Exploring Triaging and Short-Term Outcomes of Early Invasive Strategy in Non-ST Segment Elevation Acute Coronary Syndrome: A Report from Japanese Multicenter Registry
Abstract
1. Introduction
2. Methods
2.1. Data Sources
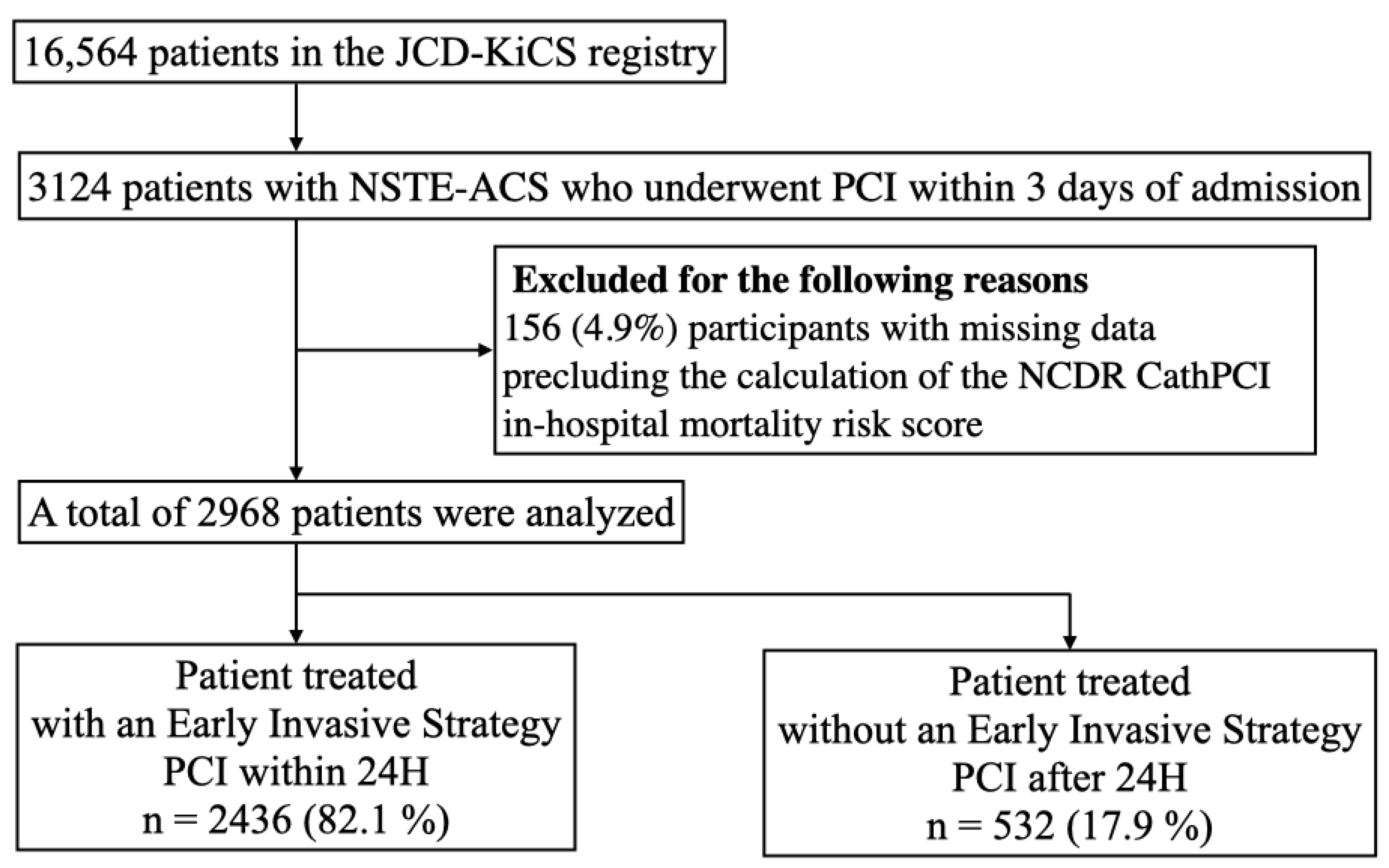
2.2. Study Population and Risk Stratification
2.3. Measured Outcomes
2.4. Statistical Analysis
3. Results
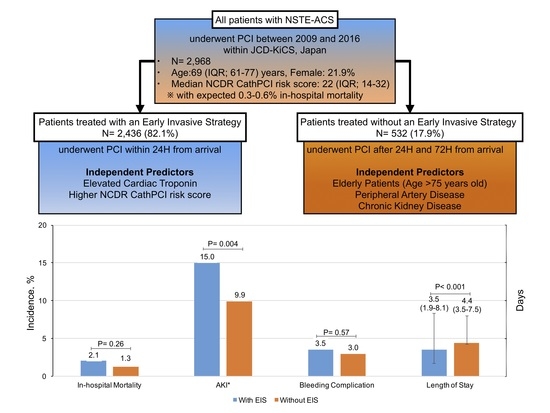
3.1. Baseline Characteristics of Patients Treated with and without EIS
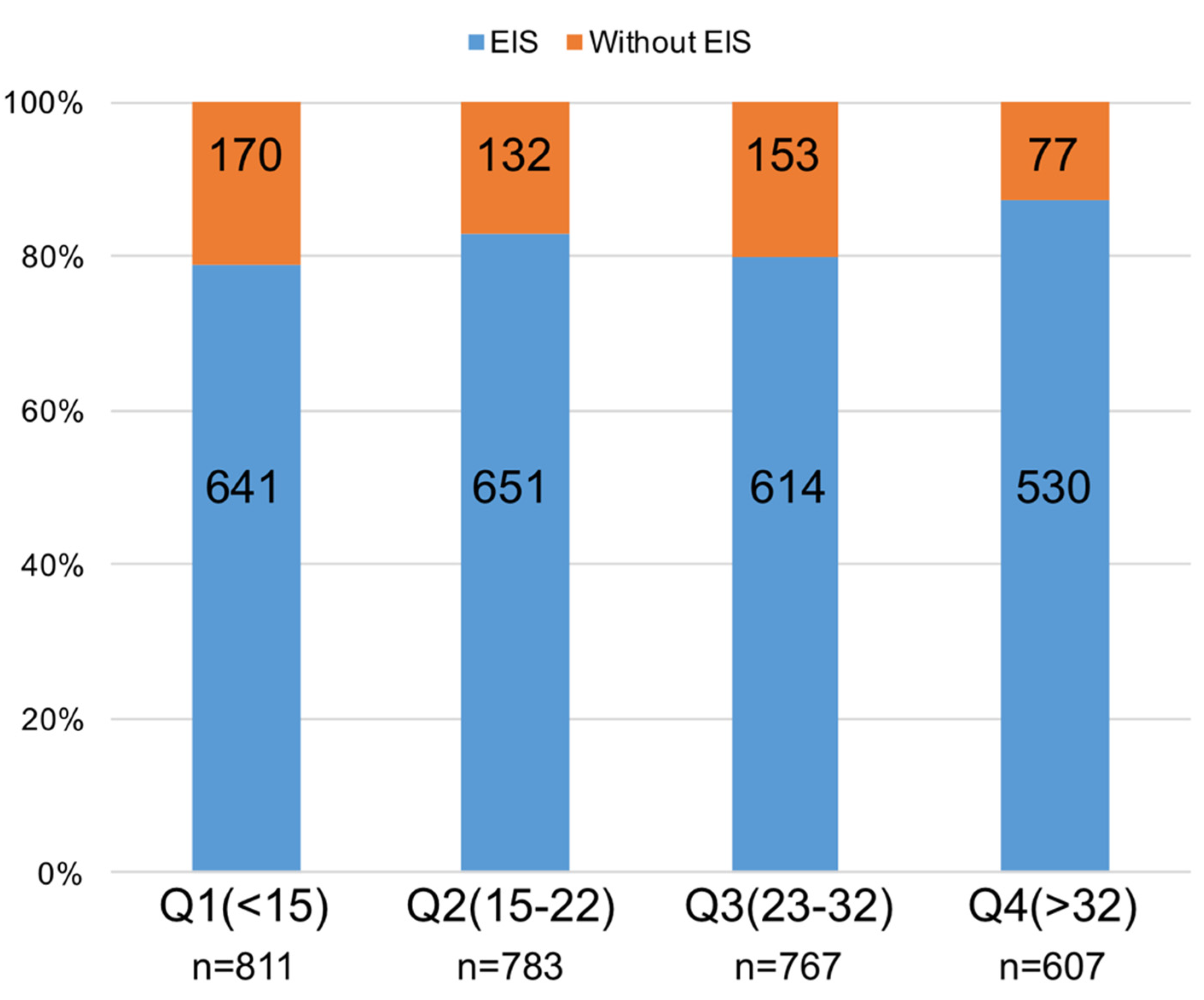
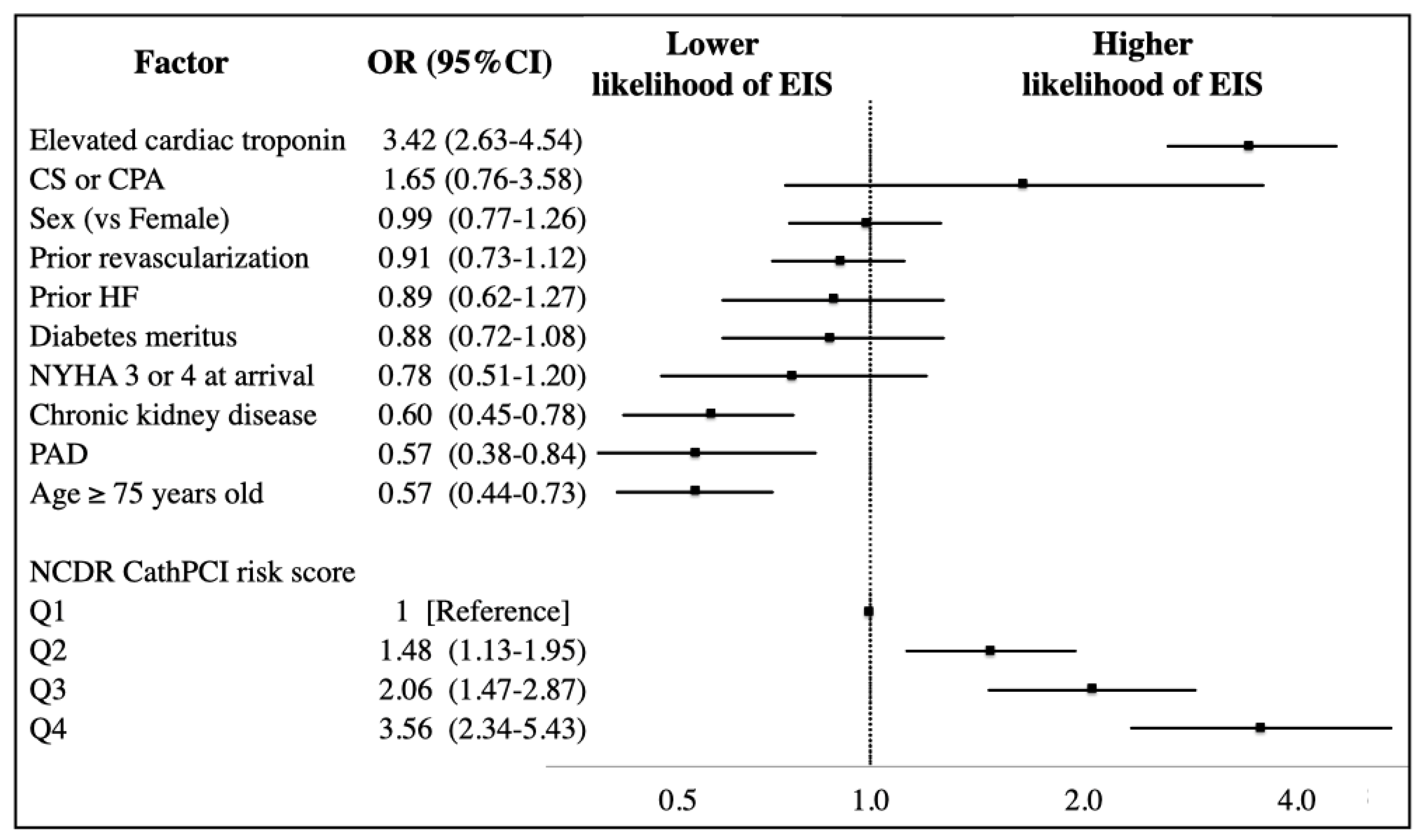
3.2. Predictors of EIS Utilization
3.3. In-Hospital Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiviott, S.D.; Morrow, D.A.; Frederick, P.D.; Antman, E.M.; Braunwald, E.; National Registry of Myocardial Infarction. Application of the Thrombolysis in Myocardial Infarction risk index in non-ST-segment elevation myocardial infarction: Evaluation of patients in the National Registry of Myocardial Infarction. J. Am. Coll. Cardiol. 2006, 47, 1553–1558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peterson, E.D.; Dai, D.; DeLong, E.R.; Brennan, J.M.; Singh, M.; Rao, S.V.; Shaw, R.E.; Roe, M.T.; Ho, K.K.; Klein, L.W.; et al. Contemporary mortality risk prediction for percutaneous coronary intervention: Results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2010, 55, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Roe, M.T.; Peterson, E.D.; Li, Y.; Chen, A.Y.; Harrington, R.A.; Greenbaum, A.B.; Berger, P.B.; Cannon, C.P.; Cohen, D.J.; et al. Utilization of early invasive management strategies for high-risk patients with