The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series
Abstract
1. Introduction
2. Methods
2.1. Study Group
2.2. Immunosuppressive Protocol
2.3. Primary Treatment Protocol of AMR
2.4. Kidney Graft Function and Protocol Biopsies
2.5. Statistics
3. Results
3.1. Baseline Characteristics
3.2. AMR Diagnosis and Treatment
3.3. Kidney Graft Function and Survival
3.4. Treatment Safety
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lefaucheur, C.; Nochy, D.; Hill, G.S.; Suberbielle-Boissel, C.; Antoine, C.; Charron, D.; Glotz, D. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am. J. Transplant. 2007, 7, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Amico, P.; Honger, G.; Mayr, M.; Steiger, J.; Hopfer, H.; Schaub, S. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation 2009, 87, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Gloor, J.M.; Winters, J.L.; Cornell, L.D.; Fix, L.A.; DeGoey, S.R.; Knauer, R.M.; Cosio, F.G.; Gandhi, M.J.; Kremers, W.; Stegall, M.D. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am. J. Transplant. 2010, 10, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Sis, B.; Racusen, L.C.; Solez, K.; Glotz, D.; Colvin, R.B.; Castro, M.C.; David, D.S.; David-Neto, E.; Bagnasco, S.M.; et al. Banff 2013 meeting report: Inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 2014, 14, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Budde, K.; Durr, M. Any progress in the tratment of antibody-mediated rejection? J. Am. Soc. Nephrol. 2018, 29, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.S.; Ying, T.D.; Wyburn, K.; Roberts, D.M.; Wyld, M.; Chadban, S.J. The treatment of antibody-mediated rejection in kidney transplantation: As updated systematic review and meta-analysis. Transplantation 2018, 102, 557–568. [Google Scholar] [CrossRef]
- Slatinska, J.; Slavcev, A.; Honsova, E.; Hruba, P.; Kratochvilova, I.; Rohal, T.; Viklicky, O. Efficacy and safety of bortezomib treatment for refractory acute antibody-mediated rejection—A pilot study. HLA 2018, 92, 47–50. [Google Scholar]
- Oblak, T.; Lindic, J.; Gubensek, J.; Kveder, R.; Rigler, A.A.; Skoberne, A.; Veceric Haler, Z.; Bosrtnar, S.; Avgustin, N.; Ponikvar, R.; et al. Treatment of antibody-mediated rejection of kidney grafts with bortezomib and/or rituximab compared to standard regimen: Experience of Slovene Natinal Center. Clin. Nephrol. 2017, 88, S91–S96. [Google Scholar] [CrossRef]
- De Sousa-Amorim, E.; Revuelta, I.; Diekmann, F.; Cofan, F.; Lozano, M.; Cid, J.; Palou, E.; Sole, M.; Campistol, J.M.; Oppenheimer, F. Bortezomib for refractory acute antibody-mediated rejection in kidney transplant recipients: A single-center case series. Nephrology 2016, 21, 700–704. [Google Scholar] [CrossRef]
- Everly, M.J.; Everly, J.J.; Susskind, B.; Brailey, P.; Arend, L.J.; Alloway, R.R.; Roy-Chaudhury, P.; Govil, A.; Moquilishetty, G.; Rike, A.H.; et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation 2008, 86, 1754–1761. [Google Scholar] [CrossRef]
