Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review
Abstract
1. Introduction
2. Literature Search
- Study sample (the number of patients in the experimental group and of participants in the control group).
- Data acquisition and processing, where the following points are analyzed:
- −
- Device, software, duration of recordings, and sampling frequency;
- −
- Recording conditions: time of the day, room (lights/voices/temperature), activities before recordings (sleep routine, physical activities, meals, drinks, using the toilet), and heart rate stabilization;
- −
- Respiratory rate during recordings and breathing control;
- HRV analysis, where the following points are analyzed:
- −
- Software, artifact correction, time series length (time/beats), information about data normality;
- −
- Frequency domain and nonlinear HRV parameters;
- HRV correction for HR.
3. Results
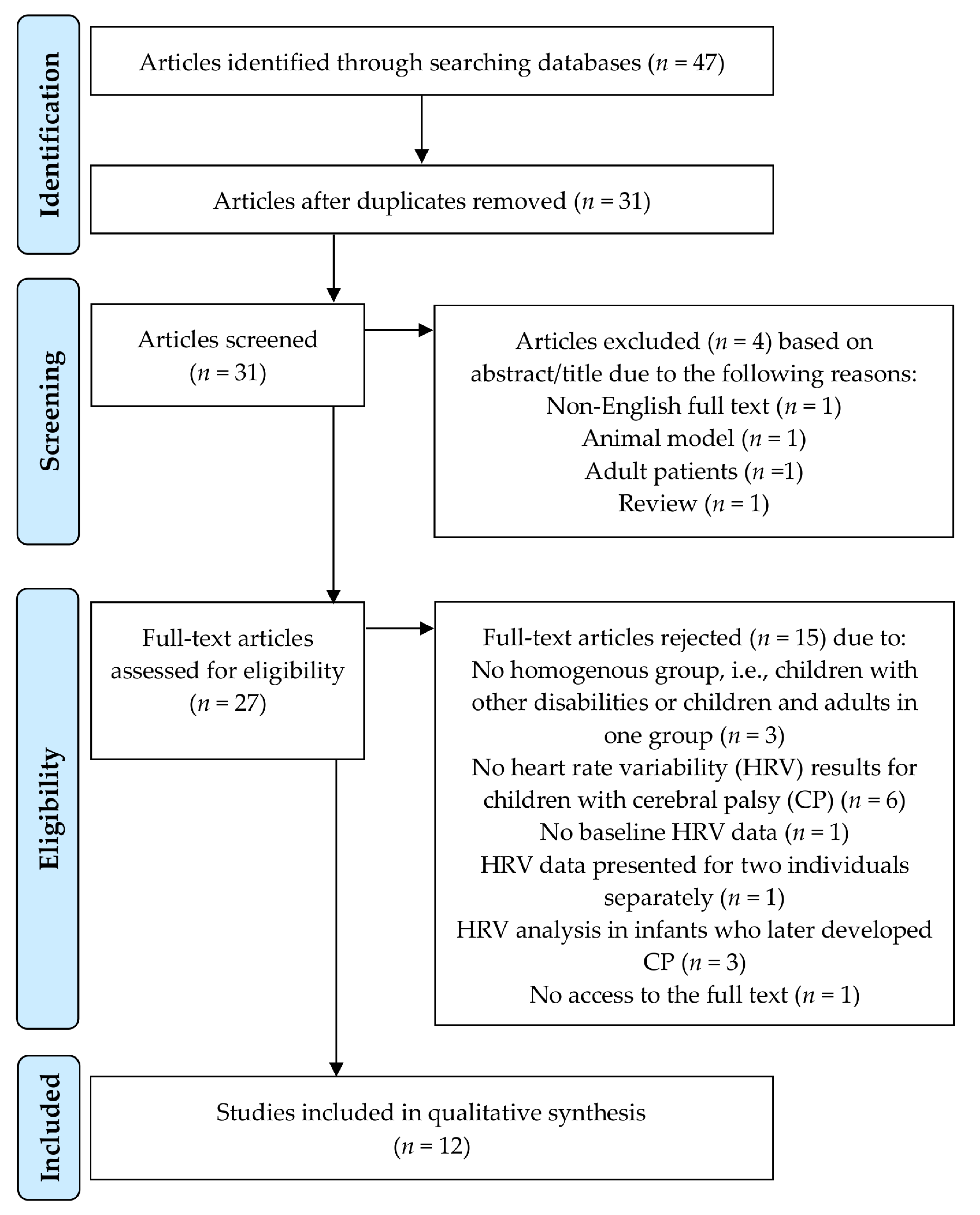
3.1. Selection of the Studies
3.2. Information Provided by the Selected Studies
3.2.1. Participants/Demographic Data
3.2.2. RR Interval Recordings
3.2.3. HRV Measurement
3.2.4. HRV Results
4. Discussion
4.1. HRV Changes in Children with CP
4.2. Compliance of the Studies to Recommendations and Guidelines
4.2.1. Study Sample
4.2.2. Data Acquisition and Processing
- Device, software, duration of recordings, and sampling frequency
- Recording conditions: time of the day, room (lights/voices/temperature), activities before recordings (sleep routine, physical activities, meals, drinks, using the toilet), and heart rate stabilization
- Respiratory rate during recordings and breathing control
4.2.3. HRV Analysis
- Software, artifact correction, time series length (time/beats), information about data normality
- Frequency domain and nonlinear HRV parameters
4.2.4. HRV Correction for HR
4.3. Lack of Methodological Information in Existing Studies on HRV in Pediatric Participants with CP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 49, 8–14. [Google Scholar] [CrossRef]
- Park, E.S.; Park, C.I.; Cho, S.-R.; Lee, J.-W.; Kim, E.J. Assessment of Autonomic Nervous System with Analysis of Heart Rate Variability in Children with Spastic Cerebral Palsy. Yonsei Med J. 2002, 43, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.F.; Chan, R.C.; Kao, C.L.; Chiu, J.W.; Liu, T.J.; Kao, N.T.; Kuo, T.B.J. Power Spectrum Analysis of Heart Rate Variability for Cerebral Palsy Patients. Am. J. Phys. Med. Rehabil. 2002, 81, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Kerppers, I.I.; Arisawa, E.A.L.; Oliveira, L.V.F.; Sampaio, L.M.M.; Oliveira, C.S. Heart rate variability in individuals with cerebral palsy. Arch. Med. Sci. 2009, 5, 45–50. [Google Scholar]
- Zamunér, A.R.; Cunha, A.B.; Da Silva, E.; Negri, A.P.; Tudella, E.; Moreno, M.A. The influence of motor impairment on autonomic heart rate modulation among children with cerebral palsy. Res. Dev. Disabil. 2011, 32, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Pastore, C.A.; Samesima, N.; Imada, R.; Reis, M.; Santos, M.T.B.R.; Ferreira, M.C.; Grupi, C.; Fumagalli, F.; Wagenführ, J.; Chammas, M. Characterization of the electrocardiographic pattern of individuals with cerebral palsy. J. Electrocardiol. 2011, 44, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Pastore, C.A.; Imada, R.; Guare, R.O.; Leite, M.; Poyares, D.; Santos, M.T.B.R. Autonomic nervous system in individuals with cerebral palsy: A controlled study. J. Oral Pathol. Med. 2011, 40, 576–581. [Google Scholar] [CrossRef]
- Kholod, H.; Jamil, A.; Katz-Leurer, M. The associations between motor ability, walking activity and heart rate and heart rate variability parameters among children with cerebral palsy and typically developed controls. Neurorehabilitation 2013, 33, 113–119. [Google Scholar] [CrossRef]
- Israeli-Mendlovic, H.; Mendlovic, J.; Katz-Leurer, M. Heart rate and heart rate variability parameters at rest, during activity and passive standing among children with cerebral palsy GMFCS IV–V. Dev. Neurorehabilit. 2014, 17, 398–402. [Google Scholar] [CrossRef]
- Amichai, T.; Katz-Leurer, M. Heart rate variability in children with cerebral palsy: Review of the literature and meta-analysis. Neurorehabilitation 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Amichai, T.; Eylon, S.; Dor-Haim, H.; Berger, I.; Katz-Leurer, M. Cardiac Autonomic System Response to Submaximal Test in Children with Cerebral Palsy. Pediatr. Phys. Ther. 2017, 29, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Amichai, T.; Eylon, S.; Berger, I.; Katz-Leurer, M. The impact of breathing rate on the cardiac autonomic dynamics among children with cerebral palsy compared to typically developed controls. Dev. Neurorehabilit. 2018, 22, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Holzer, M.; Sorek, G.; Schweizer, M.; Katz-Leurer, M. The influence of a constraint and bimanual training program using a variety of modalities on endurance and on the cardiac autonomic regulation system of children with unilateral cerebral palsy: A self-control clinical trial. Neurorehabilitation 2017, 41, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Katz-Leurer, M.; Amichai, T. Heart rate variability in children with cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 730–731. [Google Scholar] [CrossRef]
- Samuels, M.A. The Brain–Heart Connection. Circulation 2007, 116, 77–84. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.; Walker, A.M.; Horne, R.S. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 2013, 89, 145–152. [Google Scholar] [CrossRef]
