Clinical and Radiologic Features Together Better Predict Lung Nodule Malignancy in Patients with Soft-Tissue Sarcoma
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Issues
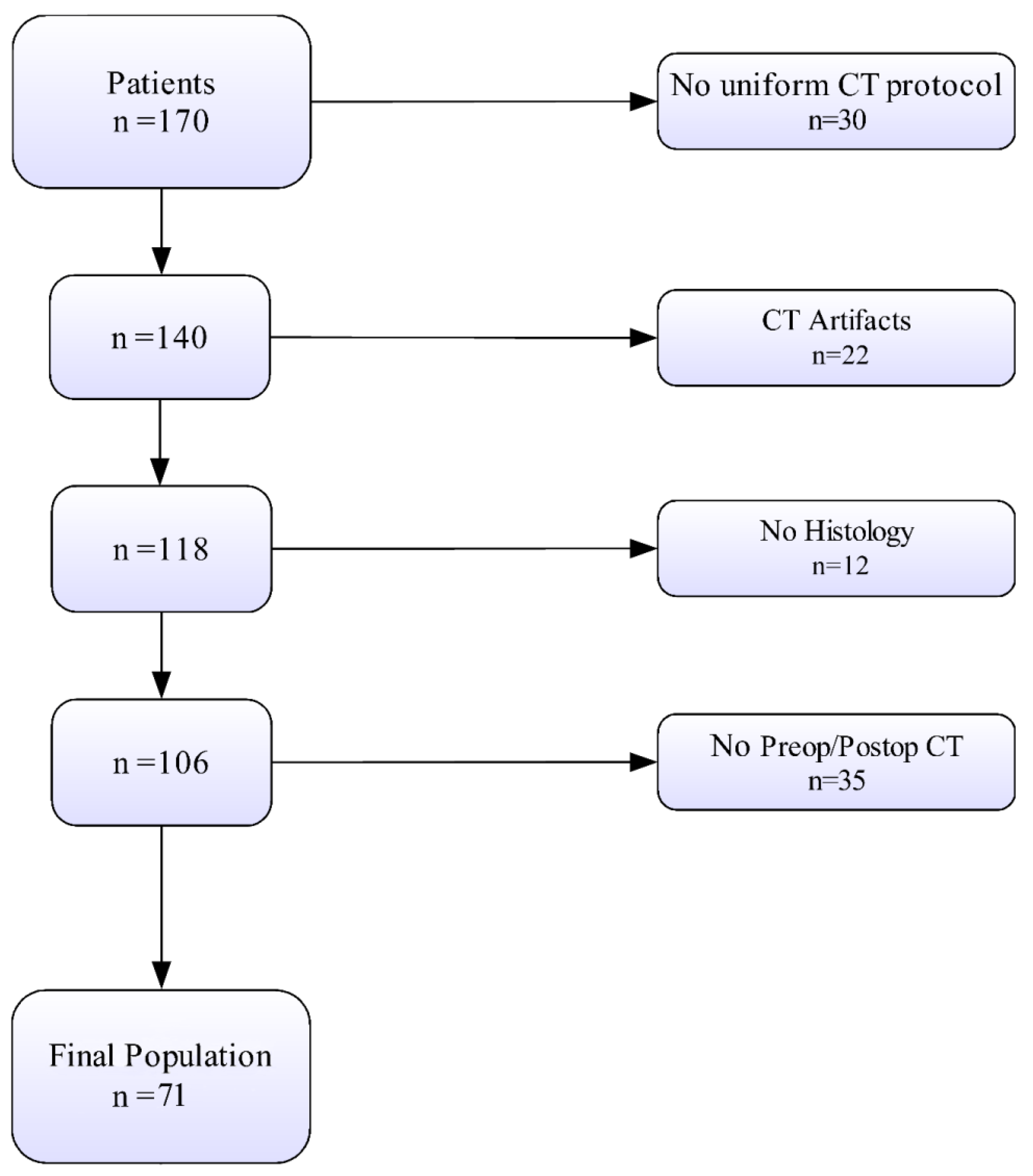
2.2. Patient Population
2.3. Definitions and Classifications
2.4. Computed Tomography (CT) Readings
2.5. Statistical Analysis
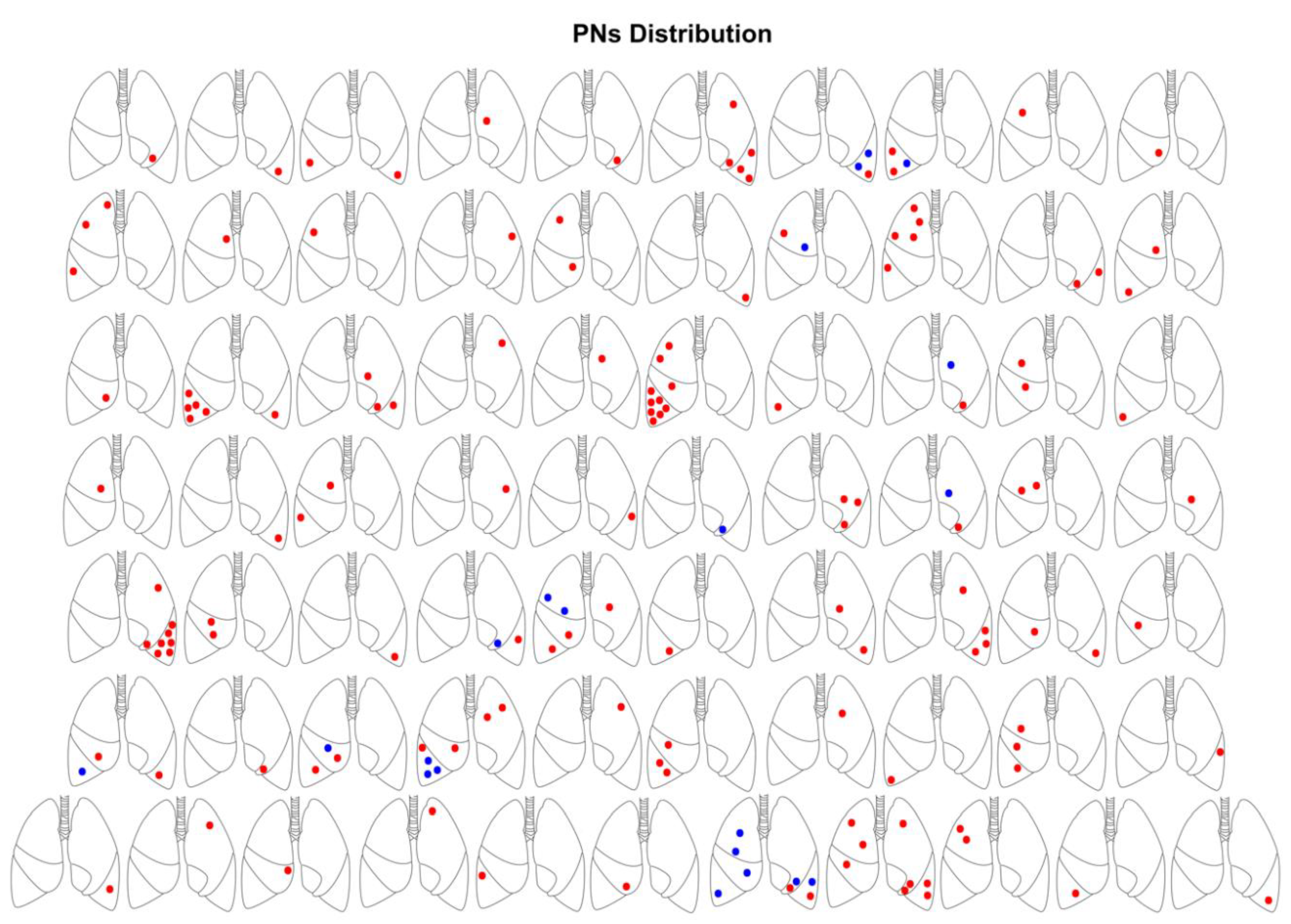
3. Results
3.1. Clinical and CT Findings
3.2. Multivariable Model Performance
3.3. Results of Multiple Regression Analysis
3.4. Interaction between Clinical and CT Features
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Inter-Observer | Intra-Observer | |
|---|---|---|
| Coefficient (95% CI) | Coefficient (95% CI) | |
| Preoperative Size (mm) | 0.8 (0.7–0.9) | 0.9 (0.8–1) |
| Preoperative Density (HU) | 0.9 (0.8–1) | 0.9 (0.8–1) |
| Preoperative Margins | 0.9 (0.8–1) | 1 |
References
- Enzinger, F.M.; Weiss, S.W. Soft Tissue Tumors; Mosby: St Louis, MI, USA, 1995. [Google Scholar]
- Doyle, L.A. Sarcoma classification: An update based on the 2013 World Health Organization Classification of Tumors of Soft Tissue and Bone. Cancer 2014, 120, 1763–1774. [Google Scholar] [CrossRef]
- van Praag, V.M.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.K.; Pollock, R.; Aston, W.; van der Hage, J.A.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised sarcoma care (PERSARC). Eur. J. Cancer 2017, 83, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, K.G.; Burt, M.E.; Jara, E.; Ginsberg, R.J.; Woodruff, J.M.; Leung, D.H.; Brennan, M.F. Pulmonary metastases from soft tissue sarcoma: Analysis of patterns of diseases and postmetastasis survival. Ann. Surg. 1999, 229, 602–610, discussion 610-602. [Google Scholar] [CrossRef] [PubMed]
- Dudeck, O.; Zeile, M.; Andreou, D.; Schnapauff, D.; Pech, M.; Wieners, G.; Ricke, J.; Reichardt, P.; Tunn, P.U. Computed tomographic criteria for the discrimination of subcentimeter lung nodules in patients with soft-tissue sarcomas. Clin. Imaging 2011, 35, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, H. Seventh Edition American Joint Committee on Cancer Staging of Cutaneous Non-Melanoma Skin Cancer. Am. J. Clin. Dermatol. 2011, 12, 155–156. [Google Scholar] [CrossRef]