- Walsh, R.C.; Brailey, P.; Girnita, A.; Alloway, R.R.; Shields, A.R.; Wall, G.E.; Sadaka, B.H.; Cardi, M.; Tevar, A.; Govil, A.; et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibitor. Transplantation 2011, 91, 1218–1226. [Google Scholar] [CrossRef]
- Sadaka, B.; Ejaz, N.S.; Shields, A.R.; Cardi, M.; Wadih, G.; Witte, D.; Abu Jawdeh, B.G.; Alloway, R.R.; Woodle, E.S. A Banff component scoring-based histologic assessment of bortezomib-based antibody-mediated rejection therapy. Transplantation 2015, 99, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Mubarak, M.; Zafar, M.N.; Musharraf, W.; Imam, M.; Aziz, T.; Rizvi, A.H. Management of plasma cell-rich acute rejection in living-related kidney transplant: Role of proteasome inhibitor. Exp. Clin. Transplant. 2019, 1, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Gubensek, J.; Buturovic-Ponikvar, J.; Kandus, A.; Arnol, M.; Lindic, J.; Kovac, D.; Rigler, A.A.; Romozi, K.; Ponikvar, R. Treatment of antibody-mediated rejection after kidney transplantation—10 years’ experience with apheresis at a single center. Ther. Apher. Dial. 2016, 20, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.C.; Everly, J.J.; Brailey, P.; Rike, A.H.; Arend, L.J.; Mogilishetty, G.; Govil, A.; Roy-Chaudhury, P.; Alloway, R.R.; Woodle, E.S. Proteasome inhibitor-based primary therapy for antibory-mediated renal allograft rejection. Transplantation 2010, 89, 277–284. [Google Scholar] [CrossRef]
- Waiser, J.; Budde, K.; Schutz, M.; Liefeldt, L.; Rudolph, B.; Schonemann, C.; Neumayer, H.-H.; Lachmann, N. Comparison between brtezomib and rituximab in the treatment of antibody-mediated renal allograft survival. Nephrol. Dial. Transplant. 2012, 27, 1246–1251. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.L.; Xu, X.G.; Shi, B.Y.; Zhang, Z.M.; Li, Z.L.; Han, Y.; Zhou, W.Q.; Chen, C.Q.; Cai, M.; et al. Bortezomib-based treatment of acute antibody-mediated rejection: A case report. Gen. Mol. Res. 2015, 14, 17951–17958. [Google Scholar] [CrossRef]
- Sureshkumar, K.K.; Hussain, S.M.; Marcus, R.J.; Ko, T.Y.; Khan, A.S.; Tom, K.; Vivas, C.A.; Parris, G.; Jasnosz, K.M.; Thai, N.L. Proteasome inhibition with bortezomib: An effective therapy for severe antibody mediated rejection after renal transplantation. Clin. Nephrol. 2012, 77, 246–253. [Google Scholar] [CrossRef]
- Perry, D.K.; Burns, J.M.; Pollinger, H.S.; Amiot, B.P.; Gloor, J.M.; Gores, G.J.; Stegall, M.D. Proteasome inhibitor causes apoptosis of normal human plasma cells preventing alloantibody production. Am. J. Transplant. 2009, 9, 201–209. [Google Scholar] [CrossRef]
- Tasaki, M.; Saito, K.; Nakagawa, Y.; Ikeda, M.; Imai, N.; Ito, Y.; Sudo, M.; Ikezumi, Y.; Yamada, T.; Hasegawa, H.; et al. Bortezomib eliminates plasma cells from a renal graft in plasma cell-rich acute rejection. Transplant. Proc. 2019, 51, 1732–1738. [Google Scholar] [CrossRef]
- Durlik, M.; Klinger, M.; Lao, M.; Rutkowski, B.; Więcek, A. The treatment of antibody-mediated rejection in kidney transplantation. In The Guidelines for Immunosuppressive Treatment in Solid Organ Transplantation; Durlik, M., Rowiński, W., Eds.; Fundacja Zjednoczeni dla Transplantacji: Warszawa, Poland, 2012; pp. 39–40. [Google Scholar]
