Sleep Disordered Breathing in Hypertrophic Cardiomyopathy—Current State and Future Directions
Abstract
:1. Introduction
2. Review Criteria
3. Prevalence of Sleep Disordered Breathing (SDB) in Hypertrophic Cardiomyopathy (HCM)
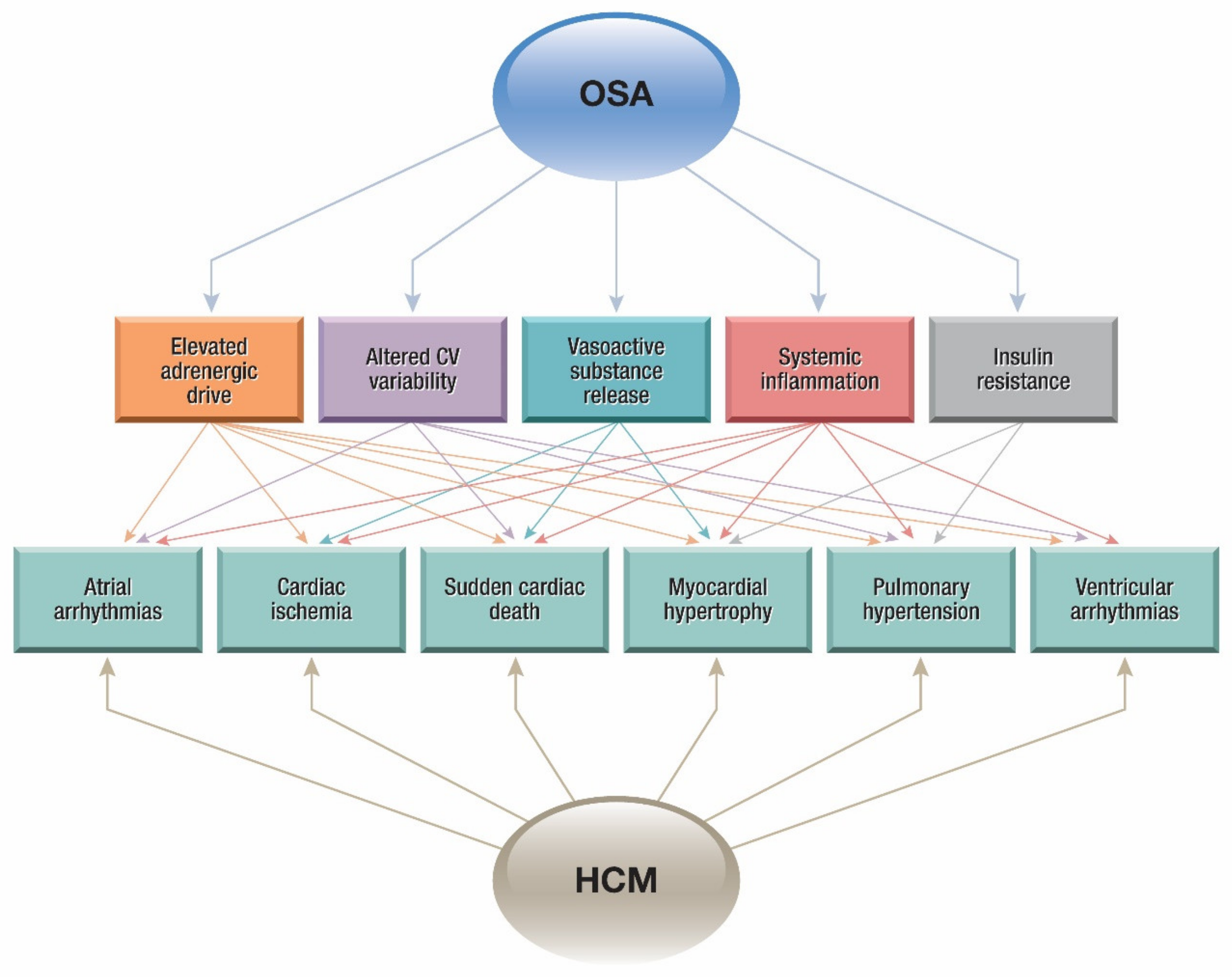
4. Cardiovascular Diseases and Obstructive Sleep Apnea (OSA)
4.1. Pathophysiology
4.1.1. Heart Failure
4.1.2. Coronary Artery Disease
4.1.3. Arrhythmias
4.1.4. Pulmonary Hypertension
5. Treatment
5.1. Current Treatment Modalities for HCM
5.2. Prevention of SCD
5.3. Therapy
5.4. Effects of OSA Treatment on HCM
6. Future Direction
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Braunwald, E.; Lambrew, C.T.; Rockoff, S.D.; Ross, J., Jr.; Morrow, A.G. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation 1964, 30, 3–119. [Google Scholar] [CrossRef]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.N.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy. Circulation 2011, 124, e783–e831. [Google Scholar]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef]
- Maron, B.J.; Spirito, P.; Wesley, Y.; Arce, J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N. Engl. J. Med. 1986, 315, 610–614. [Google Scholar] [CrossRef]
- Colan, S.D.; Lipshultz, S.E.; Lowe, A.M.; Sleeper, L.A.; Messere, J.; Cox, G.F.; Lurie, P.R.; John Orav, E.; Towbin, J.A. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: Findings from the Pediatric Cardiomyopathy Registry. Circulation 2007, 115, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Myers, K.A.; Mrkobrada, M.; Simel, D.L. Does this patient have obstructive sleep apnea? The Rational Clinical Examination systematic review. J. Am. Med. Assoc. 2013, 310, 731–741. [Google Scholar] [CrossRef]
- Venkataraman, S.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep Apnea, Hypertension and the Sympathetic Nervous System in the Adult Population. J. Clin. Med. 2020, 9, 591. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Khoo, S.-M.; Chan, M.Y.; Wong, H.-B.; Low, A.F.; Phua, Q.-H.; Richards, A.M.; Huay-Cheem, T.; Tiong-Cheng, Y. Severe Obstructive Sleep Apnea and Outcomes Following Myocardial Infarction. J. Clin. Sleep Med. 2011, 7, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, I.H.; Roberts-Thomson, K.C.; Kistler, P.M.; Edwards, G.A.; Spence, S.; Sanders, P.; Kalman, J.M. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: Implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm 2010, 7, 1263–1270. [Google Scholar] [CrossRef]