- Sender, N.S.; Govindan, R.B.; Sulemanji, M.; Al-Shargabi, T.; Lenin, R.B.; Eksioglu, Y.Z.; Du Plessis, A.J. Effects of regional brain injury on the newborn autonomic nervous system. Early Hum. Dev. 2014, 90, 893–896. [Google Scholar] [CrossRef]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.D.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef]
- Prathep, S.; Sharma, D.; Hallman, M.; Joffe, A.; Krishnamoorthy, V.; Mackensen, G.B.; Vavilala, M.S. Preliminary Report on Cardiac Dysfunction after Isolated Traumatic Brain Injury. Crit. Care Med. 2014, 42, 142–147. [Google Scholar] [CrossRef]
- Grunsfeld, A.; Fletcher, J.J.; Nathan, B.R. Cardiopulmonary complications of brain injury. Curr. Neurol. Neurosci. Rep. 2005, 5, 488–493. [Google Scholar] [CrossRef]
- Van Der Bilt, I.; Hasan, D.; Vandertop, W.P.; Wilde, A.A.; Algra, A.; Visser, F.C.; Rinkel, G.J. Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: A meta-analysis. Neurology 2009, 72, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain–Heart Interaction. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.M.; Crowley, V.E.; Hensey, O.; Broderick, J.; McGahey, A.; Gormley, J. Habitual physical activity and cardiometabolic risk factors in adults with cerebral palsy. Res. Dev. Disabil. 2014, 35, 1995–2002. [Google Scholar] [CrossRef]
- Ryan, J.M.; Hensey, O.; McLoughlin, B.; Lyons, A.; Gormley, J. Reduced Moderate-to-Vigorous Physical Activity and Increased Sedentary Behavior Are Associated with Elevated Blood Pressure Values in Children with Cerebral Palsy. Phys. Ther. 2014, 94, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.A. Role of Editing of R–R Intervals in the Analysis of Heart Rate Variability. Front. Physiol. 2012, 3, 148. [Google Scholar] [CrossRef]
- Sacha, J. Why should one normalize heart rate variability with respect to average heart rate. Front. Physiol. 2013, 4, 306. [Google Scholar] [CrossRef]
- Sacha, J. Interaction between Heart Rate and Heart Rate Variability. Ann. Noninvasive Electrocardiol. 2014, 19, 207–216. [Google Scholar] [CrossRef]
- Sacha, J. Heart rate contribution to the clinical value of heart rate variability. Kardiol. Pol. 2014, 72, 919–924. [Google Scholar] [CrossRef]
- Sacha, J. Interplay between heart rate and its variability: A prognostic game. Front. Physiol. 2014, 5, 347. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The effect of heart rate on the heart rate variability response to autonomic interventions. Front. Physiol. 2013, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Heathers, J. Everything Hertz: Methodological issues in short-term frequency-domain HRV. Front. Physiol. 2014, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Heathers, J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol. 2014, 5, 805. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef]
- Billman, G.E.; Huikuri, H.V.; Sacha, J.; Trimmel, K. An introduction to heart rate variability: Methodological considerations and clinical applications. Front. Physiol. 2015, 6, 55. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Ernst, G. Heart-Rate Variability—More than Heart Beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Ernst, G. Hidden Signals—The History and Methods of Heart Rate Variability. Front. Public Health 2017, 5, 265. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research – Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 89. [Google Scholar] [CrossRef]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart Rate Variability: An Old Metric with New Meaning in the Era of using mHealth Technologies for Health and Exercise Training Guidance. Part One: Physiology and Methods. Arrhythmia Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Froelicher, V.; Plews, D. Heart Rate Variability: An Old Metric with New Meaning in the Era of Using mHealth technologies for Health and Exercise Training Guidance. Part Two: Prognosis and Training. Arrhythmia Electrophysiol. Rev. 2018, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J.; Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthr. 2019, 38, 3. [Google Scholar] [CrossRef] [PubMed]
- De Geus, E.J.C.N.; Gianaros, P.J.; Brindle, R.C.; Jennings, J.R.; Berntson, G.G. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 2018, 56, e13287. [Google Scholar] [CrossRef]
- Li, K.; Rüdiger, H.; Ziemssen, F. Spectral Analysis of Heart Rate Variability: Time Window Matters. Front. Neurol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmid, R.M.; Zabel, M. CrossTalk proposal: Heart rate variability is a valid measure of cardiac autonomic responsiveness. J. Physiol. 2019, 597, 2595–2598. [Google Scholar] [CrossRef]
- Kemper, K.J.; Hamilton, C.; Atkinson, M. Heart Rate Variability: Impact of Differences in Outlier Identification and Management Strategies on Common Measures in Three Clinical Populations. Pediatr. Res. 2007, 62, 337–342. [Google Scholar] [CrossRef]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Xhyheri, B.; Manfrini, O.; Mazzolini, M.; Pizzi, C.; Bugiardini, R. Heart Rate Variability Today. Prog. Cardiovasc. Dis. 2012, 55, 321–331. [Google Scholar] [CrossRef]
- Billman, G.E. Heart Rate Variability – A Historical Perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef]
- Akinci, A.; Baykal, E.; Akinci, A.; Celiker, A.; Tezic, T. Heart rate variability in diabetic children: Sensitivity of the time- and frequency-domain methods. Pediatr. Cardiol. 1993, 14, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Butera, G.; Lanza, G.A.; Bossone, E.; Delogu, A.; De Rosa, G.; Marietti, G.; Rosti, L.; Carminati, M. Role of Heart Rate Variability in the Early Diagnosis of Diabetic Autonomic Neuropathy in Children. Herz 2002, 27, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Taşçılar, M.E.; Yokuşoğlu, M.; Boyraz, M.; Baysan, O.; Koz, C.; Dündaröz, R. Cardiac Autonomic Functions in Obese Children. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 60–64. [Google Scholar] [CrossRef]