- Mengel, M.; Sis, B.; Haas, M.; Colvin, R.B.; Halloran, P.F.; Racusen, L.C.; Solez, K.; Cendales, L.; Demetris, A.J.; Drachenberg, C.B.; et al. Banff 2011 meeting report: New concepts in antibody-mediated rejection. Am. J. Transplant. 2012, 12, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Loupy, A.; Lefaucheur, C.; Roufosse, C.; Glotz, D.; Seron, D.; Nankivell, B.J.; Halloran, P.F.; Colvin, R.B.; Akalin, E.; et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, ad prospects for integrative endpoints for next-generation clinical trials. Am. J. Transplant. 2018, 18, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Requiao-Moura, L.R.; de Sandes-Freitas, T.V.; Marcelo-Gomes, G.; Rangel, E.B. Bortezomib in kidney transplant: Current use and perspectives. Curr. Drug Metabol. 2017, 18, 1136–1146. [Google Scholar] [CrossRef]
- Everly, M.J.; Rebellato, L.M.; Haisch, C.E.; Ozawa, M.; Parker, K.; Briley, K.P.; Catrou, P.G.; Bolin, P.; Kendrick, W.T.; Kendrick, S.A.; et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 2013, 95, 410–417. [Google Scholar] [CrossRef]
- Morozumi, K.; Takeda, A.; Otsuka, Y.; Horike, K.; Gotoh, N.; Narumi, S.; Watarai, Y.; Kobayashi, T. Reviewing the pathogenesis of antibody-mediated rejection and renal graft pathology after kidney transplantation. Nephrology 2016, 21, 4–8. [Google Scholar] [CrossRef]
- Lemy, A.; Toungouz, M.; Abramowicz, D. Bortezomib: A new player in pre- and post-transplant desensitization? Nephrol. Dial. Transplant. 2010, 25, 3480–3489. [Google Scholar] [CrossRef]
- Hariharan, S.; McBride, M.A.; Cherikh, W.S.; Tolleris, C.B.; Bresnahan, B.A.; Johnson, C.P. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002, 62, 311–318. [Google Scholar] [CrossRef]
- Meregalli, C. An overview of bortezomib-induced neurotoxicity. Toxics 2015, 3, 294–303. [Google Scholar] [CrossRef]
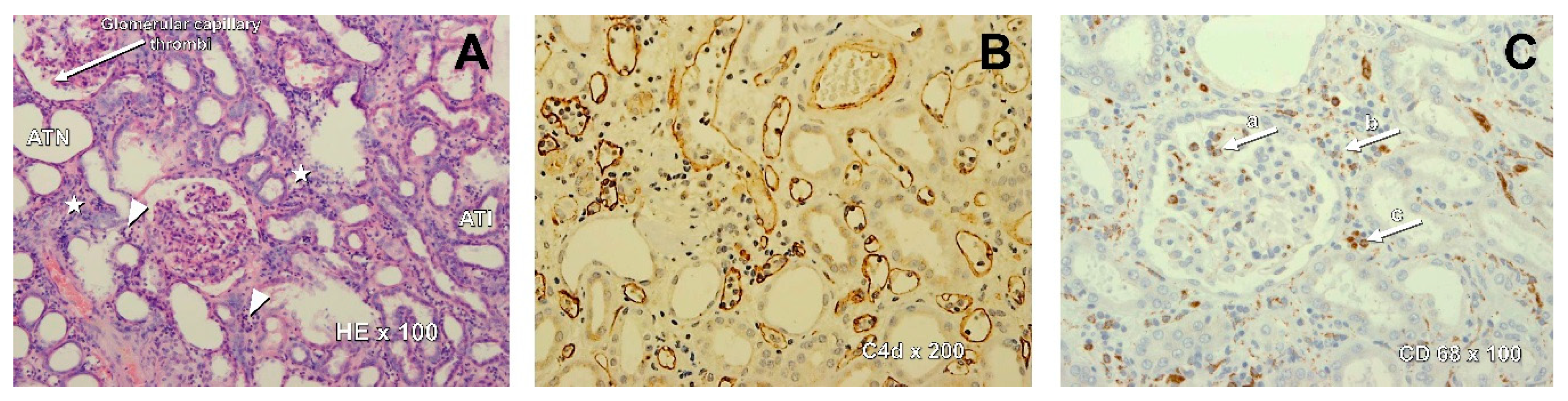
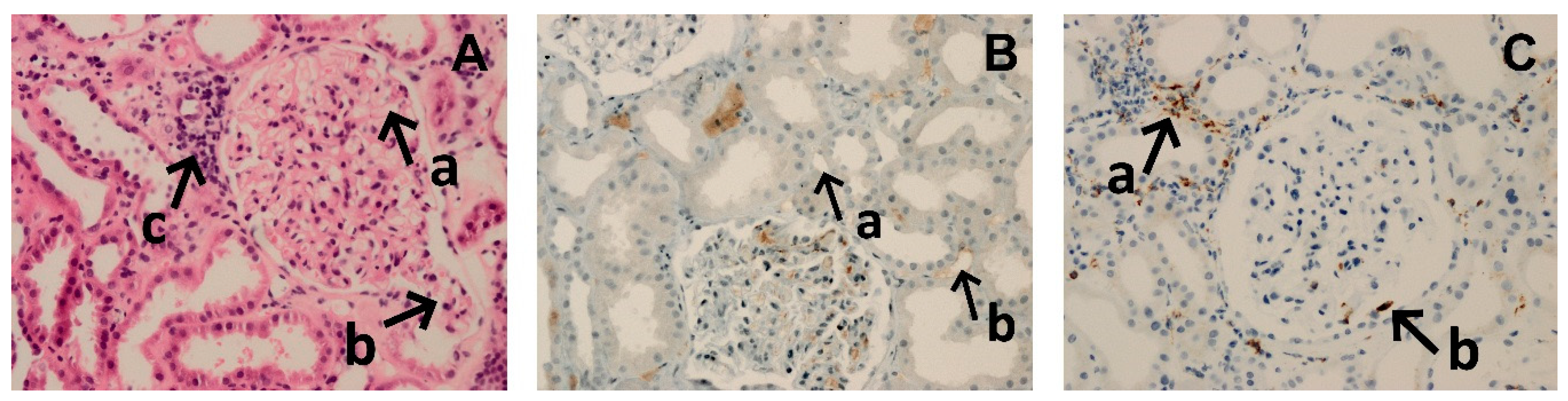
| Patient | Gender | Age (years) | Cause of ESRD | KTx No | PRA (%) | PRA max (%) | vPRA Class I | vPRA Class II | vPRA Class I+II | Pre-Treatment DSA (MFI) | Induction | Early Graft Function |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 30 | GNC | 2 | 50 | 87 | 98.108 | 0 | 98.108 | A2 (3489) | rATG | SGF |
| 2 | M | 41 | Unknown | 1 | 0 | 15 | 0 | 0 | 0 | A2 (15,101) | rATG | SGF |
| 3 | M | 61 | ADPKD | 2 | 0 | 0 | 31.732 | 0 | 31.732 | A1 (1968) | rATG | SGF |
| 4 | F | 61 | PNC | 1 | 0 | 15 | 46.798 | 99.476 | 99.607 | DR 4 (10,284) | rATG | IGF |
| 5 | M | 68 | PNC | 1 | 0 | 0 | n/a | n/a | n/a | n/a | IL-2RB | SGF |
| 6 | F | 52 | GNC | 1 | 0 | 0 | 30.335 * | 80.568 | 85.604 | DR 13 (1500) | rATG | SGF |
| 7 | M | 61 | GNC | 2 | 0 | 0 | 0 | 41.179 | 41.179 | DR10 (11,509) | None | DGF |
| 8 | F | 63 | GNC | 1 | 25 | 40 | 68.006 | 0 | 68.006 | B18 (11,321) | rATG | SGF |
| 9 | F | 56 | Unknown | 1 | 0 | 0 | 12.620 | 93.770 | 94.541 | A11 (1798) | rATG | DGF |