- Christie-Large, M.; James, S.L.; Tiessen, L.; Davies, A.M.; Grimer, R.J. Imaging strategy for detecting lung metastases at presentation in patients with soft tissue sarcomas. Eur. J. Cancer 2008, 44, 1841–1845. [Google Scholar] [CrossRef]
- Ginsberg, M.S.; Griff, S.K.; Go, B.D.; Yoo, H.H.; Schwartz, L.H.; Panicek, D.M. Pulmonary nodules resected at video-assisted thoracoscopic surgery: Etiology in 426 patients. Radiology 1999, 213, 277–282. [Google Scholar] [CrossRef]
- Fletcher, C.D. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 2014, 64, 2–11. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Staging AJCC, Reporting ER and Society AC. Manual for Staging of Cancer; American Joint Committee, 1978. [Google Scholar]
- Ferrari, A.; Chi, Y.-Y.; De Salvo, G.L.; Orbach, D.; Brennan, B.; Randall, R.L.; McCarville, M.B.; Black, J.O.; Alaggio, R.; Hawkins, D.S.; et al. Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children’s Oncology Group. Eur. J. Cancer 2017, 78, 1–6. [Google Scholar] [CrossRef]
- Chavent, M.; Saracco, J.; Kuentz-Simonet, V.; Liquet, B. ClustOfVar: An R package for the clustering of variables. J. Stat. Softw. 2012, 50, 1–16. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; van Praag, V.M.; Posch, F.; Bergovec, M.; Leitner, L.; Friesenbichler, J.; Heregger, R.; Riedl, J.M.; Pichler, M.; Gerger, A.; et al. Surgery for metachronous metastasis of soft tissue sarcoma—A magnitude of benefit analysis using propensity score methods. Eur. J. Surg. Oncol. 2019, 45, 242–248. [Google Scholar] [CrossRef]
- Brader, P.; Abramson, S.J.; Price, A.P.; Nicole, M.; Ishill, N.M.; Emily, Z.C.; Moskowitz, C.S.; Quaglia, M.P.L.; Ginsberg, M.S. Do characteristics of pulmonary nodules on computed tomography in children with known osteosarcoma help distinguish whether the nodules are malignant or benign? J. Pediatric Surg. 2011, 46, 729–735. [Google Scholar] [CrossRef]
- Caparica, R.; Mak, M.P.; Rocha, C.H.; Velho, P.H.; Viana, P.; Moura, M.R.; Menezes, M.R.; Amato, M.B.; Feher, O. Pulmonary Nodules in Patients with Nonpulmonary Cancer: Not Always Metastases. J. Glob. Oncol. 2016, 2, 138–144. [Google Scholar] [CrossRef]