- Linz, D.; Schotten, U.; Neuberger, H.-R.; Böhm, M.; Wirth, K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011, 8, 1436–1443. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [Green Version]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 686–717. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Sert Kuniyoshi, F.H.; Covassin, N.; Singh, P.; Gami, A.S.; Wang, S.; Chahal, C.A.A.; Wei, Y.; Somers, V.K. Nocturnal Hypoxemia Due to Obstructive Sleep Apnea Is an Independent Predictor of Poor Prognosis After Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003162. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Sert Kuniyoshi, F.H.; Covassin, N.; Singh, P.; Gami, A.S.; Chahal, C.A.A.; Somers, V.K. Excessive Daytime Sleepiness Independently Predicts Increased Cardiovascular Risk After Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e007221. [Google Scholar] [CrossRef] [Green Version]
- Al-Saleh, S.; Kantor, P.F.; Chadha, N.K.; Tirado, Y.; James, A.L.; Narang, I. Sleep-disordered Breathing in Children with Cardiomyopathy. Ann. Am. Thorac. Soc. 2014, 11, 770–776. [Google Scholar] [CrossRef]
- Banno, K.; Shiomi, T.; Sasanabe, R.; Otake, K.; Hasegawa, R.; Maekawa, M.; Ito, T. Sleep-Disordered Breathing in Patients with Idiopathic Cardiomyopathy. Circ. J. 2004, 68, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Eleid, M.F.; Konecny, T.; Orban, M.; Sengupta, P.P.; Somers, V.K.; Parish, J.M.; Mookadam, F.; Brady, P.A.; Sullivan, B.L.; Khandheria, B.K.; et al. High Prevalence of Abnormal Nocturnal Oximetry in Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 1805–1809. [Google Scholar] [CrossRef] [Green Version]
- Konecny, T.; Brady, P.A.; Orban, M.; Lin, G.; Pressman, G.S.; Lehar, F.; Tomas, K.; Gersh, B.J.; Tajik, A.J.; Ommen, S.R.; et al. Interactions Between Sleep Disordered Breathing and Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2010, 105, 1597–1602. [Google Scholar] [CrossRef]
- Pedrosa, R.P.; Lima, S.G.; Drager, L.F.; Genta, P.R.; Amaro, A.C.; Antunes, M.O.; Arteaga, E.; Mady, C.; Lorenzi-Filho, G. Sleep quality and quality of life in patients with hypertrophic cardiomyopathy. Cardiology 2010, 117, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Bitter, T.; Oldenburg, O.; Horstkotte, D.; Faber, L. Incidence of Sleep-Disordered Breathing in Patients with Hypertrophic Cardiomyopathy. Congest. Heart Fail. 2011, 17, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.I.; Shamoun, F.E.; Esser, H.; Parthasarathy, S.; Ackerman, M.J.; Geske, J.B.; Ommen, S.R.; Love, W.T.; Somers, V.K.; Chahl, A.C.A.; et al. Sleep Disordered Breathing in Hypertrophic Cardiomyopathy. Sleep Vigil. 2019. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef] [Green Version]