- Landis, C.; O’Neil, M.E.; Finnegan, A.; Shewokis, P.A. Calculating Heart Rate Variability from ECG Data from Youth with Cerebral Palsy During Active Video Game Sessions. J. Vis. Exp. 2019, e59230. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, B.; Ilic, S.; Dimitrijevic, L.; Milovanović, B.; Kostic, G.; Bjelakovic, L.; Lukic, S. Heart rate variability in infants with central coordination disturbance. Early Hum. Dev. 2010, 86, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Jeon, H.R.; Kim, J.; Kim, Y. Heart Rate Variability Among Children With Acquired Brain Injury. Ann. Phys. Rehabil. Med. 2017, 41, 951–960. [Google Scholar] [CrossRef][Green Version]
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Durufle-Tapin, A.; Colin, A.; Nicolas, B.; Lebreton, C.; Dauvergne, F.; Gallien, P. Analysis of the medical causes of death in cerebral palsy. Ann. Phys. Rehabil. Med. 2014, 57, 24–37. [Google Scholar] [CrossRef]
- Ryan, J.M.; Peterson, M.D.; Ryan, N.; Smith, K.J.; O’Connell, N.E.; Liverani, S.; Anokye, N.; Victor, C.; Allen, E. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 924–928. [Google Scholar] [CrossRef]
- Fatisson, J.; Oswald, V.; LaLonde, F. Influence Diagram of Physiological and Environmental Factors Affecting Heart Rate Variability: An Extended Literature Overview. Hear. Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef] [PubMed]
- Scheck, S.M.; Pannek, K.; Raffelt, D.; Fiori, S.; Boyd, R.N.; Rose, S. Structural connectivity of the anterior cingulate in children with unilateral cerebral palsy due to white matter lesions. NeuroImage Clin. 2015, 9, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Carnevali, L.; Koenig, J.; Sgoifo, A.; Ottaviani, C. Autonomic and Brain Morphological Predictors of Stress Resilience. Front. Mol. Neurosci. 2018, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Koenig, J. Resting Cerebral Blood Flow and Ethnic Differences in Heart Rate Variability: Links to Self-Reports of Affect and Affect Regulation. NeuroImage 2019, 202, 116154. [Google Scholar] [CrossRef]
- Chang, C.; Metzger, C.D.; Glover, G.H.; Duyn, J.H.; Heinze, H.-J.; Walter, M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage 2012, 68, 93–104. [Google Scholar] [CrossRef]
- Pannek, K.; Boyd, R.N.; Fiori, S.; Guzzetta, A.; Rose, S. Assessment of the structural brain network reveals altered connectivity in children with unilateral cerebral palsy due to periventricular white matter lesions. NeuroImage: Clin. 2014, 5, 84–92. [Google Scholar] [CrossRef]
- Veijalainen, A.; Haapala, E.A.; Väistö, J.; Leppänen, M.H.; Lintu, N.; Tompuri, T.; Seppälä, S.; Ekelund, U.; Tarvainen, M.P.; Westgate, K.; et al. Associations of physical activity, sedentary time, and cardiorespiratory fitness with heart rate variability in 6- to 9-year-old children: The PANIC study. Eur. J. Appl. Physiol. 2019, 119, 2487–2498. [Google Scholar] [CrossRef]
- Verschuren, O.; Peterson, M.D.; Balemans, A.C.; Hurvitz, E.A. Exercise and physical activity recommendations for people with cerebral palsy. Dev. Med. Child Neurol. 2016, 58, 798–808. [Google Scholar] [CrossRef]
- Maltais, D.B.; Pritchard-Wiart, L.; Fowler, E.; Verschuren, O.; Damiano, D.L. Health-related physical fitness for children with cerebral palsy. J. Child Neurol. 2014, 29, 1091–1100. [Google Scholar] [CrossRef]
- Keawutan, P.; Bell, K.; Oftedal, S.; Ware, R.S.; Stevenson, R.D.; Davies, P.S.W.; Boyd, R.N. Longitudinal physical activity and sedentary behaviour in preschool-aged children with cerebral palsy across all functional levels. Dev. Med. Child Neurol. 2017, 59, 852–857. [Google Scholar] [CrossRef]
- Vila, X.A.; Lado, M.J.; Cuesta-Morales, P. Evidence Based Recommendations for Designing Heart Rate Variability Studies. J. Med. Syst. 2019, 43, 311. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S. Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiol. 2016, 54, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Lachenbruch, P.A.; Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; American Statistical Association: Alexandria, VA, USA, 1988. [Google Scholar]
- Jeyhani, V.; Mahdiani, S.; Peltokangas, M.; Vehkaoja, A. Comparison of HRV parameters derived from photoplethysmography and electrocardiography signals. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milano, Italy, 25–29 August 2015; Volume 2015, pp. 5952–5955. [Google Scholar]
- Weippert, M.; Kumar, M.; Kreuzfeld, S.; Arndt, D.; Rieger, A.; Stoll, R. Comparison of three mobile devices for measuring R–R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur. J. Appl. Physiol. 2010, 109, 779–786. [Google Scholar] [CrossRef]
- Vasconcellos, F.V.; Seabra, A.; Cunha, F.; Montenegro, R.A.; Bouskela, E.; Farinatti, P.T.V. Heart rate variability assessment with fingertip photoplethysmography and polar RS800cx as compared with electrocardiography in obese adolescents. Blood Press. Monit. 2015, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, N.; Couceiro, R.; Henriques, J.; Muehlsteff, J.; Quintal, I.; Gonçalves, L.; Carvalho, P. Can PPG be used for HRV analysis? In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 2945–2949. [Google Scholar]
