Recent Advances and Clinical Outcomes of Kidney Transplantation
Abstract
1. Introduction
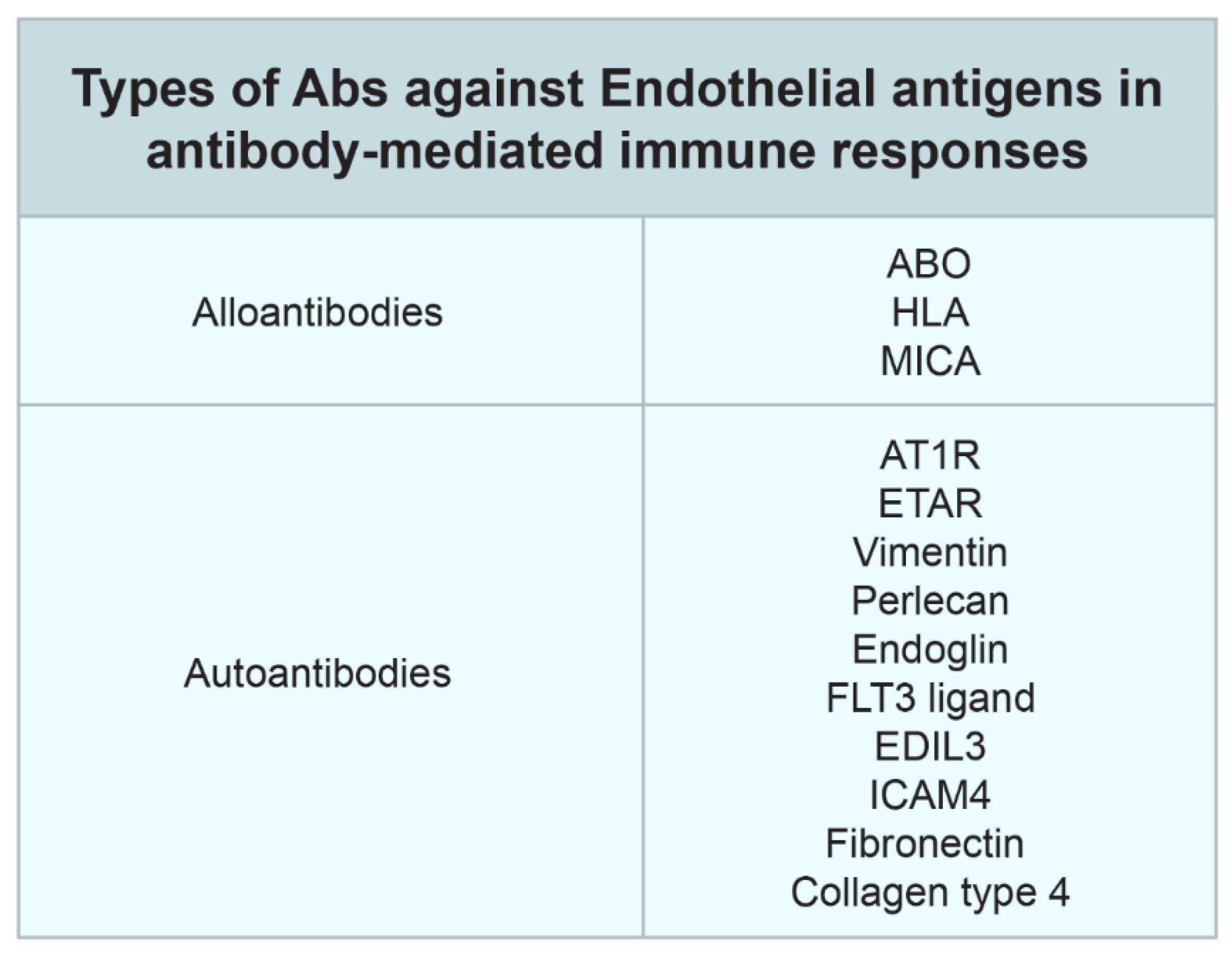
2. Non-HLA Antibodies in Transplantation
3. Active AMR
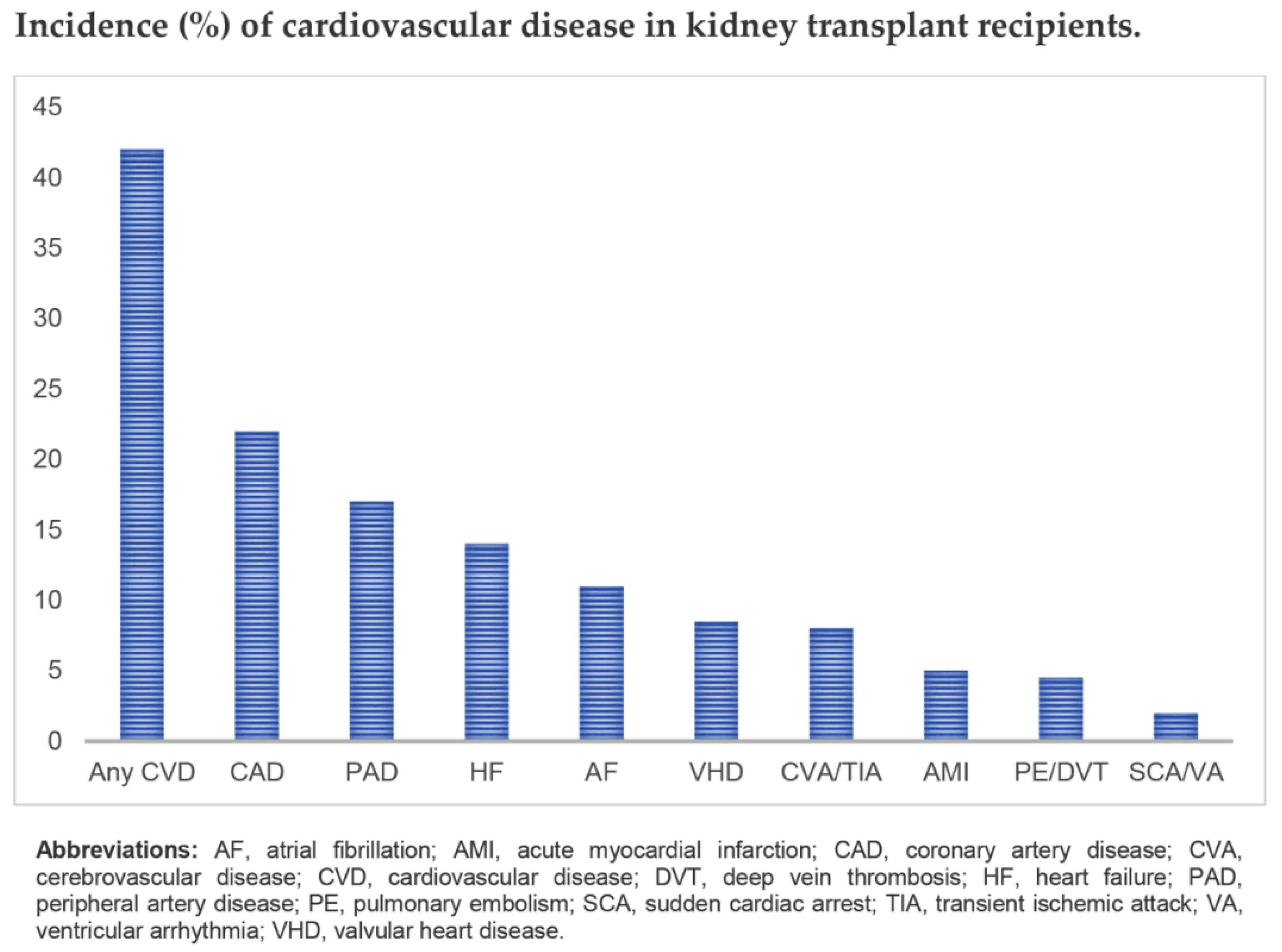
4. Cardiovascular Diseases in Kidney Transplant Recipients
5. Preexisting Diabetes and Post-Transplantation Diabetes
6. Posttransplant Malignancy
7. Infection
8. Latest Developments in Living Kidney Donation
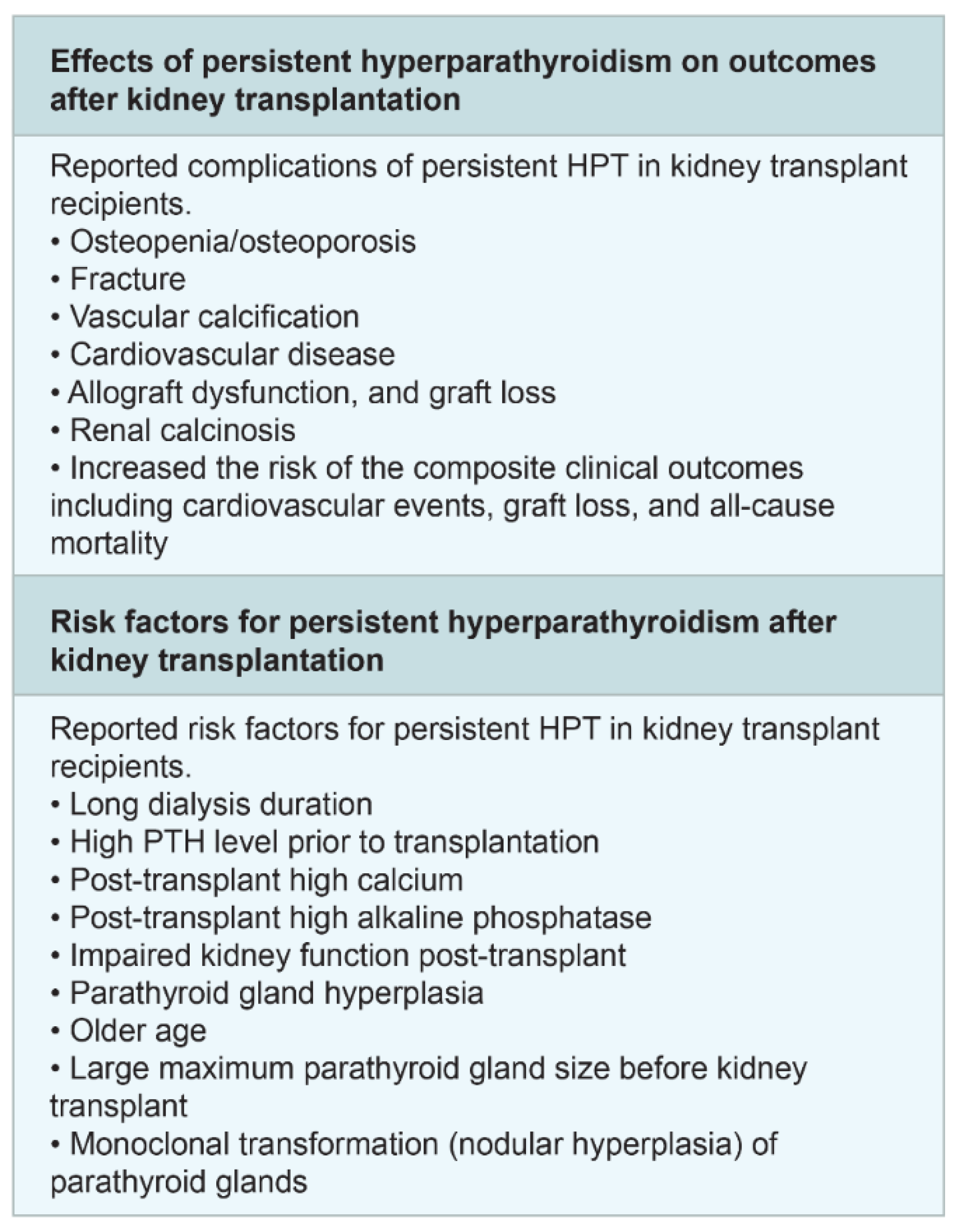
9. Post-Transplant Hyperparathyroidism and Bone Disease
10. Potential Directions and Future Scope
11. Conclusions
Funding
Conflicts of Interest
References
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Duong van Huyen, J.P.; Mooney, N.; Suberbielle, C.; Frémeaux-Bacchi, V.; Méjean, A.; Desgrandchamps, F.; et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Viklicky, O.; Novotny, M.; Hruba, P. Future developments in kidney transplantation. Curr. Opin. Organ Transplant. 2020, 25, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Faenza, A.; Fuga, G.; Nardo, B.; Donati, G.; Cianciolo, G.; Scolari, M.; Stefoni, S. Metabolic Syndrome After Kidney Transplantation. Transplant. Proc. 2007, 39, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Bucay, A.; Gordon, C.E.; Francis, J.M. Non-immunological complications following kidney transplantation. F1000Research 2019, 8, 194. [Google Scholar] [CrossRef]
- Gill, J.S.; Abichandani, R.; Kausz, A.T.; Pereira, B.J. Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int. 2002, 62, 1875–1883. [Google Scholar] [CrossRef]
- Roedder, S.; Sigdel, T.; Salomonis, N.; Hsieh, S.; Dai, H.; Bestard, O.; Metes, D.; Zeevi, A.; Gritsch, A.; Cheeseman, J.; et al. The kSORT Assay to Detect Renal Transplant Patients at High Risk for Acute Rejection: Results of the Multicenter AART Study. PLoS Med. 2014, 11, e1001759. [Google Scholar] [CrossRef]
- Garg, N.; Samaniego, M.D.; Clark, D.; Djamali, A. Defining the phenotype of antibody-mediated rejection in kidney transplantation: Advances in diagnosis of antibody injury. Transplant. Rev. 2017, 31, 257–267. [Google Scholar] [CrossRef]
- Bloom, R.D.; Bromberg, J.S.; Poggio, E.D.; Bunnapradist, S.; Langone, A.J.; Sood, P.; Matas, A.J.; Mehta, S.; Mannon, R.B.; Sharfuddin, A.; et al. Cell-Free DNA and Active Rejection in Kidney Allografts. J. Am. Soc. Nephrol. 2017, 28, 2221–2232. [Google Scholar] [CrossRef]
- Huang, E.; Sethi, S.; Peng, A.; Najjar, R.; Mirocha, J.; Haas, M.; Vo, A.; Jordan, S.C. Early clinical experience using donor-derived cell-free DNA to detect rejection in kidney transplant recipients. Arab. Archaeol. Epigr. 2019, 19, 1663–1670. [Google Scholar] [CrossRef]
- Gielis, E.M.; Ledeganck, K.J.; Dendooven, A.; Meysman, P.; Beirnaert, C.; Laukens, K.; De Schrijver, J.; Van Laecke, S.; Van Biesen, W.; Emonds, M.-P.; et al. The use of plasma donor-derived, cell-free DNA to monitor acute rejection after kidney transplantation. Nephrol. Dial. Transplant. 2019, 35, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Oellerich, M.; Shipkova, M.; Asendorf, T.; Walson, P.D.; Schauerte, V.; Mettenmeyer, N.; Kabakchiev, M.; Hasche, G.; Gröne, H.; Friede, T.; et al. Absolute quantification of donor-derived cell-free DNA as a marker of rejection and graft injury in kidney transplantation: Results from a prospective observational study. Arab. Archaeol. Epigr. 2019, 19, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Bunnapradist, S.; Bromberg, J.S.; Langone, A.J.; Hiller, D.; Yee, J.; Sninsky, J.J.; Woodward, R.; Matas, A.J. Donor-derived Cell-free DNA Identifies Antibody-mediated Rejection in Donor Specific Antibody Positive Kidney Transplant Recipients. Transplant. Direct 2018, 4, e379. [Google Scholar] [CrossRef] [PubMed]
- Sigdel, T.; Archila, F.A.; Constantin, T.; Demko, Z.; Liberto, J.M.; Damm, I.; Towfighi, P.; Navarro, S.; Kirkizlar, E.; Demko, Z.; et al. Optimizing Detection of Kidney Transplant Injury by Assessment of Donor-Derived Cell-Free DNA via Massively Multiplex PCR. J. Clin. Med. 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.; Böhmig, G.A.; Eskandary, F.; Einecke, G.; Gupta, G.; Madill-Thomsen, K.; Mackova, M.; Halloran, P.F. INTERCOMEX MMDx-Kidney study group Generating automated kidney transplant biopsy reports combining molecular measurements with ensembles of machine learning classifiers. Arab. Archaeol. Epigr. 2019, 19, 2719–2731. [Google Scholar] [CrossRef]
- Jordan, S.C.; Lorant, T.; Choi, J. IgG Endopeptidase in Highly Sensitized Patients Undergoing Transplantation. N. Engl. J. Med. 2017, 377, 1693–1694. [Google Scholar] [CrossRef]
- Lorant, T.; Bengtsson, M.; Eich, T.; Eriksson, B.-M.; Winstedt, L.; Järnum, S.; Stenberg, Y.; Robertson, A.-K.; Mosén, K.; Björck, L.; et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Arab. Archaeol. Epigr. 2018, 18, 2752–2762. [Google Scholar] [CrossRef]
- Bray, R.A.; Gebel, H.M.; Townsend, R.; Roberts, M.E.; Polinsky, M.; Yang, L.; Meier-Kriesche, H.-U.; Larsen, C.P. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Arab. Archaeol. Epigr. 2018, 18, 1783–1789. [Google Scholar] [CrossRef]
- Leibler, C.; Matignon, M.; Moktefi, A.; Samson, C.; Zarour, A.; Malard, S.; Boutin, E.; Pilon, C.; Salomon, L.; Natella, P.-A.; et al. Belatacept in renal transplant recipient with mild immunologic risk factor: A pilot prospective study (BELACOR). Arab. Archaeol. Epigr. 2019, 19, 894–906. [Google Scholar] [CrossRef]
- Kolonko, A.; Słabiak-Błaż, N.; Karkoszka, H.; Więcek, A.; Piecha, G. The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series. J. Clin. Med. 2020, 9, 529. [Google Scholar] [CrossRef]