- Narkiewicz, K.; Montano, N.; Cogliati, C.; van de Borne, P.J.H.; Dyken, M.E.; Somers, V.K. Altered Cardiovascular Variability in Obstructive Sleep Apnea. Circulation 1998, 98, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Phillips, B.G.; Narkiewicz, K.; Pesek, C.A.; Haynes, W.G.; Dyken, M.E.; Somers, V.K. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J. Hypertens. 1999, 17, 61–66. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Lam, B.; Ng, M.M.T.; Lam, W.K.; Tsang, K.W.T.; Lam, K.S.L. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 2002, 165, 670–676. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep Apnea. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Konecny, T.; Somers, V.K. Sleep-Disordered Breathing in Hypertrophic Cardiomyopathy. Chest 2014, 146, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Parker, J.D.; Newton, G.E.; Floras, J.S.; Mak, S.; Chiu, K.-L.; Rattanaumpawan, P.; Tomilson, G.; Bradley, T.D. Influence of Obstructive Sleep Apnea on Mortality in Patients with Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Fung, J.W.H.; Li, T.S.T.; Choy, D.K.L.; Yip, G.W.K.; Ko, F.W.S.; Sanderson, J.E.; Hui, D.S.C. Severe obstructive sleep apnea is associated with left ventricular diastolic dysfunction. Chest 2002, 121, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Link, M.S.; Lesser, J.R.; Chan, R.H.M.; Garberich, R.F.; Udelson, J.E.; Maron, M.S. Hypertrophic Cardiomyopathy in Adulthood Associated with Low Cardiovascular Mortality with Contemporary Management Strategies. J. Am. Coll. Cardiol. 2015, 65, 1915–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charron, P.; Elliott, P.M.; Gimeno, J.R.; Caforio, A.L.P.; Kaski, J.P.; Tavazzi, L.; Tendera, M.; Maupain, C.; Laroche, C.; Rubis, P.; et al. The Cardiomyopathy Registry of the EURObservational Research Programme of the European Society of Cardiology: Baseline data and contemporary management of adult patients with cardiomyopathies. Eur. Heart J. 2018, 39, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buda, A.J.; Pinsky, M.R.; Ingels, N.B.; Daughters, G.T.; Stinson, E.B.; Alderman, E.L. Effect of Intrathoracic Pressure on Left Ventricular Performance. N. Engl. J. Med. 1979, 301, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Okuda, N.; Ito, T.; Emura, N.; Suwa, M.; Hayashi, T.; Yoneda, H.; Kitaura, Y. Depressed myocardial contractile reserve in patients with obstructive sleep apnea assessed by tissue Doppler imaging with dobutamine stress echocardiography. Chest 2007, 131, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Basso, C. Hypertrophic cardiomyopathy and sudden death in the young: Pathologic evidence of myocardial ischemia. Adv. Cardiomyopathies 2000, 31, 988–998. [Google Scholar] [CrossRef]
- Villa, A.; Bettencourt, N.; Zarinabad, N.; Baydes, R.H.; Nagel, E.; Chiribiri, A. Stress perfusion CMR in hypertrophic cardiomyopathy: Comparison with late gadolinium enhancement. J. Cardiovasc. Magn. Reson. 2014, 16, P324. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.D.M.; Sammut, E.; Zarinabad, N.; Carr-White, G.; Lee, J.; Bettencourt, N.; Razavi, R.; Nagel, E.; Chiribiri, A. Microvascular ischemia in hypertrophic cardiomyopathy: New insights from high-resolution combined quantification of perfusion and late gadolinium enhancement. J. Cardiovasc. Magn. Reson. 2015, 18, 4. [Google Scholar] [CrossRef] [Green Version]