| 10 | M | 47 | GNC | 3 | 5 | 70 | 96.361 | 42.897 | 97.977 | A68 (13,936) | rATG | DGF |
| 11 | F | 51 | ADPKD | 1 | 0 | 0 | 75.197 | 48.049 | 86.827 | B35 (4899) | rATG | DGF |
| 12 | M | 47 | ADPKD | 1 | 0 | 0 | 0 | 0 | 0 | A2 (1992) | IL-2RB | DGF |
| 13 | F | 60 | DM | 1 | 0 | 0 | 0 | 0 | 0 | A3 (541) | IL-2RB | DGF |
| Patient | Bx POD | Banff Biopsy Scoring | C4d | Other Diagnosis | DSA | Diagnosis | Serum Creatinine Concentration (mg/dL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | (t) | (i) | (v) | (ah) | (ptc) | (cg) | (ct) | (ci) | (cv) | Pre-Treatment | Post-Treatment | 3 Month | 12 Month | 24 Month | ||||||
| 1 | 10 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | AMR | 5.7 | 2.2 | 1.1 | 1.1 | 1.1 | |
| 2 | 8 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | AMR | 2.5 | 1.8 | 1.2 | 1.3 | 1.3 | |
| 3 | 9 | 2 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | AMR | 1.8 | 0.8 | 1.1 | 0.9 | 0.8 | |
| 4 | 10 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | AMR | 0.9 | 0.7 | 0.8 | 1.1 | 0.8 | |
| 5 | 9 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | AMR susp | 1.6 | 1.3 | 1.2 | 1.4 | 1.3 | |
| 6 | 18 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | AMR | 6.2 | 1.5 | 0.9 | 0.9 | 0.9 | |
| 7 | 9 | 3 | 0 | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | TCMR | 1 | AMR susp | 12.7 | 1.5 | 1.3 | 1.5 | |
| 8 | 10 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | TCMR IIA | 1 | AMR susp | 2.7 | 1.7 | 1.5 | 1.6 | |
| 9 | 10 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | AMR | 5.9 | 1.7 | 1.7 | |||
| 10 | 10 | 2 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | AMR | 8.5 | 3.0 | 1.4 | |||
| 11 | 8 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 1 | AMR | 3.9 | 1.9 | 1.7 | |||
| 12 | 11 | 1 | 1 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | AMR susp | 4.0 | 1.2 | 1.9 | |||
| 13 | 9 | 2 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | CNI | 1 | AMR susp | 3.1 | 1.4 | 1.4 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolonko, A.; Słabiak-Błaż, N.; Karkoszka, H.; Więcek, A.; Piecha, G. The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series. J. Clin. Med. 2020, 9, 529. https://doi.org/10.3390/jcm9020529
Kolonko A, Słabiak-Błaż N, Karkoszka H, Więcek A, Piecha G. The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series. Journal of Clinical Medicine. 2020; 9(2):529. https://doi.org/10.3390/jcm9020529
Chicago/Turabian StyleKolonko, Aureliusz, Natalia Słabiak-Błaż, Henryk Karkoszka, Andrzej Więcek, and Grzegorz Piecha. 2020. "The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series" Journal of Clinical Medicine 9, no. 2: 529. https://doi.org/10.3390/jcm9020529
APA StyleKolonko, A., Słabiak-Błaż, N., Karkoszka, H., Więcek, A., & Piecha, G. (2020). The Preliminary Results of Bortezomib Used as A Primary Treatment for An Early Acute Antibody-Mediated Rejection after Kidney Transplantation—A Single-Center Case Series. Journal of Clinical Medicine, 9(2), 529. https://doi.org/10.3390/jcm9020529