- Knobbe, T.; Douwes, R.M.; Kremer, D.; Swarte, J.C.; Eisenga, M.; Gomes-Neto, A.W.; Van Londen, M.; Peters, F.; Blokzijl, H.; Nolte, I.M.; et al. Altered Gut Microbial Fermentation and Colonization with Methanobrevibacter smithii in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Osté, M.C.J.; Flores-Guerrero, J.L.; Gruppen, E.G.; Kieneker, L.M.; Connelly, M.A.; Otvos, J.; Dullaart, R.P.; Bakker, S.J.L. High Plasma Branched-Chain Amino Acids Are Associated with Higher Risk of Post-Transplant Diabetes Mellitus in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 511. [Google Scholar] [CrossRef]
- Deen, C.; Van Der Veen, A.; Gomes-Neto, A.W.; Geleijnse, J.M.; Berg, K.J.B.-V.D.; Heiner-Fokkema, M.; Kema, I.; Bakker, S.J.L. Urinary Excretion of N1-methyl-2-pyridone-5-carboxamide and N1-methylnicotinamide in Renal Transplant Recipients and Donors. J. Clin. Med. 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.G.; Groothof, D.; Vodegel, J.J.; Gacitúa, T.A.; Gomes-Neto, A.W.; Osté, M.C.J.; Pol, R.A.; Ferreccio, C.; Berger, S.P.; Chong, G.; et al. Circulating Arsenic is Associated with Long-Term Risk of Graft Failure in Kidney Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Byambasukh, O.; Osté, M.C.J.; Gomes-Neto, A.W.; Berg, E.V.D.; Navis, G.; Bakker, S.J.L.; Byambasukh, O.; Byambasukh, O. Physical Activity and the Development of Post-Transplant Diabetes Mellitus, and Cardiovascular- and All-Cause Mortality in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Lee, K.W.; Park, J.B.; Kim, K.; Jang, H.-R.; Huh, W.; Kang, E.-S. C3d-Positive Preformed DSAs Tend to Persist and Result in a Higher Risk of AMR after Kidney Transplants. J. Clin. Med. 2020, 9, 375. [Google Scholar] [CrossRef] [PubMed]
- Klont, F.; Kieneker, L.M.; Gomes-Neto, A.W.; Stam, S.P.; Hacken, N.H.T.; Kema, I.; Van Beek, A.P.; Berg, E.V.D.; Horvatovich, P.; Bischoff, R.; et al. Female Specific Association of Low Insulin-Like Growth Factor 1 (IGF1) Levels with Increased Risk of Premature Mortality in Renal Transplant Recipients. J. Clin. Med. 2020, 9, 293. [Google Scholar] [CrossRef]
- Flothow, D.; Suwelack, B.; Pavenstädt, H.; Schütte-Nütgen, K.; Reuter, S. The Effect of Proton Pump Inhibitor Use on Renal Function in Kidney Transplanted Patients. J. Clin. Med. 2020, 9, 258. [Google Scholar] [CrossRef]
- Bailey, P.K.; Caskey, F.J.; MacNeill, S.; Tomson, C.; Dor, F.; Ben-Shlomo, Y. Beliefs of UK Transplant Recipients about Living Kidney Donation and Transplantation: Findings from a Multicentre Questionnaire-Based Case–Control Study. J. Clin. Med. 2019, 9, 31. [Google Scholar] [CrossRef]
- Yang, D.; Thamcharoen, N.; Cardarelli, F. Management of Immunosuppression in Kidney Transplant Recipients Who Develop Malignancy. J. Clin. Med. 2019, 8, 2189. [Google Scholar] [CrossRef]
- Douwes, R.M.; Gomes-Neto, A.W.; Schutten, J.; Berg, E.V.D.; De Borst, M.H.; Berger, S.P.; Touw, D.; Hak, E.; Blokzijl, H.; Navis, G.; et al. Proton-Pump Inhibitors and Hypomagnesaemia in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2162. [Google Scholar] [CrossRef] [PubMed]
- Tubben, A.; Sotomayor, C.G.; Post, A.; Minovic, I.; Frelink, T.; De Borst, M.H.; Said, M.Y.; Douwes, R.M.; van den Berg, E.; Rodrigo, R.; et al. Urinary Oxalate Excretion and Long-Term Outcomes in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2104. [Google Scholar] [CrossRef] [PubMed]
- Piyasiridej, S.; Townamchai, N.; Udomkarnjananun, S.; Vadcharavivad, S.; Pongpirul, K.; Wattanatorn, S.; Sirichindakul, B.; Avihingsanon, Y.; Tungsanga, K.; Eiam-Ong, S.; et al. Plasmapheresis Reduces Mycophenolic Acid Concentration: A Study of Full AUC0-12 in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2084. [Google Scholar] [CrossRef] [PubMed]
- Gacitúa, T.A.; Sotomayor, C.G.; Groothof, D.; Eisenga, M.; Pol, R.A.; De Borst, M.H.; Gans, R.O.B.; Berger, S.P.; Rodrigo, R.; Navis, G.; et al. Plasma Vitamin C and Cancer Mortality in Kidney Transplant Recipients. J. Clin. Med. 2019, 8, 2064. [Google Scholar] [CrossRef] [PubMed]
- Deen, C.; Van Der Veen, A.; Van Faassen, M.; Minović, I.; Gomes-Neto, A.W.; Geleijnse, J.M.; Berg, K.J.B.-V.D.; Kema, I.; Bakker, S.J.L. Urinary Excretion of N1-Methylnicotinamide, as a Biomarker of Niacin Status, and Mortality in Renal Transplant Recipients. J. Clin. Med. 2019, 8, 1948. [Google Scholar] [CrossRef] [PubMed]
- Katou, S.; Globke, B.; Morgul, M.; Vogel, T.; Struecker, B.; Otto, N.; Reutzel-Selke, A.; Marksteiner, M.; Brockmann, J.; Pascher, A.; et al. Urinary Biomarkers α-GST and π-GST for Evaluation and Monitoring in Living and Deceased Donor Kidney Grafts. J. Clin. Med. 2019, 8, 1899. [Google Scholar] [CrossRef]
- Hwang, H.; Hong, K.-W.; Kim, J.; Kim, Y.; Moon, J.; Jeong, K.; Lee, S.-H. The Korean Organ Transplantation Registry Study Group; Korean Organ Transplantation Registry Study Group Validation of Identified Susceptible Gene Variants for New-Onset Diabetes in Renal Transplant Recipients. J. Clin. Med. 2019, 8, 1696. [Google Scholar] [CrossRef]
- Yepes-Calderón, M.; Sotomayor, C.G.; Kretzler, M.; Gans, R.O.B.; Berger, S.P.; Navis, G.; Ju, W.; Bakker, S.J.L. Urinary Epidermal Growth Factor/Creatinine Ratio and Graft Failure in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 1673. [Google Scholar] [CrossRef]