- Gilgen-Ammann, R.; Schweizer, T.; Wyss, T. RR interval signal quality of a heart rate monitor and an ECG Holter at rest and during exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 119, 1525–1532. [Google Scholar] [CrossRef]
- Williams, D.P.; Jarczok, M.N.; Ellis, R.J.; Hillecke, T.K.; Thayer, J.F.; Koenig, J. Two-week test-retest reliability of the Polar ® RS800CX ™ to record heart rate variability. Clin. Physiol. Funct. Imaging 2016, 37, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Heathers, J.; Kemp, A.H. On the validity of using the Polar RS800 heart rate monitor for heart rate variability research. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 112, 4179–4180. [Google Scholar] [CrossRef]
- Merri, M.; Farden, D.C.; Mottley, J.G.; Titlebaum, E.L. Sampling frequency of the electrocardiogram for spectral analysis of the heart rate variability. IEEE Trans. Biomed. Eng. 1990, 37, 99–106. [Google Scholar] [CrossRef]
- Patel, K.; Rössler, A.; Lackner, H.K.; Trozic, I.; Laing, C.; Lorr, D.; Green, D.A.; Hinghofer-Szalkay, H.; Goswami, N. Effect of postural changes on cardiovascular parameters across gender. Medicine 2016, 95, e4149. [Google Scholar] [CrossRef]
- Krejčí, J.; Botek, M.; Mc Kune, A. Stabilization period before capturing an ultra-short vagal index can be shortened to 60 s in endurance athletes and to 90 s in university students. PLoS ONE 2018, 13, e0205115. [Google Scholar] [CrossRef]
- Hirsch, J.A.; Bishop, B. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. Am. J. Physiol. Heart Circ. Physiol. 1981, 241, H620–H629. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.E.; Beightol, L.A.; Koh, J.; Eckberg, D.L. Important influence of respiration on human R-R interval power spectra is largely ignored. J. Appl. Physiol. 1993, 75, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.; Thompson, M.; Stevens, R.; Heneghan, C.; Plüddemann, A.; Maconochie, I.; Tarassenko, L.; Mant, D. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: A systematic review of observational studies. Lancet 2011, 377, 1011–1018. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Sacha, J.; Jeleń, P.; Zielinski, J.; Przybylski, J. Heart Rate and Respiratory Rate Influence on Heart Rate Variability Repeatability: Effects of the Correction for the Prevailing Heart Rate. Front. Physiol. 2016, 7, 307. [Google Scholar] [CrossRef]
- Sinnecker, D.; Dommasch, M.; Barthel, P.; Müller, A.; Dirschinger, R.J.; Hapfelmeier, A.; Huster, K.M.; Laugwitz, K.-L.; Malik, M.; Schmidt, G. Assessment of mean respiratory rate from ECG recordings for risk stratification after myocardial infarction. J. Electrocardiol. 2014, 47, 700–704. [Google Scholar] [CrossRef]
- Nielsen, L.G.; Folkestad, L.; Brodersen, J.B.; Brabrand, M. Inter-Observer Agreement in Measuring Respiratory Rate. PLoS ONE 2015, 10, e0129493. [Google Scholar] [CrossRef]
- Sandercock, G.R.; Gladwell, V.; Dawson, S.; Nunan, D.; Brodie, D.; Beneke, R. Association between RR interval and high-frequency heart rate variability acquired during short-term, resting recordings with free and paced breathing. Physiol. Meas. 2008, 29, 795–802. [Google Scholar] [CrossRef]
- Kobayashi, H. Does Paced Breathing Improve the Reproducibility of Heart Rate Variability Measurements? J. Physiol. Anthr. 2009, 28, 225–230. [Google Scholar] [CrossRef][Green Version]
- Frederiks, J.; Swenne, C.A.; TenVoorde, B.J.; Honzikova, N.; Levert, J.V.; Maan, A.C.; Schalij, M.J.; Bruschke, A.V. The importance of high-frequency paced breathing in spectral baroreflex sensitivity assessment. J. Hypertens. 2000, 18, 1635–1644. [Google Scholar] [CrossRef]
- Faes, L.; Nollo, G.; Porta, A. Information Domain Approach to the Investigation of Cardio-Vascular, Cardio-Pulmonary, and Vasculo-Pulmonary Causal Couplings. Front. Physiol. 2011, 2, 80. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Kuo, T.B.J.; Lai, C.-T.; Chu, J.-W.; Yang, C.C.H. Effects of respiratory time ratio on heart rate variability and spontaneous baroreflex sensitivity. J. Appl. Physiol. 2013, 115, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Combatalade, D.C. Don’t Add or Miss a Beat: A Guide to Cleaner Heart Rate Variability Recordings. Biofeedback 2013, 41, 121–130. [Google Scholar] [CrossRef]
- Soler, A.I.R.; Silva, L.E.V.; Fazan, R.; Murta, L.O.; Junior, L.O.M. The impact of artifact correction methods of RR series on heart rate variability parameters. J. Appl. Physiol. 2018, 124, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.; Tarassenko, L. Quantifying Errors in Spectral Estimates of HRV Due to Beat Replacement and Resampling. IEEE Trans. Biomed. Eng. 2005, 52, 630–638. [Google Scholar] [CrossRef]
- Jarrin, D.C.; McGrath, J.J.; Giovanniello, S.; Poirier, P.; Lambert, M. Measurement fidelity of heart rate variability signal processing: The devil is in the details. Int. J. Psychophysiol. 2012, 86, 88–97. [Google Scholar] [CrossRef]
- Peng, C.-K.; Havlin, S.; Stanley, H.E.; Goldberger, A.L. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos 1995, 5, 82–87. [Google Scholar] [CrossRef]
- Porta, A.; Guzzetti, S.; Montano, N.; Furlan, R.; Pagani, M.; Malliani, A.; Cerutti, S. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans. Biomed. Eng. 2001, 48, 1282–1291. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef]
- Heathers, J. The last word. Exp. Physiol. 2013, 98, 348. [Google Scholar] [CrossRef]