- Kattan, M.W.; Leung, D.H.; Brennan, M.F. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 791–796. [Google Scholar] [CrossRef]
- Ost, D.E.; Gould, M.K. Decision Making in Patients with Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2012, 185, 363–372. [Google Scholar] [CrossRef]
- Davis, S.D. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology 1991, 180, 1–12. [Google Scholar] [CrossRef]
- Rubin, G.D. Lung nodule and cancer detection in computed tomography screening. J. Thorac. Imaging 2015, 30, 130–138. [Google Scholar] [CrossRef]
- Larici, A.R.; Farchione, A.; Franchi, P.; Ciliberto, M.; Cicchetti, G.; Calandriello, L.; del Ciello, A.; Bonomo, L. Lung nodules: Size still matters. Eur. Respir. Rev. 2017, 26, 170025. [Google Scholar] [CrossRef]
- Khokhar, S.; Vickers, A.; Moore, M.S.; Mironov, S.; Stover, D.E.; Feinstein, M.B. Significance of non-calcified pulmonary nodules in patients with extrapulmonary cancers. Thorax 2006, 61, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kayton, M.L.; Huvos, A.G.; Casher, J.; Abramson, S.J.; Rosen, N.S.; Wexler, L.H.; Meyers, P.; LaQuaglia, M.P. Computed tomographic scan of the chest underestimates the number of metastatic lesions in osteosarcoma. J. Pediatric Surg. 2006, 41, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, F.; Bazzocchi, A.; Ciminari, R.; Righi, A.; Rocca, M.; Rimondi, E.; Picci, P.; Bacchi Reggiani, M.L.; Albisinni, U.; Zompatori, M.; et al. The many faces of pulmonary metastases of osteosarcoma: Retrospective study on 283 lesions submitted to surgery. Eur. J. Radiol. 2015, 84, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Matsusaka, M.; Mizumoto, K.; Mori, M.; Yoshizaki, T.; Matsubara, T.; Asanuma, K.; Sudo, A. Analysis of pulmonary nodules in patients with high-grade soft tissue sarcomas. PLoS ONE 2017, 12, e0172148. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Matsumine, A.; Niimi, R.; Matsubara, T.; Kusuzaki, K.; Maeda, M.; Tagami, T.; Uchida, A. Management of small pulmonary nodules in patients with sarcoma. Clin. Exp. Metastasis 2009, 26, 713–718. [Google Scholar] [CrossRef]
- Rissing, S.; Rougraff, B.T.; Davis, K. Indeterminate pulmonary nodules in patients with sarcoma affect survival. Clin. Orthop. Relat. Res. 2007, 459, 118–121. [Google Scholar] [CrossRef]