- Maxeiner, A.; Bichmann, A.; Oberländer, N.; El-Bandar, N.; Sugünes, N.; Ralla, B.; Biernath, N.; Liefeldt, L.; Budde, K.; Giessing, M.; et al. Native Nephrectomy before and after Renal Transplantation in Patients with Autosomal Dominant Polycystic Kidney Disease (ADPKD). J. Clin. Med. 2019, 8, 1622. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Huijink, T.M.; Pol, R.A.; El Moumni, M.; Burgerhof, J.G.; Struys, M.; Berger, S.P. Intraoperative Fluid Restriction is Associated with Functional Delayed Graft Function in Living Donor Kidney Transplantation: A Retrospective Cohort Analysis. J. Clin. Med. 2019, 8, 1587. [Google Scholar] [CrossRef]
- Thölking, G.; Schütte-Nütgen, K.; Schmitz, J.; Rovas, A.; Dahmen, M.; Bautz, J.; Jehn, U.; Pavenstädt, H.; Heitplatz, B.; Van Marck, V.; et al. A Low Tacrolimus Concentration/Dose Ratio Increases the Risk for the Development of Acute Calcineurin Inhibitor-Induced Nephrotoxicity. J. Clin. Med. 2019, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Douwes, R.M.; Neto, G.; Eisenga, M.; Vinke, J.S.J.; Borst, D.; Berg, V.D.; Berger, S.P.; Touw, D.J.; Hak, E.; Blokzijl, H.; et al. Chronic Use of Proton-Pump Inhibitors and Iron Status in Renal Transplant Recipients. J. Clin. Med. 2019, 8, 1382. [Google Scholar] [CrossRef] [PubMed]
- Neuwirt, H.; Leitner-Lechner, I.; Kerschbaum, J.; Ertl, M.; Pöggsteiner, F.; Pölt, N.; Mätzler, J.; Sprenger-Mähr, H.; Rudnicki, M.; Schratzberger, P.; et al. Efficacy and Safety of Belatacept Treatment in Renal Allograft Recipients at High Cardiovascular Risk-A Single Center Experience. J. Clin. Med. 2019, 8, 1164. [Google Scholar] [CrossRef] [PubMed]
- Lemerle, M.; Garnier, A.-S.; Planchais, M.; Brilland, B.; Delneste, Y.; Subra, J.-F.; Blanchet, O.; Blanchard, S.; Croué, A.; Duveau, A.; et al. CD45RC Expression of Circulating CD8+ T Cells Predicts Acute Allograft Rejection: A Cohort Study of 128 Kidney Transplant Patients. J. Clin. Med. 2019, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Sugünes, N.; Bichmann, A.; Biernath, N.; Peters, R.; Budde, K.; Liefeldt, L.; Schlomm, T.; Friedersdorff, F. Analysis of the Effects of Day-Time vs. Night-Time Surgery on Renal Transplant Patient Outcomes. J. Clin. Med. 2019, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Basha, J.A.; Kiel, M.; Görlich, D.; Schütte-Nütgen, K.; Witten, A.; Pavenstädt, H.; Kahl, B.C.; Dobrindt, U.; Reuter, S. Phenotypic and Genotypic Characterization of Escherichia coli Causing Urinary Tract Infections in Kidney-Transplanted Patients. J. Clin. Med. 2019, 8, 988. [Google Scholar] [CrossRef] [PubMed]
- Go, J.; Park, S.C.; Yun, S.-S.; Ku, J.; Park, J.; Shim, J.-W.; Lee, H.; Kim, Y.; Moon, Y.E.; Hong, S.H.; et al. Exposure to Hyperchloremia Is Associated with Poor Early Recovery of Kidney Graft Function after Living-Donor Kidney Transplantation: A Propensity Score-Matching Analysis. J. Clin. Med. 2019, 8, 955. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Uchida, J.; Nishide, S.; Kabei, K.; Kosoku, A.; Maeda, K.; Iwai, T.; Naganuma, T.; Takemoto, Y.; Nakatani, T. Comparison of Glucose Tolerance between Kidney Transplant Recipients and Healthy Controls. J. Clin. Med. 2019, 8, 920. [Google Scholar] [CrossRef]
- Suarez, M.L.G.; Thongprayoon, C.; Mao, M.A.; Leeaphorn, N.; Bathini, T.; Cheungpasitporn, W. Outcomes of Kidney Transplant Patients with Atypical Hemolytic Uremic Syndrome Treated with Eculizumab: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 919. [Google Scholar] [CrossRef]
- Bellini, M.I.; Charalampidis, S.; Stratigos, I.; Dor, F.; Papalois, V. Dor The Effect of Donors’ Demographic Characteristics in Renal Function Post-Living Kidney Donation. Analysis of a UK Single Centre Cohort. J. Clin. Med. 2019, 8, 883. [Google Scholar] [CrossRef]
- Attias, P.; Melica, G.; Boutboul, D.; De Castro, N.; Audard, V.; Stehlé, T.; Gaube, G.; Fourati, S.; Botterel, F.; Fihman, V.; et al. Epidemiology, Risk Factors, and Outcomes of Opportunistic Infections after Kidney Allograft Transplantation in the Era of Modern Immunosuppression: A Monocentric Cohort Study. J. Clin. Med. 2019, 8, 594. [Google Scholar] [CrossRef] [PubMed]
- Schütte-Nütgen, K.; Thölking, G.; Steinke, J.; Pavenstädt, H.; Schmidt, R.; Suwelack, B.; Reuter, S. Correction: Fast Tac Metabolizers at Risk-It is Time for a C/D Ratio Calculation. J. Clin. Med. 2019, 8, 1870. [Google Scholar] [CrossRef] [PubMed]
- Chewcharat, A.; Thongprayoon, C.; Bathini, T.; Aeddula, N.R.; Boonpheng, B.; Kaewput, W.; Watthanasuntorn, K.; Lertjitbanjong, P.; Sharma, K.; Torres-Ortiz, A.; et al. Incidence and Mortality of Renal Cell Carcinoma after Kidney Transplantation: A Meta-Analysis. J. Clin. Med. 2019, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Ungprasert, P.; Wijarnpreecha, K.; Kaewput, W.; Leeaphorn, N.; Bathini, T.; Chebib, F.T.; Kroner, P. Subarachnoid Hemorrhage in Hospitalized Renal Transplant Recipients with Autosomal Dominant Polycystic Kidney Disease: A Nationwide Analysis. J. Clin. Med. 2019, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Hyun, H.S.; Park, E.; Moon, K.C.; Min, S.I.; Ha, J.; Ha, I.S.; Cheong, H.I.; Ahn, Y.H.; Kang, H.G. Higher Incidence of BK Virus Nephropathy in Pediatric Kidney Allograft Recipients with Alport Syndrome. J. Clin. Med. 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Calderón, M.; Sotomayor, C.G.; Gomes-Neto, A.W.; Gans, R.O.B.; Berger, S.P.; Rimbach, G.; Esatbeyoglu, T.; Rodrigo, R.; Geleijnse, J.M.; Navis, G.; et al. Plasma Malondialdehyde and Risk of New-Onset Diabetes after Transplantation in Renal Transplant Recipients: A Prospective Cohort Study. J. Clin. Med. 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.; Pol, R.A.; Struys, M.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef]
- Alcendor, D.J. BK Polyomavirus Virus Glomerular Tropism: Implications for Virus Reactivation from Latency and Amplification during Immunosuppression. J. Clin. Med. 2019, 8, 1477. [Google Scholar] [CrossRef]