- Pichon, A.; Roulaud, M.; Antoine-Jonville, S.; De Bisschop, C.; Denjean, A. Spectral analysis of heart rate variability: Interchangeability between autoregressive analysis and fast Fourier transform. J. Electrocardiol. 2006, 39, 31–37. [Google Scholar] [CrossRef]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med Boil. Eng. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Perkiömäki, J.S.; Maestri, R.; Pinna, G.D. Clinical impact of evaluation of cardiovascular control by novel methods of heart rate dynamics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Sassi, R.; Cerutti, S.; Lombardi, F.; Malik, M.; Huikuri, H.V.; Peng, C.-K.; Schmidt, G.; Yamamoto, Y.; Gorenek, B.; Lip, G.Y.; et al. Advances in heart rate variability signal analysis: Joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Schulz, S.; Schroeder, R.; Baumert, M.; Caminal, P. Methods derived from nonlinear dynamics for analysing heart rate variability. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 367, 277–296. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Sacha, J.; Jeleń, P.; Pawłowski, M.; Werner, B.; Dąbrowski, M.J. Interaction Between Heart Rate Variability and Heart Rate in Pediatric Population. Front. Physiol. 2015, 6, 475. [Google Scholar] [CrossRef]
- Gąsior, J.S.; Sacha, J.; Pawłowski, M.; Zieliński, J.; Jeleń, P.; Tomik, A.; Ksiazczyk, T.; Werner, B.; Dąbrowski, M.J. Normative Values for Heart Rate Variability Parameters in School-Aged Children: Simple Approach Considering Differences in Average Heart Rate. Front. Physiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Sacha, J.; Grzeszczak, W. Left ventricular mass index determines variability of the sinus rhythm in essential hypertension. New insight into heart period fluctuations via corrected spectral analysis. Folia Cardiol. 2001, 8, 487–497. [Google Scholar]
- Sacha, J.; Pluta, W. Different methods of heart rate variability analysis reveal different correlations of heart rate variability spectrum with average heart rate. J. Electrocardiol. 2005, 38, 47–53. [Google Scholar] [CrossRef]
- Sacha, J.; Pluta, W. Alterations of an average heart rate change heart rate variability due to mathematical reasons. Int. J. Cardiol. 2008, 128, 444–447. [Google Scholar] [CrossRef]
- Sacha, J.; Barabach, S.; Statkiewicz-Barabach, G.; Sacha, K.; Müller, A.; Piskorski, J.; Barthel, P.; Schmidt, G. How to select patients who will not benefit from ICD therapy by using heart rate and its variability? Int. J. Cardiol. 2013, 168, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Barabach, S.; Statkiewicz-Barabach, G.; Sacha, K.; Müller, A.; Piskorski, J.; Barthel, P.; Schmidt, G. How to strengthen or weaken the HRV dependence on heart rate — Description of the method and its perspectives. Int. J. Cardiol. 2013, 168, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Sobon, J.; Sacha, K.; Barabach, S. Heart rate impact on the reproducibility of heart rate variability analysis. Int. J. Cardiol. 2013, 168, 4257–4259. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Barabach, S.; Statkiewicz-Barabach, G.; Sacha, K.; Müller, A.; Piskorski, J.; Barthel, P.; Schmidt, G. Gender differences in the interaction between heart rate and its variability — How to use it to improve the prognostic power of heart rate variability. Int. J. Cardiol. 2014, 171, e42–e45. [Google Scholar] [CrossRef]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.-B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisløff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical Characterization of the Underappreciated and Important Relationship Between Heart Rate Variability and Heart Rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef]
- Silva, L.E.V.; Salgado, H.C.; Fazan, R. Mean Heart Rate Level Does Not Affect All Heart Rate Variability Indices. Hypertens. 2017, 69, e21–e22. [Google Scholar] [CrossRef]
- Huikuri, H.V.; Mäkikallio, T.; Airaksinen, J.; Mitrani, R.; Castellanos, A.; Myerburg, R.J. Measurement of heart rate variability: A clinical tool or a research toy? J. Am. Coll. Cardiol. 1999, 34, 1878–1883. [Google Scholar] [CrossRef]
- Eckberg, D.L. Sympathovagal Balance. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef]
- Moak, J.P.; Goldstein, D.S.; Eldadah, B.A.; Saleem, A.; Holmes, C.; Pechnik, S.; Sharabi, Y. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Hear. Rhythm. 2007, 4, 1523–1529. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Bentho, O.; Park, M.-Y.; Sharabi, Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 2011, 96, 1255–1261. [Google Scholar] [CrossRef]
- Rahman, F.; Pechnik, S.; Gross, D.J.; Sewell, L.; Goldstein, D.S. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Heathers, J. Sympathovagal balance from heart rate variability: An obituary. Exp. Physiol. 2012, 97, 556. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Del Paso, G.A.R.; Langewitz, W.; Mulder, L.J.M.; Van Roon, A.; Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Dan, B. Understanding the autonomic nervous system in cerebral palsy. Dev. Med. Child Neurol. 2017, 59, 668. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Ahmet, I.; Liu, J.; Lyashkov, A.E.; Guiriba, T.-R.; Okamoto, Y.; Ziman, B.D.; Lakatta, E.G. Synchronization of sinoatrial node pacemaker cell clocks and its autonomic modulation impart complexity to heart beating intervals. Hear. Rhythm. 2014, 11, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.D.; Davis, R.B.; Goldberger, A.L. Heart Rate Fragmentation: A Symbolic Dynamical Approach. Front. Physiol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.D.; Davis, R.B.; Goldberger, A.L. Heart Rate Fragmentation: A New Approach to the Analysis of Cardiac Interbeat Interval Dynamics. Front. Physiol. 2017, 8, 255. [Google Scholar] [CrossRef]