- Fernlund, E.; Schlegel, T.T.; Platonov, P.G.; Carlson, J.; Carlsson, M.; Liuba, P. Peripheral microvascular function is altered in young individuals at risk for hypertrophic cardiomyopathy and correlates with myocardial diastolic function. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1351–H1358. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Wolfson, J.K.; Epstein, S.E.; Roberts, W.C. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 1986, 8, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary Microvascular Dysfunction and Prognosis in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Girolami, F.; Torricelli, F.; Camici, P.G. Relevance of Coronary Microvascular Flow Impairment to Long-Term Remodeling and Systolic Dysfunction in Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 1043–1048. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.A.; Yaggi, H.K.; Concato, J.; Mohsenin, V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath 2010, 14, 131–136. [Google Scholar] [CrossRef]
- Butt, M.; Khair, O.A.; Dwivedi, G.; Shantsila, A.; Shantsila, E.; Lip, G.Y.H. Myocardial Perfusion by Myocardial Contrast Echocardiography and Endothelial Dysfunction in Obstructive Sleep Apnea. Hypertension 2011, 58, 417–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siontis, K.C.; Geske, J.B.; Ong, K.; Nishimura, R.A.; Ommen, S.R.; Gersh, B.J. Atrial fibrillation in hypertrophic cardiomyopathy: Prevalence, clinical correlations, and mortality in a large high-risk population. J. Am. Heart Assoc. 2014, 3, e001002. [Google Scholar] [CrossRef] [Green Version]
- Bonow, R.O.; Frederick, T.M.; Bacharach, S.L.; Green, M.V.; Goose, P.W.; Maron, B.J.; Rosing, D.R. Atrial systole and left ventricular filling in hypertrophic cardiomyopathy: Effect of verapamil. Am. J. Cardiol. 1983, 51, 1386–1391. [Google Scholar] [CrossRef]
- Burstein, B.; Nattel, S. Atrial Fibrosis: Mechanisms and Clinical Relevance in Atrial Fibrillation. J. Am. Coll. Cardiol. 2008, 51, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Linz, D.; Schotten, U.; Neuberger, H.-R.; Böhm, M.; Wirth, K. Combined blockade of early and late activated atrial potassium currents suppresses atrial fibrillation in a pig model of obstructive apnea. Heart Rhythm 2011, 8, 1933–1939. [Google Scholar] [CrossRef]
- Lammers, W.J.; Kirchhof, C.; Bonke, F.I.; Allessie, M.A. Vulnerability of rabbit atrium to reentry by hypoxia. Role of inhomogeneity in conduction and wavelength. Am. J. Physiol. 1992, 262, H47–H55. [Google Scholar] [CrossRef]