- Bellini, M.I.; Nozdrin, M.; Yiu, J.; Papalois, V. Machine Perfusion for Abdominal Organ Preservation: A Systematic Review of Kidney and Liver Human Grafts. J. Clin. Med. 2019, 8, 1221. [Google Scholar] [CrossRef]
- Visser, I.J.; van der Staaij, J.P.T.; Muthusamy, A.; Willicombe, M.; Lafranca, J.A.; Dor, F. Timing of Ureteric Stent Removal and Occurrence of Urological Complications after Kidney Transplantation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 689. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Acharya, P.; Aeddula, N.R.; Torres-Ortiz, A.; Bathini, T.; Sharma, K.; Ungprasert, P.; Watthanasuntorn, K.; Suarez, M.L.G.; Salim, S.A.; et al. Effects of denosumab on bone metabolism and bone mineral density in kidney transplant patients: A systematic review and meta-analysis. Arch Osteoporos. 2019, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Wijarnpreecha, K.; Mitema, D.G.; Mao, M.A.; Nissaisorakarn, P.; Podboy, A.; Kittanamongkolchai, W.; Sakhuja, A.; Erickson, S.B. Decline in prevalence and risk of helicobacter pylori in kidney transplant recipients: A systematic review and meta-analysis. J. Evid.-Based Med. 2017, 10, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Boonpheng, B.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Mao, M.A.; Cheungpasitporn, W. Proton pump inhibitors and adverse effects in kidney transplant recipients: A meta-analysis. World J. Transplant. 2019, 9, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Chokesuwattanaskul, R.; Bathini, T.; Khoury, N.J.; Sharma, K.; Ungprasert, P.; Prasitlumkum, N.; Aeddula, N.R.; Watthanasuntorn, K.; Salim, S.A.; et al. Epidemiology and Prognostic Importance of Atrial Fibrillation in Kidney Transplant Recipients: A Meta-Analysis. J. Clin. Med. 2018, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; A Mao, M.; A Mao, S.; D’Costa, M.R.; Kittanamongkolchai, W.; Kashani, K.B. Contrast-induced acute kidney injury in kidney transplant recipients: A systematic review and meta-analysis. World J. Transplant. 2017, 7, 81–87. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; A Mao, M.; Kittanamongkolchai, W.; Sathick, I.J.J.; Dhondup, T.; Erickson, S.B. Incidence of kidney stones in kidney transplant recipients: A systematic review and meta-analysis. World J. Transplant. 2016, 6, 790–797. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Khoury, N.J.; Bathini, T.; Aeddula, N.R.; Boonpheng, B.; Leeaphorn, N.; Ungprasert, P.; Bruminhent, J.; Lertjitbanjong, P.; Watthanasuntorn, K.; et al. BK polyomavirus genotypes in renal transplant recipients in the United States: A meta-analysis. J. Evid. Based Med. 2019, 12, 291–299. [Google Scholar] [CrossRef]
- Schinstock, C.; Gandhi, M.; Cheungpasitporn, W.; Mitema, D.; Prieto, M.; Dean, P.; Cornell, L.; Cosio, F.; Stegall, M. Kidney Transplant with Low Levels of DSA or Low Positive B-Flow Crossmatch. Transplant. 2017, 101, 2429–2439. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Kremers, W.K.; Lorenz, E.; Amer, H.; Cosio, F.G.; Stegall, M.D.; Gandhi, M.J.; Schinstock, C.A. De novo donor-specific antibody following BK nephropathy: The incidence and association with antibody-mediated rejection. Clin. Transplant. 2018, 32, e13194. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Mitema, D.G.; Mao, M.A.; Sakhuja, A.; Kittanamongkolchai, W.; Gonzalez-Suarez, M.L.; Erickson, S.B. The effect of aspirin on kidney allograft outcomes; a short review to current studies. J. Nephropathol. 2017, 6, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Harindhanavudhi, T.; Edmonds, P.; Erickson, S.B. Hypomagnesemia linked to new-onset diabetes mellitus after kidney transplantation: A systematic review and meta-analysis. Endocr. Res. 2016, 41, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Thongprayoon, C.; Cheungpasitporn, W.; Markovic, S.N.; Herrmann, S.M. Systematic Review of the Safety of Immune Checkpoint Inhibitors Among Kidney Transplant Patients. Kidney Int. Rep. 2019, 5, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Vijayvargiya, P.; Anthanont, P.; Erickson, S.B. The Risk for New-Onset Diabetes Mellitus after Kidney Transplantation in Patients with Autosomal Dominant Polycystic Kidney Disease: A Systematic Review and Meta-Analysis. Can. J. Diabetes 2016, 40, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Khoury, N.J.; Thongprayoon, C.; Craici, I.M. Is Remote Ischemic Conditioning of Benefit to Patients Undergoing Kidney Transplantation? J. Investig. Surg. 2017, 32, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Erickson, S.B. Outcomes of living kidney donors with monoclonal gammopathy of undetermined significance. Ren. Fail. 2015, 37, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Ungprasert, P.; Erickson, S.B. Outcomes of Living Kidney Donors with Rheumatoid Arthritis. Prog. Transplant. 2015, 25, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Kaewput, W.; Sharma, K.; Wijarnpreecha, K.; Leeaphorn, N.; Ungprasert, P.; Sakhuja, A.; Rivera, F.H.C.; Cheungpasitporn, W. Outcomes of kidney transplantation in patients with hepatitis B virus infection: A systematic review and meta-analysis. World J. Hepatol. 2018, 10, 337–346. [Google Scholar] [CrossRef]
- Schinstock, C.; Cosio, F.; Cheungpasitporn, W.; Dadhania, D.M.; Everly, M.J.; Samaniego-Picota, M.D.; Cornell, L.; Stegall, M.D. The Value of Protocol Biopsies to Identify Patients with De Novo Donor-Specific Antibody at High Risk for Allograft Loss. Arab. Archaeol. Epigr. 2017, 17, 1574–1584. [Google Scholar] [CrossRef]