non-ST-segment elevation acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. JAMA 2004, 292, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, N.; Sawano, M.; Ueda, I.; Fukuda, K.; Kohsaka, S. Consequence of reimbursement policy alteration for urgent PCI in Japan. Lancet 2018, 391, 2208–2209. [Google Scholar] [CrossRef]
- Desai, N.R.; Bradley, S.M.; Parzynski, C.S.; Nallamothu, B.K.; Chan, P.S.; Spertus, J.A.; Patel, M.R.; Ader, J.; Soufer, A.; Krumholz, H.M.; et al. Appropriate Use Criteria for Coronary Revascularization and Trends in Utilization, Patient Selection, and Appropriateness of Percutaneous Coronary Intervention. JAMA 2018, 314, 2045–2053. [Google Scholar] [CrossRef]
- Gruberg, L.; Mintz, G.S.; Mehran, R.; Dangas, G.; Lansky, A.J.; Kent, K.M.; Pichard, A.D.; Satler, L.F.; Leon, M.B. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J. Am. Coll. Cardiol. 2000, 36, 1542–1548. [Google Scholar] [CrossRef]
- Kohsaka, S.; Miyata, H.; Ueda, I.; Masoudi, F.A.; Peterson, E.D.; Maekawa, Y.; Kawamura, A.; Fukuda, K.; Roe, M.T.; Rumsfeld, J.S.; et al. An international comparison of patients undergoing percutaneous coronary intervention: A collaborative study of the National Cardiovascular Data Registry (NCDR) and Japan Cardiovascular Database-Keio interhospital Cardiovascular Studies (JCD-KiCS). Am. Heart J. 2015, 170, 1077–1085. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef]