| First Author and Year of Publication | Experimental Group | Control Group | RR Intervals Acquisition | ||||
|---|---|---|---|---|---|---|---|
| Software for RR Intervals Acquisition, Sampling Frequency, and Duration of Recordings | Time of the Day and Room (Lights/Voices/Temperature) | Activities (Sleep Routine, Physical Activities, Meals, Drinks, Using the Toilet before Recordings) and Instructions Given. Time Reported for Rest or Heart Rate Stabilization before Recordings | Respiratory Rate (Breathing Control) during Recordings | Position during Recordings | |||
| Park et al., 2002 [2] | 12 children with CP (7♂). Age: 6–11 years; 5: quadriplegia, 4: diplegia, 3: hemiplegia | 12 normally developed children (7♂). Age: 5–12 years | Software: software developed by the authors Sampling: 1000 Hz Duration: 3 min | Measurements were carried out at about 3:00 pm in a quiet room at room temperature 20–24 °C. | Subjects had a very light lunch. 10 min | Subjects were instructed to breathe with a metronome at 15 breaths/min (0.25 Hz). | Supine, 70° head-up tilt using a tilt table |
| Yang et al., 2002 [3] | 30 children with CP (18♂). Age: 4–10 years; 7: quadriplegia, 23: diplegia | 30 age- and sex-matched normally developed children | Software: software developed by one of the authors Sampling: 256 Hz Duration: 288 s | Not reported | Not reported 15 min | Not reported | Supine, head-up tilt (angle not specified) |
| Ferreira et al., 2011 [7] | 90 children with CP (58♂). Age: 3–15 years; 31: quadriplegia, 31: diplegia, 6: hemiplegia | 35 individuals matched by age | Software: Electrocardiography (ECG) Holter monitoring (SEER Light, GE Medical Systems, Milwaukee, WI, USA) Sampling: 250 Hz Duration: 24 h | 24 h monitoring | Not reported Not reported | Not reported | Not applicable |
| Zamunér et al., 2011 [5] | 12 children with CP (7♂). Age: 4–13 years; 4: quadriplegia, 6: diplegia, 2: hemiplegia | 16 children with typical motor development (5♂) | Software: Nerve–Express system software (Heart Rhythm Instruments, Inc., Metuchen, NJ, EUA) Sampling: not reported Duration: 15 min | Not reported | The children and their parents were given instructions to avoid consumption of stimulating beverages, to suspend any major physical activity, to have light meals, and to have a good night’s rest. All children were familiarized with the experimental proceedings during a pilot test conducted a week prior to the study procedures. The children were asked not to talk or to move during data collection. Not reported | The children maintained spontaneous breathing, presenting 10 to 20 breaths per minute. | Supine, standing |
| Kholod et al., 2013 [8] | 26 children with CP (12♂). Age: 8–14 years; 13: quadriplegia, 9: diplegia, 4: hemiplegia, 2: athetoid signs. GMFCS I-V | 16 typically developed children (6♂) matched for age | Software: 12-lead digital ECG Holter recorder (DR180 Digital Recorder; NorthEast Monitoring Inc. Maynard, Mass) Sampling: not reported Duration: lack of precise description. The ECG was continuously monitored throughout the test procedure. | All procedures were performed in a quiet room, with the temperature between 21–26°C. | Before data collection, each subject was familiarized with the study protocol. Every attempt was made to control external factors: similar assessment time, restriction of activity, and/or heavy meal prior to the Holter recording. Lack of precise description | Not reported | Supine, during walking |
| Israeli-Mendlovic et al., 2014 [9] | 30 children with CP (17♂). Age: 6–12 years; 25: quadriplegia, 5: dyskinesia. GMFCS IV-V | No control group | Software: Polar Advanced Heart Rate Monitor (RC800CX) Sampling: not reported Duration: supine—10 min, GMFM assessment, rest—5 min, highest activity achieved in the GMFM assessment performed over and over again for 2 min, standing—10 min | All procedures were performed in a quiet room, with the temperature between 21–26 °C. | Before data collection, each subject was familiarized with the study protocol. Lack of precise description | Not reported | Supine, during activities (GMFM assessment), standing |
| Amichai et al., 2017 [11] | 20 children with CP (12♂). Age: 6–11 years; 12: diplegia, 8: hemiplegia. GMFCS I-III | No control group | Software: Polar Advanced Heart Rate Monitor (RC800CX) Sampling: Not reported. Duration: lack of precise description. No information whether 5 min were dedicated for a rest or RR interval recording. | Not reported | The children were asked to sit quietly at rest for 5 min and then to walk on the treadmill. Not reported | Not reported | Sitting, walking |
| Cohen-Holzer et al., 2017 [13] | 24 children with unilateral CP (16♂). Age: 6–10 years; GMFCS I-II | No control group | Software: Polar Advanced Heart Rate Monitor (RS800CX) Sampling: not reported Duration: not reported | Not reported | Not reported Not reported | Not reported | Not reported |
| Kim et al., 2017 [56] | 13 children with CP (8♂) considered as control group. Mean age: 7.5 years (1.9–16.0); GMFCS I-III | CP children were considered the control group for the children with acute brain injury | Software: not reported Sampling: not reported Duration: 5 min | Noise-free environment. Data were collected between 1:00 and 3:00 PM. The room temperature during data collection was 24–26 °C. | Not reported 30 min | Not reported | Supine |