- Leef, J.L., 3rd; Klein, J.S. The solitary pulmonary nodule. Radiol. Clin. North. Am. 2002, 40, 123–143. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Elias, A.; Lee, C.Y.; Nakache, Y.P.; Li, C.S.; Shah, D.R.; Boutin, R.D.; Canter, R.J. Predictors of residual disease after unplanned excision of soft tissue sarcomas. J. Surg. Res. 2017, 208, 26–32. [Google Scholar] [CrossRef]
- Johnson, G.D.; Smith, G.; Dramis, A.; Grimer, R.J. Delays in referral of soft tissue sarcomas. Sarcoma 2008, 2008, 378574. [Google Scholar] [CrossRef]



| Age | 50.00 (42.50, 61.50) | |
| Female sex | 25 (35.2) | |
| * Chemotherapy | 45 (63.4) | |
| * Radiotherapy | 56 (78.9) | |
| Primary Tumor | ||
| Size (mm) | 9.00 (6.00, 12.25) | |
| Histology | ||
| Synovial Sarcoma | 13 (18.3) | |
| Undifferentiated Pleiomorphic Sarcoma | 6 (8.5) | |
| Myxofibrosarcoma | 4 (5.6) | |
| Extra skeletal Myxoid Chondrosarcoma | 5 (7.0) | |
| Epithelioid Sarcoma | 3 (4.2) | |
| Leiomyosarcoma | 3 (4.2) | |
| Extra Skeletal Ewing Sarcoma | 3 (4.2) | |
| Solitary Fibrous Tumor | 1 (1.4) | |
| Epithelioid Hemangioendothelioma | 1 (1.4) | |
| MPSNT | 3 (4.2) | |
| Adult Fibrosarcoma | 1 (1.4) | |
| Dedifferentiated Liposarcoma | 2 (2.8) | |
| Pleomorphic Liposarcoma | 1 (1.4) | |
| Clear Cell Sarcoma of the soft tissue | 3 (4.2) | |
| Dermatofibrosarcoma Protuberans | 1 (1.4) | |
| Undifferentiated Epithelioid Sarcoma | 2 (2.8) | |
| Myxoid Liposarcoma | 2 (2.8) | |
| Undifferentiated Sarcoma | 1 (1.4) | |
| Undifferentiated Spindle-cell Sarcoma | 15 (21.1) | |
| Pleiomorphic Rhabdomyosarcoma | 1 (1.4) | |
| Depth | ||
| Deep | 47 (66.1) | |
| Superficial | 16 (22.6) | |
| Mixed | 8 (11.3) | |
| Site | ||
| Lower limb | 39 (54.9) | |
| Upper limb | 20 (28.2) | |
| Abdominal wall | 1 (1.4) | |
| Back | 6 (8.5) | |
| Surgery | R0 | 52 (73.2) |
| R1–R2 | 19 (26.8) | |
| MTS Surgery | Neck | 1 (1.4) |
| Gluteus | 3 (4.2) | |
| Pelvis | 1 (1.4) | |
| Wedge Resection | 63 (88.7) | |
| Segmentectomy | 3 (4.2) | |
| Lobectomy | 1 (1.4) | |
| Wedge + Segmentectomy | 2 (2.8) | |
| Wedge + Lobectomy | 2 (2.8) | |
| Open Surgery | 70 (98.6) | |