- Katsuki, T.; Sawano, M.; Ueda, I.; Ikemura, N.; Shimoji, K.; Noma, S.; Suzuki, M.; Numasawa, Y.; Hayashida, K.; Yuasa, S.; et al. C-Reactive Protein in Non-St Elevation Myocardial Infarction Patients Is Useful in Improving Discrimination of Conventional Risk Score: A Report from Multicenter Pci Registry. J. Am. Coll. Cardiol. 2017, 69, 294. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Kohsaka, S.; Miyata, H.; Ueda, I.; Maekawa, Y.; Fukuda, K.; Cohen, D.J.; Kennedy, K.F.; Rumsfeld, J.S.; Spertus, J.A. Performance and Validation of the U.S. NCDR Acute Kidney Injury Prediction Model in Japan. J. Am. Coll. Cardiol. 2016, 67, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Sandberg, K.R. Epidemiology of contrast-induced nephropathy. Rev. Cardiovasc. Med. 2003, 4 (Suppl. 5), S3–S9. [Google Scholar]
- Slocum, N.K.; Grossman, P.M.; Moscucci, M.; Smith, D.E.; Aronow, H.D.; Dixon, S.R.; Share, D.; Gurm, H.S. The changing definition of contrast-induced nephropathy and its clinical implications: Insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am. Heart J. 2012, 163, 829–834. [Google Scholar] [CrossRef]
- Ando, G.; Cortese, B.; Russo, F.; Rothenbuhler, M.; Frigoli, E.; Gargiulo, G.; Briguori, C.; Vranckx, P.; Leonardi, S.; Guiducci, V.; et al. Acute Kidney Injury After Radial or Femoral Access for Invasive Acute Coronary Syndrome Management: AKI-MATRIX. J. Am. Coll. Cardiol. 2017, 69, 2592–2603. [Google Scholar] [CrossRef]
- Subherwal, S.; Peterson, E.D.; Dai, D.; Thomas, L.; Messenger, J.C.; Xian, Y.; Brindis, R.G.; Feldman, D.N.; Senter, S.; Klein, L.W.; et al. Temporal trends in and factors associated with bleeding complications among patients undergoing percutaneous coronary intervention: A report from the National Cardiovascular Data CathPCI Registry. J. Am. Coll. Cardiol. 2012, 59, 1861–1869. [Google Scholar] [CrossRef]