- Lin, Y.K.; Lai, M.S.; Chen, Y.C.; Cheng, C.C.; Huang, J.H.; Chen, S.A.; Chen, Y.-J.; Lin, C.-I. Hypoxia and reoxygenation modulate the arrhythmogenic activity of the pulmonary vein and atrium. Clin. Sci. 2012, 122, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasai, T.; Bradley, T.D. Obstructive Sleep Apnea and Heart Failure. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Orban, M.; Bruce, C.J.; Pressman, G.S.; Leinveber, P.; Romero-Corral, A.; Korinek, J.; Konecny, T.; Villarraga, H.R.; Kara, T.; Caples, S.M.; et al. Dynamic Changes of Left Ventricular Performance and Left Atrial Volume Induced by the Mueller Maneuver in Healthy Young Adults and Implications for Obstructive Sleep Apnea, Atrial Fibrillation, and Heart Failure. Am. J. Cardiol. 2008, 102, 1557–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somers, V.; Javaheri, S. Cardiovascular effects of sleep-related breathing disorders. Princ. Pract. Sleep Med. 2017, 1243–1252. [Google Scholar] [CrossRef]
- Monahan, K.; Storfer-Isser, A.; Mehra, R.; Shahar, E.; Mittleman, M.; Rottman, J.; Punjabi, N.; Sanders, M.; Quan, S.F.; Resnick, H.; et al. Triggering of Nocturnal Arrhythmias by Sleep-Disordered Breathing Events. J. Am. Coll. Cardiol. 2009, 54, 1797–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of Nocturnal Arrhythmias with Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef]
- Wang, S.; Cui, H.; Song, C.; Zhu, C.; Wu, R.; Meng, L.; Yu, Q.; Huang, X.; Wang, S. Obstructive sleep apnea is associated with nonsustained ventricular tachycardia in patients with hypertrophic obstructive cardiomyopathy. Heart Rhythm 2019, 16, 694–701. [Google Scholar] [CrossRef]
- Monserrat, L.; Elliott, P.M.; Gimeno, J.R.; Sharma, S.; Penas-Lado, M.; McKenna, W.J. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2003, 42, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lian, Z.; Rowin, E.J.; Maron, B.J.; Maron, M.S.; Link, M.S. Prognostic Implications of Nonsustained Ventricular Tachycardia in High-Risk Patients with Hypertrophic Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2017, 10, e004604. [Google Scholar] [CrossRef]
- McLaughlin, V.V.; Archer, S.L.; Badesch, D.B.; Barst, R.J.; Farber, H.W.; Lindner, J.R.; Mathier, M.A.; McGoon, M.D.; Park, M.H.; Rosenson, R.S.; et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. J. Am. Coll. Cardiol. 2009, 53, 1573–1619. [Google Scholar] [CrossRef]
- Wu, X.; Cui, H.; Xiao, M.H.; Lu, J.; Zhu, C.S.; Wang, S.Y.; Huang, X.H. Prevalence of pulmonary hypertension in patients with hypertrophic obstructive cardiomyopathy: A case-control study. Zhonghua Xin Xue Guan Bing Za Zhi 2016, 44, 1010–1014. [Google Scholar]