- Leeaphorn, N.; Thongprayoon, C.; Chon, W.J.; Cummings, L.S.; Mao, M.A.; Cheungpasitporn, W. Outcomes of kidney retransplantation after graft loss as a result of BK virus nephropathy in the era of newer immunosuppressant agents. Arab. Archaeol. Epigr. 2019. [Google Scholar] [CrossRef]
- Chewcharat, A.; Chang, Y.T.; Thongprayoon, C.; Crisafio, A.; Bathini, T.; Mao, M.A.; Cheungpasitporn, W. Efficacy and safety of febuxostat for treatment of asymptomatic hyperuricemia among kidney transplant patients: A meta-analysis of observational studies. Clin. Transplant. 2020, e13820. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Thongprayoon, C.; Edmonds, P.; Bruminhent, J.; Tangdhanakanond, K. The effectiveness and safety of rituximab as induction therapy in ABO-compatible non-sensitized renal transplantation: A systematic review and meta-analysis of randomized controlled trials. Ren. Fail. 2015, 37, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Mao, M.A.; Kittanamongkolchai, W.; Sathick, I.J.J.; Erickson, S.B. The Effect of Renin-angiotensin System Inhibitors on Kidney Allograft Survival: A Systematic Review and Meta-analysis. N. Am. J. Med. Sci. 2016, 8, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Ungprasert, P.; Wijarnpreecha, K.; Mao, M.A.; Aeddula, N.R.; Kaewput, W.; Bathini, T.; Kroner, P.T. Hepatitis A hospitalizations among kidney transplant recipients in the United States: Nationwide inpatient sample 2005–2014. Eur. J. Gastroenterol. Hepatol. 2020, 32, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Chebib, F.T.; Cornell, L.D.; Brodin, M.L.; Nasr, S.H.; Schinstock, C.; Stegall, M.D.; Amer, H. Intravitreal Antivascular Endothelial Growth Factor Therapy May Induce Proteinuria and Antibody Mediated Injury in Renal Allografts. Transplantation 2015, 99, 2382–2386. [Google Scholar] [CrossRef]
- Farouk, S.; Zhang, Z.; Menon, M. Non-HLA donor–recipient mismatches in kidney transplantation—A stone left unturned. Arab. Archaeol. Epigr. 2019, 20, 19–24. [Google Scholar] [CrossRef]
- Starzl, T.E.; Marchioro, T.L.; Holmes, J.H.; Hermann, G.; Brittain, R.S.; Stonington, O.H.; Talmage, D.W.; Waddell, W.R. Renal homografts in patients with major donor-recipient blood group incompatibilities. Surgery 1964, 55, 195–200. [Google Scholar]
- Sumitran-Holgersson, S.; Wilczek, H.E.; Holgersson, J.; Soderstrom, K. Identification of the nonclassical HLA molecules, mica, as targets for humoral immunity associated with irreversible rejection of kidney allografts1. Transplantation 2002, 74, 268–277. [Google Scholar] [CrossRef]
- Dragun, D.; Muller, D.N.; Brasen, J.H.; Fritsche, L.; Nieminen-Kelha, M.; Dechend, R.; Kintscher, U.; Rudolph, B.; Hoebeke, J.; Eckert, D.; et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 2005, 352, 558–569. [Google Scholar] [CrossRef]
- Banasik, M.; Boratyńska, M.; Kościelska-Kasprzak, K.; Kamińska, D.; Zmonarski, S.; Mazanowska, O.; Krajewska, M.; Bartoszek, D.; Żabińska, M.; Myszka-Kozłowska, M.; et al. Non-HLA Antibodies: Angiotensin II Type 1 Receptor (Anti-AT1R) and Endothelin-1 Type A Receptor (Anti-ETAR) Are Associated With Renal Allograft Injury and Graft Loss. Transplant. Proc. 2014, 46, 2618–2621. [Google Scholar] [CrossRef]
- Besarani, D.; Cerundolo, L.; Smith, J.D.; Procter, J.; Barnardo, M.C.N.; Roberts, I.S.D.; Friend, P.J.; Rose, M.L.; Fuggle, S.V. Role of Anti-Vimentin Antibodies in Renal Transplantation. Transplantation 2014, 98, 1. [Google Scholar] [CrossRef]
- Cardinal, H.; Dieudé, M.; Brassard, N.; Qi, S.; Patey, N.; Soulez, M.; Beillevaire, D.; Echeverry, F.; Daniel, C.; Durocher, Y.; et al. Antiperlecan Antibodies Are Novel Accelerators of Immune-Mediated Vascular Injury. Arab. Archaeol. Epigr. 2013, 13, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Sigdel, T.K.; Delville, M.; Hsieh, S.-C.; Dai, H.; Bagnasco, S.; Montgomery, R.A.; Sarwal, M.M. Endothelial Cell Antibodies Associated with Novel Targets and Increased Rejection. J. Am. Soc. Nephrol. 2014, 26, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Sablik, K.A.; Van Groningen, M.C.C.-; Damman, J.; Roelen, D.L.; Betjes, M. Banff lesions and renal allograft survival in chronic-active antibody mediated rejection. Transplant. Immunol. 2019, 56, 101213. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Wen, M.-C.; Wu, M.-J.; Chen, C.-H.; Yu, T.-M.; Chuang, Y.-W.; Huang, S.-T.; Tsai, S.-F.; Lo, Y.-C.; Ho, H.-C.; et al. Treatment of chronic active antibody-mediated rejection in renal transplant recipients-a single center retrospective study. BMC Nephrol. 2020, 21, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Gheith, O.; Al-Otaibi, T.; Mostafa, M.; Rida, S.; Sobhy, I.; Halim, M.A.; Mahmoud, T.; Abdul-Hameed, M.; Maher, A.; et al. Management of Chronic Active Antibody-Mediated Rejection in Renal Transplant Recipients: Single-Center Experience. Exp. Clin. Transplant. 2019, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Arab. Archaeol. Epigr. 2017, 17, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.A.; Aubert, O.; Haas, M.; Huang, E.; Zhang, X.; Choi, J.; Peng, A.; Najjar, R.; Sethi, S.; Ammerman, N.; et al. Clinical Relevance of Posttransplant DSAs in Patients Receiving Desensitization for HLA-incompatible Kidney Transplantation. Transplantation 2019, 103, 2666–2674. [Google Scholar] [CrossRef]
- Pottebaum, A.A.; Venkatachalam, K.; Liu, C.; Brennan, D.C.; Murad, H.; Malone, A.F.; Alhamad, T. Efficacy and Safety of Tocilizumab in the Treatment of Acute Active Antibody-mediated Rejection in Kidney Transplant Recipients. Transplant. Direct 2020, 6, e543. [Google Scholar] [CrossRef]