- Shoji, S.; Kohsaka, S.; Kumamaru, H.; Sawano, M.; Shiraishi, Y.; Ueda, I.; Noma, S.; Suzuki, M.; Numasawa, Y.; Hayashida, K.; et al. Stroke After Percutaneous Coronary Intervention in the Era of Transradial Intervention. Circ. Cardiovasc. Interv. 2018, 11, e006761. [Google Scholar] [CrossRef]
- Mehta, S.R.; Granger, C.B.; Boden, W.E.; Steg, P.G.; Bassand, J.P.; Faxon, D.P.; Afzal, R.; Chrolavicius, S.; Jolly, S.S.; Widimsky, P.; et al. Early versus delayed invasive intervention in acute coronary syndromes. N. Engl. J. Med. 2009, 360, 2165–2175. [Google Scholar] [CrossRef]
- Kofoed, K.F.; Kelbæk, H.; Hansen, P.R.; Torp-Pedersen, C.; Høfsten, D.; Kløvgaard, L.; Holmvang, L.; Helqvist, S.; Jørgensen, E.; Galatius, S.; et al. Early Versus Standard Care Invasive Examination and Treatment of Patients with Non-ST-Segment Elevation Acute Coronary Syndrome: The VERDICT (Very EaRly vs Deferred Invasive evaluation using Computerized Tomography)—Randomized Controlled Trial. Circulation 2018, 138, 2741–2750. [Google Scholar] [CrossRef]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E.; Chavey, W.E.; Fesmire, F.M.; Hochman, J.S.; Levin, T.N.; et al. ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction: Executive Summary. Circulation 2007, 116, 803–877. [Google Scholar] [CrossRef]
- Puymirat, E.; Simon, T.; Cayla, G.; Cottin, Y.; Elbaz, M.; Coste, P.; Lemesle, G.; Motreff, P.; Popovic, B.; Khalife, K.; et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017, 136, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Lafrance, J.P.; Miller, D.R. Acute kidney injury associates with increased long-term mortality. J. Am. Soc. Nephrol. 