- Musumeci, M.B.; Mastromarino, V.; Casenghi, M.; Tini, G.; Francia, P.; Maruotti, A.; Romaniello, A.; Magri, D.; Lillo, R.; Adduci, C.; et al. Pulmonary hypertension and clinical correlates in hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 248, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kanbayashi, K.; Minami, Y.; Haruki, S.; Maeda, R.; Itani, R.; Ashihara, K.; Hagiwara, N. Association of elevated pulmonary artery systolic pressure with stroke and systemic embolic events in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2017, 240, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Geske, J.B.; Hebl, V.B.; Nishimura, R.A.; Schaff, H.V.; Ackerman, M.J.; Klarich, K.W.; Siontis, K.C.; Coutinho, T.; Dearani, J.A.; et al. Pulmonary hypertension is associated with worse survival in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 604–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerbass, F.B.; Salemi, V.M.C.; Pedrosa, R.P.; Portilho, N.D.P.; Ferreira-Filho, J.C.A.; Moriya, H.T.; Murillo, O.A.; Arteaga-Fernandez, E.; Drager, L.F.; Lorenzi-Filho, G. Acute Effects of Nasal CPAP in Patients with Hypertrophic Cardiomyopathy. Chest 2016, 150, 1050–1058. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Anastasakis, A.; Rapezzi, C.; Biagni, E.; Gimeno, J.R.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Ommen, S.R.; Christiaans, I.; Arbustini, E.; Garcia-Pavia, P.; Cecchi, F.; Olivotta, I.; Kitaoka, H.; Gotsman, I.; et al. International External Validation Study of the 2014 European Society of Cardiology Guidelines on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy (EVIDENCE-HCM). Circulation 2018, 137, 1015–1023. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, C.; Akhtar, M.M.; Anastasiou, Z.; Guttmann, O.P.; Vriesendorp, P.A.; Michels, M.; Magri, D.; Autore, C.; Fernandez, A.; Ochoa, J.P.; et al. Effectiveness of the 2014 European Society of Cardiology guideline on sudden cardiac death in hypertrophic cardiomyopathy: A systematic review and meta-analysis. Heart 2019, 105, 623–631. [Google Scholar] [CrossRef]
- Weng, Z.; Yao, J.; Chan, R.H.; He, J.; Yang, X.; Zhou, Y.; He, Y. Prognostic Value of LGE-CMR in HCM. JACC Cardiovasc. Imaging 2016, 9, 1392–1402. [Google Scholar] [CrossRef]
- Sedaghat-Hamedani, F.; Kayvanpour, E.; Tugrul, O.F.; Lai, A.; Amr, A.; Haas, J.; Proctor, T.; Ehlermann, P.; Jensen, K.; Katus, H.A.; et al. Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: A meta-analysis on 7675 individuals. Clin. Res. Cardiol. 2018, 107, 30–41. [Google Scholar] [CrossRef]
- Steriotis, A.K.; Sharma, S. Risk Stratification in Hypertrophic Cardiomyopathy. Eur. Cardiol. 2015, 10, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Hedner, J.; Norum, J.; Kraiczi, H.; Carlson, J. Increased Incidence of Cardiovascular Disease in Middle-aged Men with Obstructive Sleep Apnea: A 7-year follow-up. Am. J. Respir. Crit. Care Med. 2002, 166, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Milleron, O. Benefits of obstructive sleep apnoea treatment in coronary artery disease: A long-term follow-up study. Eur. Heart J. 2004, 25, 728–734. [Google Scholar] [CrossRef]