- Eskandary, F.; Duerr, M.; Budde, K.; Doberer, K.; Reindl-Schwaighofer, R.; Waiser, J.; Wahrmann, M.; Regele, H.; Spittler, A.; Lachmann, N.; et al. Clazakizumab in late antibody-mediated rejection: Study protocol of a randomized controlled pilot trial. Trials 2019, 20, 37. [Google Scholar] [CrossRef]
- Jordan, S.C.; Ammerman, N.; Choi, J.; Huang, E.; Peng, A.; Sethi, S.; Najjar, R.; Toyoda, M.; Lim, K.; Louie, S.; et al. Novel Therapeutic Approaches to Allosensitization and Antibody-mediated Rejection. Transplantation 2019, 103, 262–272. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Aubert, O.; Haas, M.; Loupy, A.; Huang, E.; Peng, A.; Kim, I.; Louie, S.; Ammerman, N.; et al. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Arab. Archaeol. Epigr. 2018, 18, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Orandi, B.J.; Racusen, L.; Jackson, A.M.; Garonzik-Wang, J.M.; Shah, T.; Woodle, E.S.; Sommerer, C.; Fitts, D.; Rockich, K.; et al. Plasma-Derived C1 Esterase Inhibitor for Acute Antibody Mediated Rejection Following Kidney Transplantation: Results of a Randomized, Double-Blind, Placebo-Controlled Pilot Study. Arab. Archaeol. Epigr. 2016, 16, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Böhmig, G.A.; Eskandary, F.; Doberer, K.; Halloran, P.F. The therapeutic challenge of late antibody-mediated kidney allograft rejection. Transplant. Int. 2019, 32, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Mathew, R.O.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular disease in the kidney transplant recipient: Epidemiology, diagnosis and management strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Diekmann, F.; Graziani, G. Hypertension in kidney transplant recipients. Transplant. Int. 2011, 24, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.; Jones, C.M.; Hughes, M.G.; Eng, M.; Marvin, M.R. The Impact of Recipient Obesity on Outcomes After Renal Transplantation. Ann. Surg. 2013, 257, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Holdaas, H.; Fellström, B.; Jardine, A. Clinical Practice Guidelines for Managing Dyslipidemias in Kidney Transplant Patients: Lessons to be Learnt From the Assessment of LescolR in Renal Transplantation (ALERT) Trial. Arab. Archaeol. Epigr. 2005, 5, 1574–1575. [Google Scholar] [CrossRef]
- Kasiske, B.L. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients. Am. J. Transplant. 2005, 5, 1576. [Google Scholar] [CrossRef]
- Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Arab. Archaeol. Epigr. 2009, 9, S1–S155. [CrossRef]
- Mallamaci, F.; D’Arrigo, G.; Tripepi, R.; Leonardis, D.; Porto, G.; Testa, A.; Elhafeez, S.A.; Mafrica, A.; Versace, M.C.; Provenzano, P.F.; et al. Office, standardized and 24-h ambulatory blood pressure and renal function loss in renal transplant patients. J. Hypertens. 2018, 36, 119–125. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Anjum, S.; Shah, R.; Skogen, J.; Kandaswamy, C.; Danielson, B.; O’Shaughnessy, E.A.; Dahl, D.C.; Silkensen, J.R.; Sahadevan, M.; et al. Hypertension after kidney transplantation. Am. J. Kidney Dis. 2004, 43, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, N.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Cosio, F.G.; Kudva, Y.; Van Der Velde, M.; Larson, T.S.; Textor, S.C.; Griffin, M.D.; Stegall, M.D. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005, 67, 2415–2421. [Google Scholar] [CrossRef] [PubMed]
- Weinrauch, L.; Claggett, B.; Liu, J.; Finn, P.V.; Weir, M.R.; E Weiner, D.; A D’Elia, J. Smoking and outcomes in kidney transplant recipients: A post hoc survival analysis of the FAVORIT trial. Int. J. Nephrol. Renov. Dis. 2018, 11, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Holdaas, H.; Fellström, B.; Jardine, A.; Nyberg, G.; Grönhagen-Riska, C.; Madsen, S.; Neumayer, H.-H.; Cole, E.; Maes, B.; Ambühl, P.; et al. Beneficial effect of early initiation of lipid-lowering therapy following renal transplantation. Nephrol. Dial. Transplant. 2005, 20, 974–980. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicoletto, B.B.; Fonseca, N.K.O.; Manfro, R.C.; Gonçalves, L.F.S.; Leitao, C.; Souza, G.C. Effects of Obesity on Kidney Transplantation Outcomes. Transplantation 2014, 98, 167–176. [Google Scholar] [CrossRef]
- Weiner, D.E.; A Carpenter, M.; Levey, A.S.; Ivanova, A.; Cole, E.H.; Hunsicker, L.; Kasiske, B.L.; Kim, S.J.; Kusek, J.W.; Bostom, A.G. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: The FAVORIT trial. Arab. Archaeol. Epigr. 2012, 12, 2437–2445. [Google Scholar] [CrossRef]
- Fernández-Fresnedo, G.; Escallada, R.; Rodrigo, E.; De Francisco, A.L.M.; Cotorruelo, J.G.; De Castro, S.S.; Zubimendi, J.A.; Ruiz, J.C.; Arias, M. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation 2002, 73, 1345–1348. [Google Scholar] [CrossRef]
- Hiremath, S.; A Fergusson, D.; Fergusson, N.; Bennett, A.; Knoll, G.A. Renin-Angiotensin System Blockade and Long-term Clinical Outcomes in Kidney Transplant Recipients: A Meta-analysis of Randomized Controlled Trials. Am. J. Kidney Dis. 2017, 69, 78–86. [Google Scholar] [CrossRef]
- Paoletti, E.; Bellino, D.; Signori, A.; Pieracci, L.; Marsano, L.; Russo, R.; Massarino, F.; Ravera, M.; Fontana, I.; Carta, A.; et al. Regression of asymptomatic cardiomyopathy and clinical outcome of renal transplant recipients: A long-term prospective cohort study. Nephrol. Dial. Transplant. 2015, 31, 1168–1174. [Google Scholar] [CrossRef][Green Version]