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Numasawa, Y.; Higashi, T.; Ueda, I.; Suzuki, M.; Hayashida, K.; Yuasa, S.; Maekawa, Y.; Fukuda, K.; Kohsaka, S. Predictors of high cost after percutaneous coronary intervention: A review from Japanese multicenter registry overviewing the influence of procedural complications. Am. Heart J. 2017, 194, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Freda, B.J.; Tang, W.H.W.; Van Lente, F.; Peacock, W.F.; Francis, G.S. Cardiac troponins in renal insufficiency. J. Am. Coll. Cardiol. 2002, 40, 2065–2071. [Google Scholar] [CrossRef]
- Inohara, T.; Kohsaka, S.; Miyata, H.; Ueda, I.; Ishikawa, S.; Ohki, T.; Nishi, Y.; Hayashida, K.; Maekawa, Y.; Kawamura, A.; et al. Appropriateness ratings of percutaneous coronary intervention in Japan and its association with the trend of noninvasive testing. JACC Cardiovasc. Interv. 2014, 7, 1000–1009. [Google Scholar] [CrossRef]
- Miyachi, H.; Takagi, A.; Miyauchi, K.; Yamasaki, M.; Tanaka, H.; Yoshikawa, M.; Saji, M.; Suzuki, M.; Yamamoto, T.; Shimizu, W.; et al. Current characteristics and management of ST elevation and non-ST elevation myocardial infarction in the Tokyo metropolitan area: From the Tokyo CCU network registered cohort. Heart Vessels 2016, 31, 1740–1751. [Google Scholar] [CrossRef]
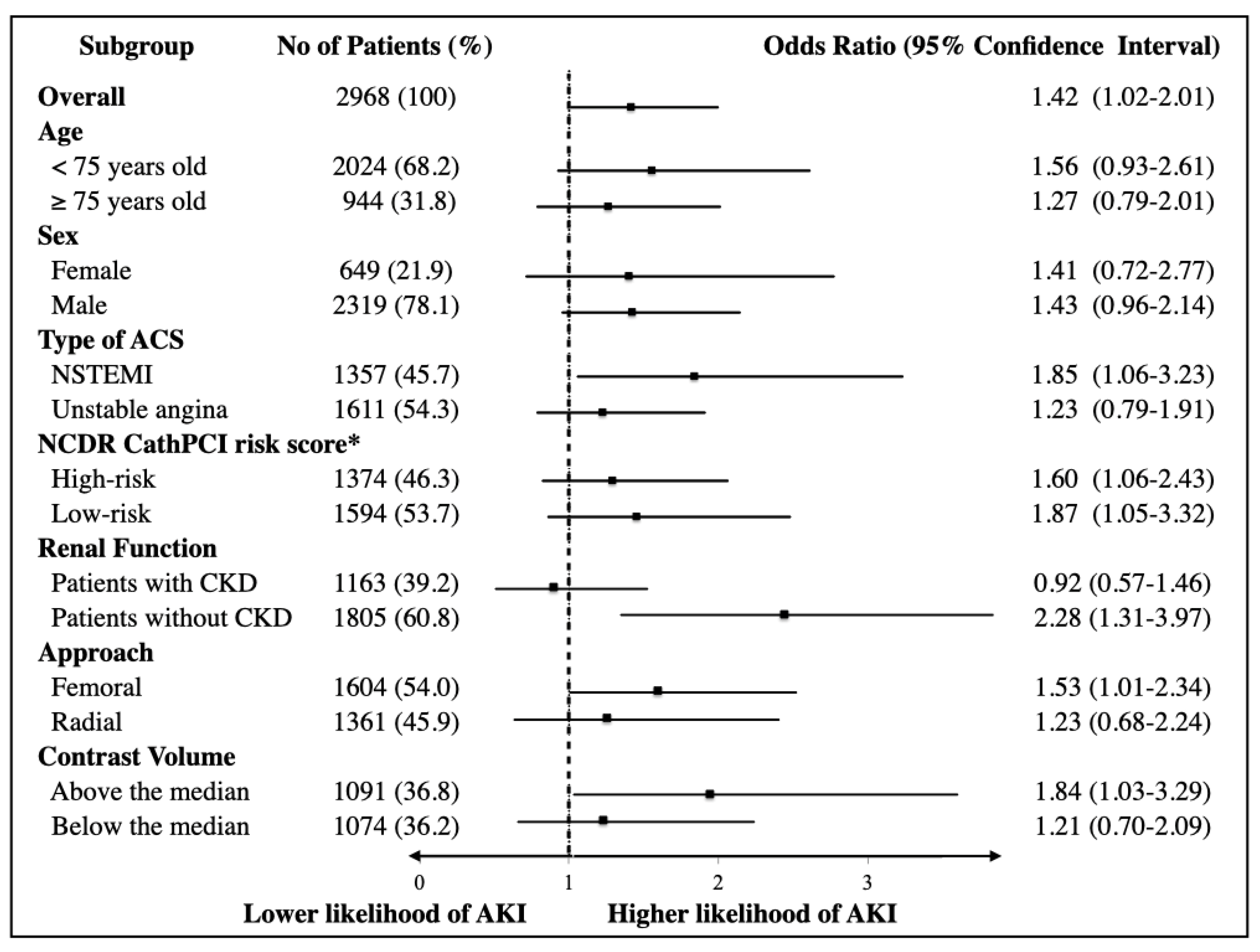
| Number (%) | Treated with an EIS (PCI ≤ 24 h; n = 2436) | Treated with Delayed PCI (PCI 24–72 h; n = 532) | p Value |
|---|---|---|---|
| Male | 1907 (78.3) | 412 (77.4) | 0.67 |