| Amichai et al., 2019 [12] | 20 children with CP (15♂). Age: 6–11 years; 11: diplegia, 7: hemiplegia, 2: quadriplegia. GMFCS I–III | 20 typically developed children (14♂) matched for age and gender | Software: Polar Advanced Heart Rate Monitor (RS800CX) Sampling: not reported Duration: HRV data were recorded throughout the entire session. | Not reported | Not reported Not reported | The children were asked to lie down quietly on the back for 5 min, then to sit quietly in a resting state for 5 min, followed by a paced breathing training (15 min). Paced breathing and breathing rate were evaluated using the ProRelax software (ver. 5.1) and a chest belt | Supine (HRV), sitting (HRV and breathing manipulation) |
| Katz-Leurer et al., 2019 [14] | 110 children with CP (66♂). Age: 6–11 years; GMFCS I-V | 35 typically developed children matched for age | Software: Polar Advanced Heart Rate Monitor RC800CX Sampling: not reported Duration: 10 min | Testing was performed in the morning hours, in a quiet room with the temperature between 21–26 °C. | The children were asked not to consume a heavy meal, drink caffeinated beverages, or perform physical activities for at least 2 h before testing. Not reported | Not reported | Not reported |
| Landis et al., 2019 [54] | 10 children with CP (4♂). Mean age: 15.5 ± 3.6 years; 4: diplegia, 6: hemiplegia. GMFCS II-III | No control group | Software: Heart rate monitor (name of the software not reported) Sampling: 250 Hz Duration: 5 min; conditioning phase (10 min) divided into two 5 min phases | Not reported | Not reported 5 min | Not reported | Sitting |
| First Author and Year of Publication | Software | Artifact Correction | Time Series Length (Time/Beats) | Information about Data Normality | Time Domain Parameters (Units) | Frequency Domain Parameters and Bands (Units) | Frequency Analysis Method with Details | Nonlinear Parameters |
|---|---|---|---|---|---|---|---|---|
| Park et al., 2002 [2] | Software developed by the authors. | Modified spatial velocity algorithm to detect QRS peaks. The signals were passed through a band pass filter of 0.1–150 Hz to eliminate unwanted noise signals. | Not reported | Not mentioned | Did not perform time domain analysis. | LF: 0.05–0.15 Hz (ms2, nu) HF: 0.15–0.40 Hz (ms2, nu) TP (ms2) LF/HF | Cubic spline interpolation method. Autoregressive model using the Burg’s maximum entropy method. | Did not perform nonlinear analysis. |
| Yang et al., 2002 [3] | Software developed by one of the authors | For the RR interval rejection procedure, a temporary mean and the standard deviation of all RR intervals were first calculated as the standard reference. Each RR interval was then validated with respect to this reference. If the standard score of an RR value exceeded 3, it was considered erroneous or non-stationary and was thus rejected. The valid RR values were then resampled and interpolated at the rate of 7.11 Hz to accomplish continuity in the time domain. | 288 s/2048 data points | Not mentioned | Did not perform time domain analysis. | LF: 0.04–0.15 Hz (nu) HF: 0.15–0.40 Hz (nu) | Fast Fourier transformation. Resulting power spectrum was corrected for attenuation resulting from the sampling process and the Hamming window. | Did not perform nonlinear analysis. |
| Ferreira et al., 2011 [7] | Not reported | Data were processed and analyzed using a 250 Hz sampling frequency (GE MARS 7.1 equipment with MARS 7.1; GE Medical System software). | Normal RR intervals over a period of at least 18 h of the analyzable signal were analyzed. | Not mentioned | SDNN (ms) pNN50 (%) | VLF: 0.003–0.04 Hz (ms2) LF: 0.04–0.15 Hz (ms2) HF: 0.15–0.40 Hz (ms2) TP (ms2) LF/HF | Not reported | Did not perform nonlinear analysis. |
| Zamunér et al., 2011 [5] | Nerve–Express system software | Not reported | 5 min | Data distribution was tested using the Shapiro–Wilk test, and the normality hypothesis of all variables was rejected. | Did not perform time domain analysis. | LF: 0.04–0.15 Hz (nu) HF: 0.15–0.40 Hz (nu) | Authors reported to select the highest stability section RR intervals and to perform an autoregressive spectral analysis. | Did not perform nonlinear analysis. |
| Kholod et al., 2013 [8] | NorthEast Monitoring’s Holter LX Enhanced Plus Software (version 5.2 Beta) | RR intervals were visually inspected and then filtered with the HRV software to eliminate undesirable noise or premature beats. | Not reported | Normality distribution checked (method not specified). | SDNN (ms) RMSSD (ms) | Did not perform frequency domain analysis. | Not applicable | Did not perform nonlinear analysis. |
| Israeli-Mendlovic et al., 2014 [9] | Not reported | Beat intervals were visually inspected and then filtered with the HRV software to eliminate undesirable noise. | Not reported | Not mentioned | SDNN (ms) RMSSD (ms) | LF: 0.04–0.15 Hz (nu) HF: 0.15–0.40 Hz (nu) LF/HF | Not reported | Did not perform nonlinear analysis. |
| Amichai et al., 2017 [11] | Not reported | The interbeat intervals were visually inspected and filtered with the HRV software to eliminate noise. | Not reported | Not mentioned | SDNN [ms] RMSSD (ms) | LF/HF | Not reported | SD1 (ms) SD2 (ms) |
| Cohen-Holzer et al., 2017 [13] | Kubios heart rate variability software version 2.0; Biosignal Analysis and Medical Imaging Group | Not reported | Not reported | Not mentioned | SDNN [ms] RMSSD (ms) | Did not perform frequency domain analysis. | Not applicable | Did not perform nonlinear analysis. |
| Kim et al., 2017 [56] | SA-6000 device (Medicore Co., Seoul, Korea) | Abnormal beats, significant pauses, and areas of artifact were automatically rejected by using a computerized algorithm. | 5 min | Not mentioned | SDNN [ms] RMSSD (ms) | LF: 0.04–0.15 Hz (ms2, nu) HF: 0.15–0.40 Hz (ms2, nu) TP (ms2) LF/HF | Fast Fourier transform | ApEn |