- Ketteler, M.; Block, G.A.; Evenepoel, P.; Fukagawa, M.; Herzog, C.A.; McCann, L.; Moe, S.M.; Shroff, R.; Tonelli, M.; Toussaint, N.D.; et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: What’s changed and why it matters. Kidney Int. 2017, 92, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Rigatto, C. Electrocardiographic Left Ventricular Hypertrophy in Renal Transplant Recipients: Prognostic Value and Impact of Blood Pressure and Anemia. J. Am. Soc. Nephrol. 2003, 14, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Israni, A.K.; Snyder, J.J.; Skeans, M.A.; Peng, Y.; MacLean, J.R.; Weinhandl, E.D.; Kasiske, B.L. For the PORT Investigators Predicting Coronary Heart Disease after Kidney Transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Arab. Archaeol. Epigr. 2010, 10, 338–353. [Google Scholar] [CrossRef]
- Kasiske, B.L.; A Chakkera, H.; Roel, J. Explained and unexplained ischemic heart disease risk after renal transplantation. J. Am. Soc. Nephrol. 2000, 11, 1735–1743. [Google Scholar] [PubMed]
- Gaston, R.S.; Basadonna, G.; Cosio, F.G.; Davis, C.L.; Kasiske, B.L.; Larsen, J.; Leichtman, A.B.; Delmonico, F. Transplantation in the diabetic patient with advanced chronic kidney disease: A task force report. Am. J. Kidney Dis. 2004, 44, 529–542. [Google Scholar] [CrossRef]
- Balla, A.; Chobanian, M. New-onset diabetes after transplantation: A review of recent literature. Curr. Opin. Organ. Transplant. 2009, 14, 375–379. [Google Scholar] [CrossRef]
- Munagala, M.R.; Phancao, A. Managing Cardiovascular Risk in the Post Solid Organ Transplant Recipient. Med. Clin. N. Am. 2016, 100, 519–533. [Google Scholar] [CrossRef]
- Beshyah, S.A.; Beshyah, A.S.; Beshyah, W.S.; Yaghi, S. Use of SGLT2 Inhibitors in Diabetic Renal Transplant Recipients: A Mixed Method Exploratory Exercise. Int. J. Diabetes Metab. 2018, 21, 16–21. [Google Scholar] [CrossRef]
- Conte, C.; Secchi, A. Post-transplantation diabetes in kidney transplant recipients: An update on management and prevention. Acta Diabetol. 2018, 55, 763–779. [Google Scholar] [CrossRef]
- Campistol, J.M. Minimizing the Risk of Posttransplant Malignancy. Transplant. Proc. 2008, 40, S40–S43. [Google Scholar] [CrossRef] [PubMed]
- Briggs, J.D. Causes of death after renal transplantation. Nephrol. Dial. Transplant. 2001, 16, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Rama, I.; Grinyó, J.M. Malignancy after renal transplantation: The role of immunosuppression. Nat. Rev. Nephrol. 2010, 6, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, G.B.; Peng, F.H.; Xie, X.B. Cancer risks in recipients of renal transplants: A meta-analysis of cohort studies. Oncotarget 2018, 9, 15375–15385. [Google Scholar] [CrossRef] [PubMed]
- White, S.L.; Rawlinson, W.; Boan, P.; Sheppeard, V.; Wong, G.; Waller, K.; Opdam, H.; Kaldor, J.; Fink, M.; Verran, D.; et al. Infectious Disease Transmission in Solid Organ Transplantation: Donor Evaluation, Recipient Risk, and Outcomes of Transmission. Transplant. Direct 2019, 5, e416. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Patton, P.R.; Reed, A.; Hemming, A.W.; Van Der Werf, W.J.; Pfaff, W.W.; Srinivas, T.R.; Scornik, J.C. The changing causes of graft loss and death after kidney transplantation. Transplantation 2002, 73, 1923–1928. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Madariaga, H.M.; Thomas, B.S.; Lerma, E. Kidney transplantation for the primary care provider. Disease 2020, 66, 100869. [Google Scholar] [CrossRef]
- Chesdacha, S.; Thongprayoon, C.; Bruminhent, J.; Cheungpasitporn, W. Efficacy and adverse effects of cidofovir for treatment of BK virus infection in kidney transplant recipients. J. Nephropharmacol. 2017, 7, 10–17. [Google Scholar] [CrossRef]
- Vanichanan, J.; Udomkarnjananun, S.; Avihingsanon, Y.; Jutivorakool, K. Common viral infections in kidney transplant recipients. Kidney Res. Clin. Pract. 2018, 37, 323–337. [Google Scholar] [CrossRef]
- Hilbrands, L.B. Latest developments in living kidney donation. Curr. Opin. Organ. Transplant. 2020, 25, 74–79. [Google Scholar] [CrossRef]
- A Montgomery, R.; Gentry, S.; Marks, W.H.; Warren, D.S.; Hiller, J.; Houp, J.; A Zachary, A.; Melancon, J.K.; Maley, W.R.; Rabb, H.; et al. Domino paired kidney donation: A strategy to make best use of live non-directed donation. Lancet 2006, 368, 419–421. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Pham, T.A.; Melcher, M.L. Living Kidney Donation: Strategies to Increase the Donor Pool. Surg. Clin. N. Am. 2019, 99, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.S.; Montgomery, R.A. Incompatible kidney transplantation: Lessons from a decade of desensitization and paired kidney exchange. Immunol. Res. 2010, 47, 257–264. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, M.; Kal-van Gestel, J.A.; van de Wetering, J.; Kho, M.L.; Middel-de Sterke, S.; Betjes, M.G.H.; Zuidema, W.C.; Roelen, D.; Glorie, K.; Roodnat, J.I. Creating Options for Difficult-to-match Kidney Transplant Candidates. Transplantation 2020. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.F.; Rodrigue, J.R.; Veatch, R.M. Ethical and Logistical Issues Raised by the Advanced Donation Program “Pay It Forward” Scheme. J. Med. Philos. 2017, 42, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Doshi, M.D.; Ortigosa-Goggins, M.; Garg, A.X.; Li, L.; Poggio, E.D.; Winkler, C.A.; Kopp, J.B. APOL1 Genotype and Renal Function of Black Living Donors. J. Am. Soc. Nephrol. 2018, 29, 1309–1316. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Cheungpasitporn, W. Persistent hyperparathyroidism after kidney transplantation; updates on the risk factors and its complications. J. Parathyr. Dis. 2017, 6, 26–28. [Google Scholar] [CrossRef]