| Age, median (Q1–Q3) | 69 (60–76) | 71 (63–78) | <0.001 |
| BMI, median (Q1–Q3) | 23.7 (21.5–25.9) | 23.8 (22.0–26.1) | 0.83 |
| Family history of CAD | 299 (12.3) | 66 (12.5) | 0.93 |
| Current smoker | 856 (35.2) | 165 (31.0) | 0.069 |
| Past medical history | |||
| Hypertension | 1836 (75.4) | 416 (78.2) | 0.16 |
| Diabetes mellitus | 945 (38.8) | 223 (41.9) | 0.18 |
| Dyslipidemia | 1610 (66.1) | 359 (67.5) | 0.53 |
| CKD | 928 (38.1) | 235 (44.2) | 0.009 |
| eGFR, median (Q1–Q3) | 69.0 (48.5–92.9) | 65.4 (43.9–82.1) | <0.001 |
| HD | 127 (5.2) | 31 (5.8) | 0.56 |
| PAD | 151 (6.2) | 47 (8.8) | 0.027 |
| COPD | 81 (3.3) | 17 (3.2) | 0.88 |
| Prior heart failure | 152 (6.2) | 35 (6.6) | 0.77 |
| Prior MI | 433 (17.8) | 127 (23.9) | 0.001 |
| Prior PCI/CABG | 725 (29.8) | 198 (37.2) | 0.001 |
| Situation at arrival | |||
| NSTEMI | 1201 (51.3) | 156 (33.3) | <0.001 |
| Unstable angina | 1235 (50.7) | 376 (70.7) | <0.001 |
| Heart failure | 268 (11.0) | 63 (11.8) | 0.57 |
| NYHA 3 or 4 | 159 (6.5) | 34 (6.4) | 0.90 |
| EF, median (Q1–Q3) | 60 (50–68) | 61 (53.2–69) | 0.77 |
| EF <40% | 121 (10.1) | 24 (7.4) | 0.13 |
| Cardiogenic shock | 75 (3.1) | 8 (1.5) | 0.046 |
| CPA | 57 (2.3) | 1 (0.2) | 0.001 |
| Elevated cardiac troponin | 922 (39.4) | 74 (15.8) | <0.001 |
| NCDR CathPCI risk score, median (Q1–Q3) | 22 (14–32) | 22 (12–31) | 0.002 |
| NCDR CathPCI risk score, mean, (SD) | 24.3 (14.1) | 21.9 (12.7) | <0.001 |
| Procedure characteristics | |||
| Femoral approach | 1321 (54.2) | 283 (53.2) | 0.77 |
| Use of IABP | 169 (6.9) | 28 (5.3) | 0.15 |
| Use of VA-ECMO | 21 (0.9) | 1 (0.2) | 0.10 |