| VATS | 1 (1.4) | |
| Two-stage MTS | 14 (19.7) |
| Overall | Malignant | Benign | p | |
|---|---|---|---|---|
| Lesions (n) | 160 | 139 | 21 | |
| Size (mm) | 6.50 (4.0–12.0) | 7.6 (4.4–13.0) | 3.5 (3.0–5.5) | <0.001 † |
| 0–5 mm | 59 (36.9) | 44 (31.7) | 15 (71.4) | |
| 5.1–10 mm | 52 (32.5) | 46 (33.1) | 6 (28.6) | 0.002 ‡ |
| 10.1–20 mm | 34 (21.2) | 34 (24.5) | 0 (0.0) | |
| >20 mm | 15 (9.4) | 15 (10.8) | 0 (0.0) | |
| Shape | ||||
| Round | 105 (65.6) | 98 (69.1) | 9 (42.9) | |
| Elongated | 25 (15.6) | 18 (12.9) | 7 (33.3) | |
| Complex | 19 (11.9) | 15 (10.8) | 4 (19.0) | 0.09 ‡ |
| Spiculated | 10 (6.2) | 9 (6.5) | 1 (4.8) | |
| Atypical | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cavitated | 1 (0.6) | 1 (0.7) | 0 (0.0) | |
| Density | ||||
| Solid | 95 (59.4) | 84 (60.4) | 11 (52.4) | |
| Ground-glass | 33 (20.6) | 26 (18.7) | 7 (33.3) | 0.325 ‡ |
| Mixed | 28 (17.5) | 26 (18.7) | 2 (9.5) | |
| Calcified | 4 (2.5) | 3 (2.2) | 1 (4.8) | |
| Margins | ||||
| Well defined | 115 (71.9) | 105 (75.5) | 10 (47.6) | 0.017 § |
| Ill defined | 45 (28.1) | 34 (24.5) | 11 (52.4) | |
| Side | ||||
| Right | 84 (52.5) | 71 (51.1) | 13 (61.9) | 0.489 § |
| Left | 76 (47.5) | 68 (48.9) | 8 (38.1) | |
| Lobe | ||||
| Upper | 52 (32.5) | 45 (32.4) | 7 (33.3) | |
| Middle | 36 (22.5) | 31 (22.3) | 5 (23.8) | >9 ‡ |
| Lower | 72 (45.0) | 63 (45.3) | 9 (42.9) | |
| Location | ||||
| Pleural | 26 (16.2) | 22 (15.8) | 4 (19.0) | |
| Subpleural | 91 (56.9) | 81 (58.3) | 10 (47.6) | <658 ‡ |
| Parenchymal | 40 (25.0) | 33 (23.7) | 7 (33.3) | |
| Hilar | 3 (1.9) | 3 (2.2) | 0 (0.0) | |
| * Size vs. previous CT | ||||
| Reduction | 9 (5.6) | 9 (6.5) | 0 (0.0) | |
| Unvaried | 53 (33.1) | 36 (25.9) | 17 (81.0) | <0.001 ‡ |
| Increase | 68 (42.5) | 66 (47.5) | 2 (9.5) | |
| New Onset | 30 (18.8) | 28 (20.1) | 2 (9.5) | |
| * Density vs. previous CT | ||||
| Reduction | 12 (7.5) | 12 (8.6) | 0 (0.0) | |
| Unvaried | 133 (83.1) | 112 (80.6) | 21 (100.0) | 0.086 ‡ |
| Increase | 15 (9.4) | 15 (10.8) | 0 (0.0) | |
| * Chemotherapy | ||||
| No | 109 (68.1) | 95 (74.2) | 14 (77.8) | >9 § |
| Yes | 37 (25.3) | 33 (25.8) | 4 (22.2) |
| OR | 95% CI | p | |
|---|---|---|---|
| Multiple analysis | |||