- Yoo, K.D.; Noh, J.; Lee, H.; Kim, D.K.; Lim, C.S.; Kim, Y.H.; Lee, J.P.; Kim, G.; Kim, Y.S. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: A Multicenter Cohort Study. Sci. Rep. 2017, 7, 8904. [Google Scholar] [CrossRef] [PubMed]
- Mark, E.; Goldsman, D.; Gurbaxani, B.; Keskinocak, P.; Sokol, J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS ONE 2019, 14, e0209068. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Massie, A.B.; Thomas, A.G.; Bahn, G.; Luo, X.; Jackson, K.R.; Ottmann, S.E.; Brennan, D.C.; Desai, N.M.; Coresh, J.; et al. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Arab. Archaeol. Epigr. 2018, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Atallah, D.M.; Badawy, M.; El-Sayed, A.; Ghoneim, M.A. Predicting kidney transplantation outcome based on hybrid feature selection and KNN classifier. Multimed. Tools Appl. 2019, 78, 20383–20407. [Google Scholar] [CrossRef]
- Nematollahi, M.; Akbari, R.; Nikeghbalian, S.; Salehnasab, C. Classification Models to Predict Survival of Kidney Transplant Recipients Using Two Intelligent Techniques of Data Mining and Logistic Regression. Int. J. Organ. Transplant. Med. 2017, 8, 119–122. [Google Scholar] [PubMed]
- Tapak, L.; Hamidi, O.; Amini, P.; Poorolajal, J. Prediction of Kidney Graft Rejection Using Artificial Neural Network. Healthc. Inform. Res. 2017, 23, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Shahmoradi, L.; Langarizadeh, M.; Pourmand, G.; Fard, Z.A.; Borhani, A. Comparing Three Data Mining Methods to Predict Kidney Transplant Survival. Acta Inform. Med. 2016, 24, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.; Sylvain, T.; Cardinal, H.; Lodi, A.; Bengio, Y. Deep Learning for Patient-Specific Kidney Graft Survival Analysis. arXiv 2017, arXiv:1705.10245. [Google Scholar]
- Topuz, K.; Zengul, F.D.; Dag, A.; Almehmi, A.; Yildirim, M.B. Predicting graft survival among kidney transplant recipients: A Bayesian decision support model. Decis. Support Syst. 2018, 106, 97–109. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Kaewput, W.; Kovvuru, K.; Hansrivijit, P.; Kanduri, S.R.; Bathini, T.; Chewcharat, A.; Leeaphorn, N.; Gonzalez-Suarez, M.L.; Cheungpasitporn, W. Promises of Big Data and Artificial Intelligence in Nephrology and Transplantation. J. Clin. Med. 2020, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Forbes, R.C.; Rybacki, D.B.; Johnson, T.B.; Hannah-Gillis, A.; Shaffer, D.; Hale, D.A. A Cost Comparison for Telehealth Utilization in the Kidney Transplant Waitlist Evaluation Process. Transplantation 2018, 102, 279–283. [Google Scholar] [CrossRef]
- Andrew, N.; Barraclough, K.A.; Long, K.; Fazio, T.N.; Holt, S.; Kanhutu, K.; Hughes, P.D. Telehealth model of care for routine follow up of renal transplant recipients in a tertiary centre: A case study. J. Telemed. Telecare 2018. [Google Scholar] [CrossRef]
| Cardiovascular Risk Factor | Suggested Management | Reference |
|---|---|---|
| Traditional risk factors | ||
| Hypertension |
| [109,110,111,112] |
| Diabetes |
| [109,113] |
| Cigarette smoking |
| [109,114] |
| Dyslipidemia |
| [109,115] |
| Obesity |
| [109,116] |
| Non-traditional risk factors | ||
| eGFR < 45 ml/min/1.73m2 |
| [109,117] |
| Proteinuria |
| [109,118,119] |
| Left ventricular hypertrophy |
| [109,120] |
| Anemia |
| [109,121,122] |
| Acute rejection episodes |
| [109,123,124] |
| Cancer | Standardized Incidence Ratio (95% CI) |
|---|---|
| Lip cancer | 29.45 (17.85–48.59) |
| Non-melanoma skin cancer | 12.14 (6.37–23.13) |
| Renal cell carcinoma | 10.77 (6.40–18.12) |
| Non-Hodgkin lymphoma | 10.66 (8.54–13.31) |
| Thyroid cancer | 5.04 (3.79–6.71) |
| Hodgkin lymphoma | 4.90 (3.09–7.78) |
| Urinary bladder cancer | 3.52 (1.48–8.37) |
| Melanoma | 2.48 (1.08–5.67) |
| Hepatocellular carcinoma | 2.45 (1.63–3.66) |
| Gastric cancer | 1.93 (1.60–2.34) |
| Colon cancer | 1.85 (1.53–2.23) |
| Lung cancer | 1.68 (1.29–2.19) |
| Ovarian cancer | 1.60 (1.23–2.07) |
| Pancreatic cancer | 1.53 (1.23–1.91) |
| Breast cancer | 1.11 (1.11–1.24) |
| <1 Month | 1–6 Month | >6 Month |
Bacterial infection *
| Bacterial infection
| Bacterial infection
|
Viral infection
| ||
Viral infection
| ||
Viral infection
| ||
Fungal infection
| ||
Fungal infection
| ||
Fungal infection
| ||
Parasitic infection
| ||
Parasitic infection
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongprayoon, C.; Hansrivijit, P.; Leeaphorn, N.; Acharya, P.; Torres-Ortiz, A.; Kaewput, W.; Kovvuru, K.; Kanduri, S.R.; Bathini, T.; Cheungpasitporn, W. Recent Advances and Clinical Outcomes of Kidney Transplantation. J. Clin. Med. 2020, 9, 1193. https://doi.org/10.3390/jcm9041193
Thongprayoon C, Hansrivijit P, Leeaphorn N, Acharya P, Torres-Ortiz A, Kaewput W, Kovvuru K, Kanduri SR, Bathini T, Cheungpasitporn W. Recent Advances and Clinical Outcomes of Kidney Transplantation. Journal of Clinical Medicine. 2020; 9(4):1193. https://doi.org/10.3390/jcm9041193
Chicago/Turabian StyleThongprayoon, Charat, Panupong Hansrivijit, Napat Leeaphorn, Prakrati Acharya, Aldo Torres-Ortiz, Wisit Kaewput, Karthik Kovvuru, Swetha R. Kanduri, Tarun Bathini, and Wisit Cheungpasitporn. 2020. "Recent Advances and Clinical Outcomes of Kidney Transplantation" Journal of Clinical Medicine 9, no. 4: 1193. https://doi.org/10.3390/jcm9041193
APA StyleThongprayoon, C., Hansrivijit, P., Leeaphorn, N., Acharya, P., Torres-Ortiz, A., Kaewput, W., Kovvuru, K., Kanduri, S. R., Bathini, T., & Cheungpasitporn, W. (2020). Recent Advances and Clinical Outcomes of Kidney Transplantation. Journal of Clinical Medicine, 9(4), 1193. https://doi.org/10.3390/jcm9041193