| LMT lesion | 96 (3.9) | 25 (4.7) | 0.42 |
| LAD lesion | 1242 (51.0) | 264 (49.6) | 0.56 |
| LCX lesion | 656 (26.9) | 130 (24.4) | 0.23 |
| RCA lesion | 716 (29.4) | 171 (32.1) | 0.21 |
| Multivessel PCI | 267 (11.0) | 54 (10.2) | 0.58 |
| Fluoroscopy time, min, median, (Q1–Q3) | 23.0 (16.0–35.1) | 23.0 (15.3–35.1) | 0.82 |
| Contrast volume, mL median, (Q1–Q3) | 160 (125–200) | 150 (119.7–200) | 0.007 |
| Number (%) | Treated with an EIS (PCI ≤ 24 h; n = 2436) | Treated with a Delayed PCI (PCI 24–72 h; n = 532) | p Value |
|---|---|---|---|
| In-hospital mortality | 50 (2.1) | 7 (1.3) | 0.26 |
| Acute kidney injury* | 329 (15.0) | 46 (9.9) | 0.004 |
| Acute kidney injury requiring dialysis | 23 (0.9) | 6 (1.1) | 0.69 |
| Bleeding complication within 72 h | 85 (3.5) | 16 (3.0) | 0.57 |
| Stroke | 9 (0.4) | 1 (0.2) | 0.51 |
| Length of stay, days (IQR) | 3.5 (1.9–8.1) | 4.4 (3.5–7.5) | <0.001 |
| Variables | Adjusted OR | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Early invasive strategy | 1.43 | 1.02 | 2.01 | 0.04 |
| Male (vs. female) | 0.71 | 0.55 | 0.92 | 0.011 |
| Age (≥75 years old) | 2.00 | 1.48 | 2.70 | <0.001 |
| Prior heart failure | 1.80 | 1.27 | 2.55 | 0.001 |
| Diabetes mellitus | 1.32 | 1.05 | 1.67 | 0.018 |
| Peripheral artery disease | 0.75 | 0.46 | 1.23 | 0.25 |
| Chronic kidney disease* | 0.84 | 0.62 | 1.13 | 0.25 |
| NYHA 3 or 4 | 2.09 | 1.44 | 3.05 | <0.001 |
| Elevated cardiac troponin | 1.73 | 1.37 | 2.18 | <0.001 |
| Prior PCI/CABG | 0.63 | 0.48 | 0.84 | 0.001 |