| Clinical Features | |||
| Synovial Sarcoma | 6.47 | 6.18–31.5 | <0.001 |
| Grade II | 4.76 | 3.2–36.6 | <0.001 |
| Grade III | 10.04 | 8.3–51.1 | <0.001 |
| Surgical margins R1 | 7.6 | 2.4–20.9 | <0.001 |
| Surgical Margins R2 | 14.2 | 4.8-80.6 | <0.001 |
| CT Features | |||
| Size | 9.22 | 2.97–43.96 | <0.001 |
| Well-defined Margins | 1.23 | 1.05–2.91 | 0.03 |
| Size vs. Baseline CT | 2.33 | 1.19–5.54 | 0.002 |
| New Onset Nodule | 4.65 | 1.26–13.5 | 0.03 |
| Single analysis (Clinical) | |||
| Leiomyosarcoma | 2.1 | 1.3–5.4 | 0.02 |
| Grade III | 4.3 | 1.9–12.4 | 0.009 |
| Surgical margins R2 | 4.8 | 2.1–13.7 | 0.008 |
| Single analysis (CT) | |||
| Size | 2.9 | 1.4–8.5 | 0.01 |
| New Onset Nodule | 1.5 | 1.1–4.1 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetta, C.; Giugliano, A.; Tonetti, L.; Rocca, M.; Longhi, A.; Londero, F.; Parise, G.; Parise, O.; Micali, L.R.; La Meir, M.; et al. Clinical and Radiologic Features Together Better Predict Lung Nodule Malignancy in Patients with Soft-Tissue Sarcoma. J. Clin. Med. 2020, 9, 1209. https://doi.org/10.3390/jcm9041209
Tetta C, Giugliano A, Tonetti L, Rocca M, Longhi A, Londero F, Parise G, Parise O, Micali LR, La Meir M, et al. Clinical and Radiologic Features Together Better Predict Lung Nodule Malignancy in Patients with Soft-Tissue Sarcoma. Journal of Clinical Medicine. 2020; 9(4):1209. https://doi.org/10.3390/jcm9041209
Chicago/Turabian StyleTetta, Cecilia, Antonio Giugliano, Laura Tonetti, Michele Rocca, Alessandra Longhi, Francesco Londero, Gianmarco Parise, Orlando Parise, Linda Renata Micali, Mark La Meir, and et al. 2020. "Clinical and Radiologic Features Together Better Predict Lung Nodule Malignancy in Patients with Soft-Tissue Sarcoma" Journal of Clinical Medicine 9, no. 4: 1209. https://doi.org/10.3390/jcm9041209
APA StyleTetta, C., Giugliano, A., Tonetti, L., Rocca, M., Longhi, A., Londero, F., Parise, G., Parise, O., Micali, L. R., La Meir, M., Maessen, J. G., & Gelsomino, S. (2020). Clinical and Radiologic Features Together Better Predict Lung Nodule Malignancy in Patients with Soft-Tissue Sarcoma. Journal of Clinical Medicine, 9(4), 1209. https://doi.org/10.3390/jcm9041209