| Amichai et al., 2019 [12] | Not reported | The interbeat intervals were visually inspected and then filtered using the HRV software to eliminate undesirable noise. | 5 min | The Kolmogorov–Smirnov test was performed for all outcome measures. | SDNN [ms] RMSSD (ms) | LF: 0.04–0.15 Hz (ms2) HF: 0.15–0.40 Hz (ms2) LF/HF | Fast Fourier transform | Did not perform nonlinear analysis. |
| Katz-Leurer et al., 2019 [14] | Not reported | Not reported | Not reported | Not mentioned | mRR (ms) SDNN (ms) RMSSD (ms) | LF/HF | Not reported | Did not perform nonlinear analysis. |
| Landis et al., 2019 [54] | Not reported | The aim of this study was to generate a method for calculating HRV from ECG waveforms. Preliminary R peak detection and peak correction described with details. | 5 min | Not mentioned | avNN (s) RMSSD (ms) SDNN (ms) NN50 (count) pNN50 (%) | LF (RR)* HF (RR) LF/HF (RR) LF/HF (ECG)** Units not reported | Fast Fourier transform | Did not perform nonlinear analysis. |
| First Author and Year of Publication | Main Results and Conclusion Related to HRV |
|---|---|
| Park et al., 2002 [2] | Main results
Vagal withdrawal and sympathetic activation, which occur during a head-up tilt position, are not sufficient to overcome the orthostatic stress arising in children with spastic CP. |
| Yang et al., 2002 [3] | Main results
The disturbed balance of activity between the sympathetic and parasympathetic nervous system observed in the study might result from the loss of hemispheric influence in patients with CP; however, further investigation is clearly necessary. |
| Ferreira et al., 2011 [7] | Main results The group of children with CP presented higher HF and LF values and lower LF/HF compared to the controls. Conclusions Individuals with CP present an increased cardiovascular risk, a disturbed sympathovagal balance that could contribute to the salivary secretion alterations observed. |
| Zamunér et al., 2011 [5] | Main results
Children with CP present lower HRV indices, indicating sympathovagal imbalance. The decrease of HRV in children with CP is related to the motor impairment level. |
| Kholod et al., 2013 [8] | Main results
Among children with CP, the cardiac autonomic mechanism is less efficient at rest and less adaptive to exercise and activity as compared to typically developed children. |
| Israeli-Mendlovic et al., 2014 [9] | Main results
HR autonomic regulation system has an opportunity to be influenced by training in children with CP GMFCS IV. |
| Amichai et al., 2017 [11] | Main results
Further studies are needed to assess the possible influence of exercise protocols on the cardiac autonomic system. |
| Cohen-Holzer et al., 2017 [13] | Main results Significant reduction in HR and an increase in RMSSD 3 months post-intervention.Conclusions An intensive hybrid program (10 days, 6 h per day) effectively improved the cardiac autonomic regulation system. |
| Kim et al., 2017 [56] | Main results
Patients with acute brain injury have higher sympathetic excitatory activity and more dominant sympathetic power than the parasympathetic power compared to the control group. Presence of paroxysmal sympathetic hyperactivity symptoms were noted among the children with acute brain injury compared to the age- and sex-matched control group of children with CP. |
| Amichai et al., 2019 [12] | Main results
Children with CP have the ability to perform paced breathing training in order to influence their respiratory rate. HRV parameters increase during a paced breathing practice in children with CP, showing an impact on the cardiac autonomic control system. |
| Katz-Leurer et al., 2019 [14] | Main results
Assessing appropriate protocols for improving autonomic regulation in children with CP is the next step needed. |
| Landis et al., 2019 [54] | Main results The authors presented an active video game data collection protocol and a methodology to calculate HRV from the ECG data obtained via an HR monitor. Conclusions The proposed methodology allows to extract RR intervals and HRV measures from ECG waveforms during gaming physical activities in youths with CP. The method is currently tailored towards active video game sessions in a specific game, but could easily be adapted to other protocols and ECG devices for future experiments. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsior, J.S.; Zamunér, A.R.; Silva, L.E.V.; Williams, C.A.; Baranowski, R.; Sacha, J.; Machura, P.; Kochman, W.; Werner, B. Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review. J. Clin. Med. 2020, 9, 1141. https://doi.org/10.3390/jcm9041141
Gąsior JS, Zamunér AR, Silva LEV, Williams CA, Baranowski R, Sacha J, Machura P, Kochman W, Werner B. Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review. Journal of Clinical Medicine. 2020; 9(4):1141. https://doi.org/10.3390/jcm9041141
Chicago/Turabian StyleGąsior, Jakub S., Antonio Roberto Zamunér, Luiz Eduardo Virgilio Silva, Craig A. Williams, Rafał Baranowski, Jerzy Sacha, Paulina Machura, Wacław Kochman, and Bożena Werner. 2020. "Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review" Journal of Clinical Medicine 9, no. 4: 1141. https://doi.org/10.3390/jcm9041141
APA StyleGąsior, J. S., Zamunér, A. R., Silva, L. E. V., Williams, C. A., Baranowski, R., Sacha, J., Machura, P., Kochman, W., & Werner, B. (2020). Heart Rate Variability in Children and Adolescents with Cerebral Palsy—A Systematic Literature Review. Journal of Clinical Medicine, 9(4), 1141. https://doi.org/10.3390/jcm9041141