| Cardiogenic shock/CPA | 2.29 | 1.34 | 3.91 | 0.002 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikemura, N.; Shiraishi, Y.; Sawano, M.; Ueda, I.; Numasawa, Y.; Noma, S.; Suzuki, M.; Momiyama, Y.; Hayashida, K.; Yuasa, S.; et al. Exploring Triaging and Short-Term Outcomes of Early Invasive Strategy in Non-ST Segment Elevation Acute Coronary Syndrome: A Report from Japanese Multicenter Registry. J. Clin. Med. 2020, 9, 1106. https://doi.org/10.3390/jcm9041106
Ikemura N, Shiraishi Y, Sawano M, Ueda I, Numasawa Y, Noma S, Suzuki M, Momiyama Y, Hayashida K, Yuasa S, et al. Exploring Triaging and Short-Term Outcomes of Early Invasive Strategy in Non-ST Segment Elevation Acute Coronary Syndrome: A Report from Japanese Multicenter Registry. Journal of Clinical Medicine. 2020; 9(4):1106. https://doi.org/10.3390/jcm9041106
Chicago/Turabian StyleIkemura, Nobuhiro, Yasuyuki Shiraishi, Mitsuaki Sawano, Ikuko Ueda, Yohei Numasawa, Shigetaka Noma, Masahiro Suzuki, Yukihiko Momiyama, Kentaro Hayashida, Shinsuke Yuasa, and et al. 2020. "Exploring Triaging and Short-Term Outcomes of Early Invasive Strategy in Non-ST Segment Elevation Acute Coronary Syndrome: A Report from Japanese Multicenter Registry" Journal of Clinical Medicine 9, no. 4: 1106. https://doi.org/10.3390/jcm9041106
APA StyleIkemura, N., Shiraishi, Y., Sawano, M., Ueda, I., Numasawa, Y., Noma, S., Suzuki, M., Momiyama, Y., Hayashida, K., Yuasa, S., Miyata, H., Fukuda, K., & Kohsaka, S. (2020). Exploring Triaging and Short-Term Outcomes of Early Invasive Strategy in Non-ST Segment Elevation Acute Coronary Syndrome: A Report from Japanese Multicenter Registry. Journal of Clinical Medicine, 9(4), 1106. https://doi.org/10.3390/jcm9041106