1.06) |
| LDCT, digits coded | 0.08 (–0.08; 0.24) | 0.02 (–0.15; 0.19) | 0.09 (–0.08; 0.25) | 0.02 (–0.15; 0.19) | 0.01 (–0.15; 0.17) |
| PLTi, pictures remembered | –0.02 (–0.07; 0.03) | –0.03 (–0.08; 0.02) | –0.02 (–0.07; 0.03) | –0.04 (–0.09; 0.01) | 0.01 (–0.04; 0.05) |
| PLTd, pictures remembered | 0.00 (–0.07; 0.07) | –0.03 (–0.10; 0.04) | 0.00 (–0.07; 0.07) | –0.04 (–0.11; 0.04) | 0.00 (–0.07; 0.07) |
| QT (per SD) | QTc (per SD) | JT (per SD) | JTc (per SD) | QRS (per SD) | |
|---|---|---|---|---|---|
| Brain MRI Measurements | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) |
| Fully adjusted 1 | |||||
| White matter hypertensities | |||||
| Total lesion volume, mL/y | –0.21 (–2.87; 2.44) | –0.01 (–1.81; 1.79) | –0.45 (–2.82; 1.93) | –0.19 (–1.85; 1.46) | 0.41 (–1.25; 2.07) |
| Subcortical, mL/y | –0.08 (–0.60; 0.44) | –0.04 (–0.39; 0.31) | –0.13 (–0.59; 0.34) | –0.07 (–0.40; 0.25) | 0.09 (–0.24; 0.42) |
| Periventricular, mL/y | –0.13 (–2.42; 2.15) | 0.03 (–1.52; 1.58) | –0.32 (–2.37; 1.73) | –0.12 (–1.55; 1.31) | 0.32 (–1.11; 1.75) |
| Brain atrophy | |||||
| Intracranial volume, mL/y | –4.0 (–44.3; 36.3) | 4.8 (–23.0; 32.6) | –5.9 (–41.8; 30.1) | 2.1 (–23.4; 27.6) | 3.9 (–21.2; 29.0) |
| Parenchymal volume, mL/y | –3.7 (–34.3; 26.8) | 3.2 (–17.9; 24.3) | –6.2 (–33.5; 21.1) | –0.5 (–18.9; 19.8) | 4.8 (–14.3; 23.8) |
| Of % atrophy (ICV-Par/ICV ×100) | 0.04 (–1.05; 1.14) | 0.02 (–0.72; 0.76) | 0.11 (–0.87; 1.08) | 0.07 (–0.61; 0.74) | –0.11 (–0.79; 0.57) |
| Grey matter volume, mL/y | –1.12 (–3.19; 0.95) | –0.68 (–2.06; 0.71) | –1.19 (–3.03; 0.64) | –0.81 (–2.10; 0.49) | 0.48 (–0.96; 1.91) |
| Number of microbleeds | 0.33 (–0.28; 0.94) | 0.19 (–0.21; 0.59) | 0.37 (–0.17; 0.91) | 0.23 (–0.14; 0.60) | –0.14 (–0.51; 0.23) |
| Number of deep white matter microbleeds | 0.01 (–0.08; 0.10) | 0.01 (–0.05; 0.07) | 0.02 (–0.06; 0.10) | 0.02 (–0.03; 0.08) | –0.02 (–0.08; 0.03) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zonneveld, M.H.; Noordam, R.; van der Grond, J.; Sabayan, B.; Mooijaart, S.P.; Mcfarlane, P.W.; Jukema, J.W.; Trompet, S. Ventricular Repolarization is Associated with Cognitive Function, but Not with Cognitive Decline and Brain Magnetic Resonance Imaging (MRI) Measurements in Older Adults. J. Clin. Med. 2020, 9, 911. https://doi.org/10.3390/jcm9040911
Zonneveld MH, Noordam R, van der Grond J, Sabayan B, Mooijaart SP, Mcfarlane PW, Jukema JW, Trompet S. Ventricular Repolarization is Associated with Cognitive Function, but Not with Cognitive Decline and Brain Magnetic Resonance Imaging (MRI) Measurements in Older Adults. Journal of Clinical Medicine. 2020; 9(4):911. https://doi.org/10.3390/jcm9040911
Chicago/Turabian StyleZonneveld, Michelle H., Raymond Noordam, Jeroen van der Grond, Behnam Sabayan, Simon P. Mooijaart, Peter W. Mcfarlane, J. Wouter Jukema, and Stella Trompet. 2020. "Ventricular Repolarization is Associated with Cognitive Function, but Not with Cognitive Decline and Brain Magnetic Resonance Imaging (MRI) Measurements in Older Adults" Journal of Clinical Medicine 9, no. 4: 911. https://doi.org/10.3390/jcm9040911
APA StyleZonneveld, M. H., Noordam, R., van der Grond, J., Sabayan, B., Mooijaart, S. P., Mcfarlane, P. W., Jukema, J. W., & Trompet, S. (2020). Ventricular Repolarization is Associated with Cognitive Function, but Not with Cognitive Decline and Brain Magnetic Resonance Imaging (MRI) Measurements in Older Adults. Journal of Clinical Medicine, 9(4), 911. https://doi.org/10.3390/jcm9040911