- Drager, L.F.; Bortolotto, L.A.; Figueiredo, A.C.; Silva, B.C.; Krieger, E.M.; Lorenzi-Filho, G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest 2007, 131, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Cloward, T.V.; Walker, J.M.; Farney, R.J.; Anderson, J.L. Left Ventricular Hypertrophy Is a Common Echocardiographic Abnormality in Severe Obstructive Sleep Apnea and Reverses with Nasal Continuous Positive Airway Pressure. Chest 2003, 124, 594–601. [Google Scholar] [CrossRef]
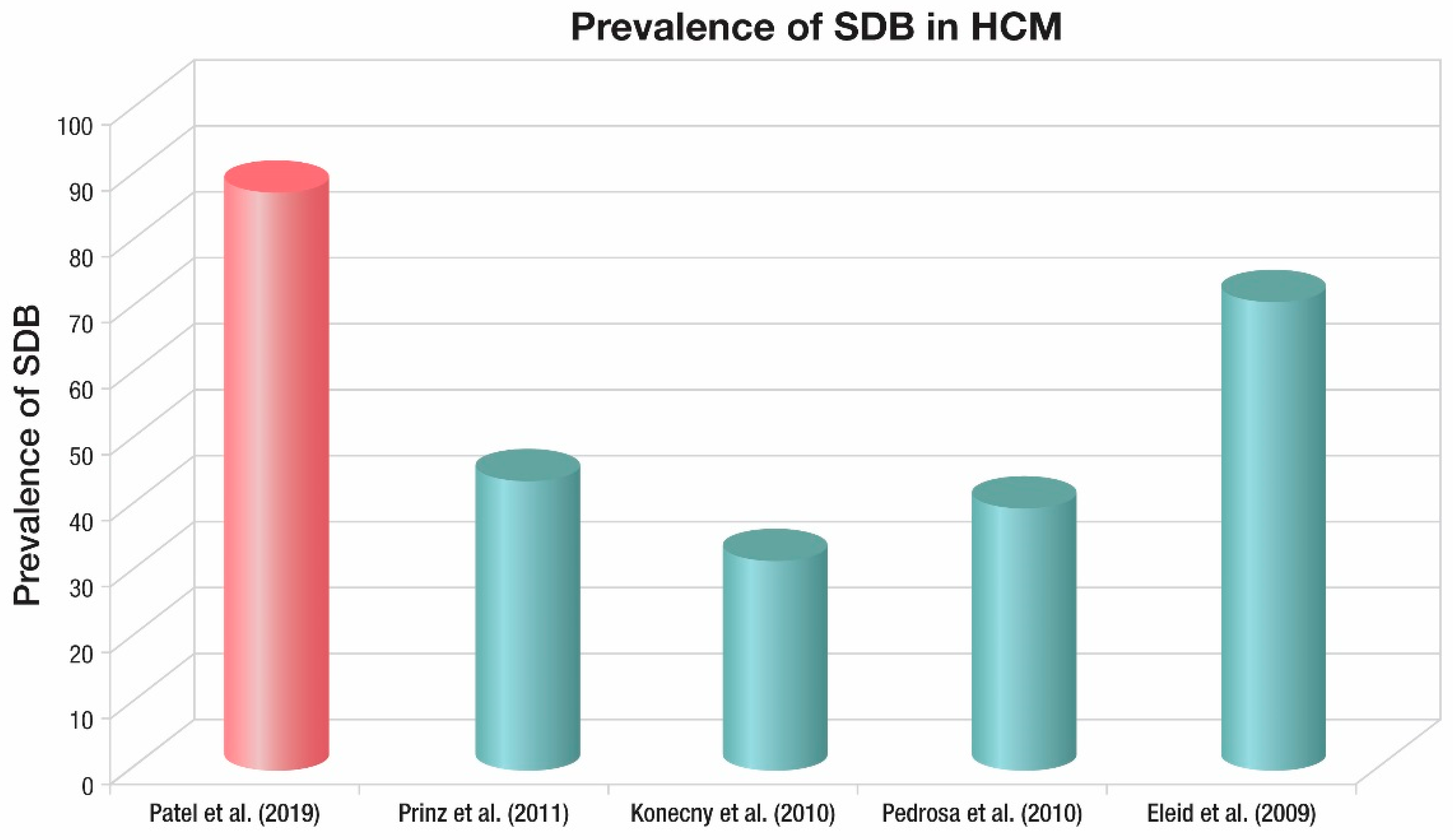
| Author | Prinz et al. | Konecny et al. | Pedrosa et al. | Eleid et al. |
|---|---|---|---|---|
| Year published | 2011 | 2010 | 2010 | 2009 |
| Patients (n) | 63 | 91 | 80 | 100 |
| HCM patients n (%) | 63 (100) | 91 (100) | 80 (100) | 100 (100) |
| Age (years) | 59 (34–85) | 52 (20–83) | 47 (32–58) | 55 (44–75) |
| Male (%) | 63 | 68 | 49 | 59 |
| OSA n (%) | 44 | 32 | 40 | 71 |
| BMI mean (range) (kg/m2) | 26.9 (21.4–32.4) | 31 (26–36) | 26.4 (17–35.8) | 31.1 (24.6–37.6) |
| Method used | Portable monitor | Overnight oximetry | Portable monitor | Overnight oximetry |
| Criteria used | American Academy of Sleep Medicine 1999 criteria | >5 events/hr of decrease in O2 saturation of at least 4%, with a threshold of 90% | American Academy of Sleep Medicine 1999 criteria | >5 events/hr of decrease in O2 saturation of at least 4%, with a threshold of 90% |
| RDI/ODI (events/h) | 34.8 (2.3–67.3) | 8.6 | 9.2 (4.1–24.8) | N/A |
| Nadir SpO2 (%) | N/A | N/A | 84 (78–88) | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkataraman, S.; Karim, S.; Rajendran, A.; Chahal, C.A.A.; Somers, V.K. Sleep Disordered Breathing in Hypertrophic Cardiomyopathy—Current State and Future Directions. J. Clin. Med. 2020, 9, 901. https://doi.org/10.3390/jcm9040901
Venkataraman S, Karim S, Rajendran A, Chahal CAA, Somers VK. Sleep Disordered Breathing in Hypertrophic Cardiomyopathy—Current State and Future Directions. Journal of Clinical Medicine. 2020; 9(4):901. https://doi.org/10.3390/jcm9040901
Chicago/Turabian StyleVenkataraman, Shreyas, Shahid Karim, Aiswarya Rajendran, C. Anwar A. Chahal, and Virend K. Somers. 2020. "Sleep Disordered Breathing in Hypertrophic Cardiomyopathy—Current State and Future Directions" Journal of Clinical Medicine 9, no. 4: 901. https://doi.org/10.3390/jcm9040901
APA StyleVenkataraman, S., Karim, S., Rajendran, A., Chahal, C. A. A., & Somers, V. K. (2020). Sleep Disordered Breathing in Hypertrophic Cardiomyopathy—Current State and Future Directions. Journal of Clinical Medicine, 9(4), 901. https://doi.org/10.3390/jcm9040901





