The Role of Subthreshold Micropulse Yellow Laser as an Alternative Option for the Treatment of Refractory Postoperative Cystoid Macular Edema
Abstract
1. Introduction
2. Experimental Section
2.1. Study Participants
2.2. Study Evaluations
2.3. Laser Treatment
2.4. Statistical Analysis
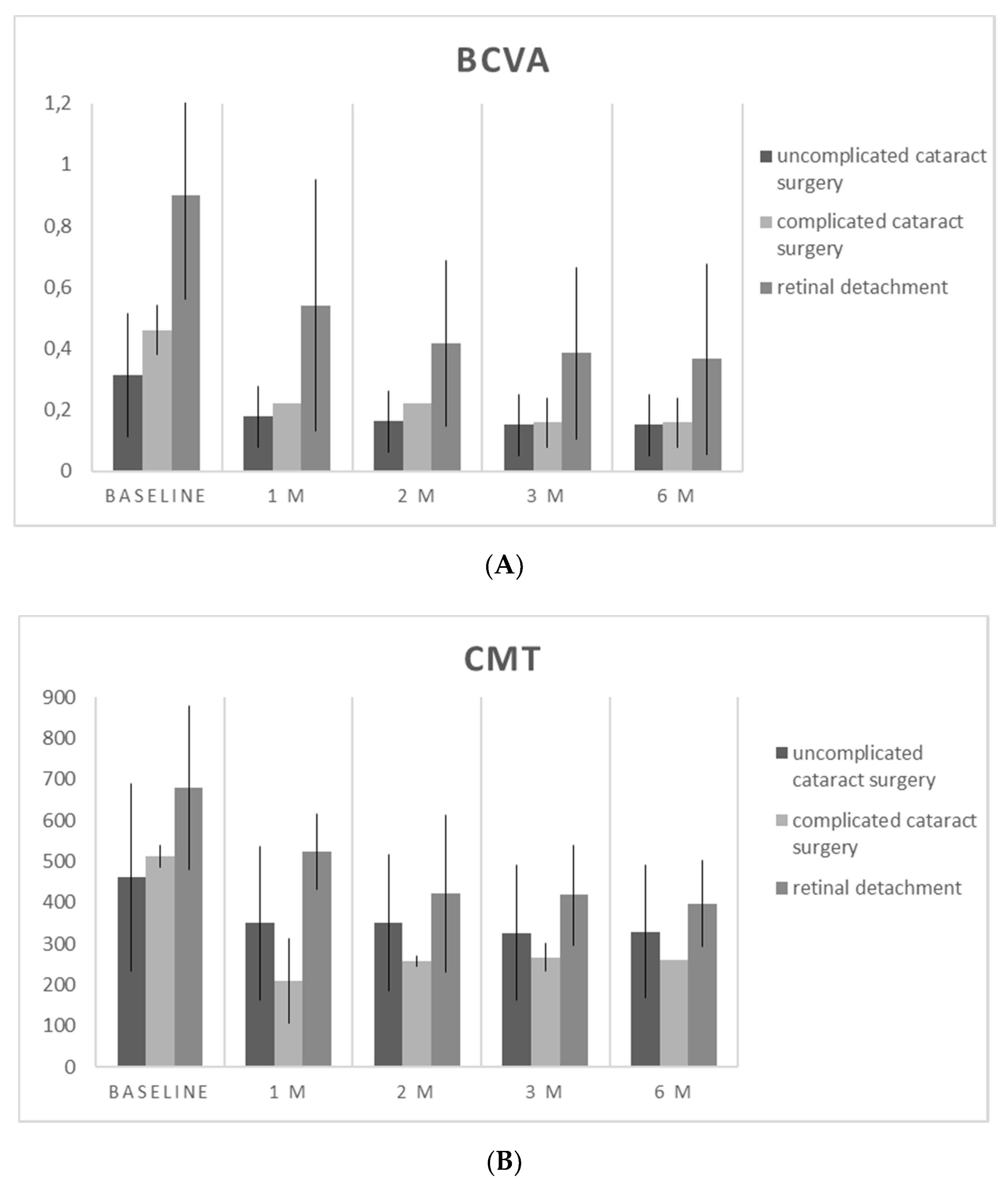
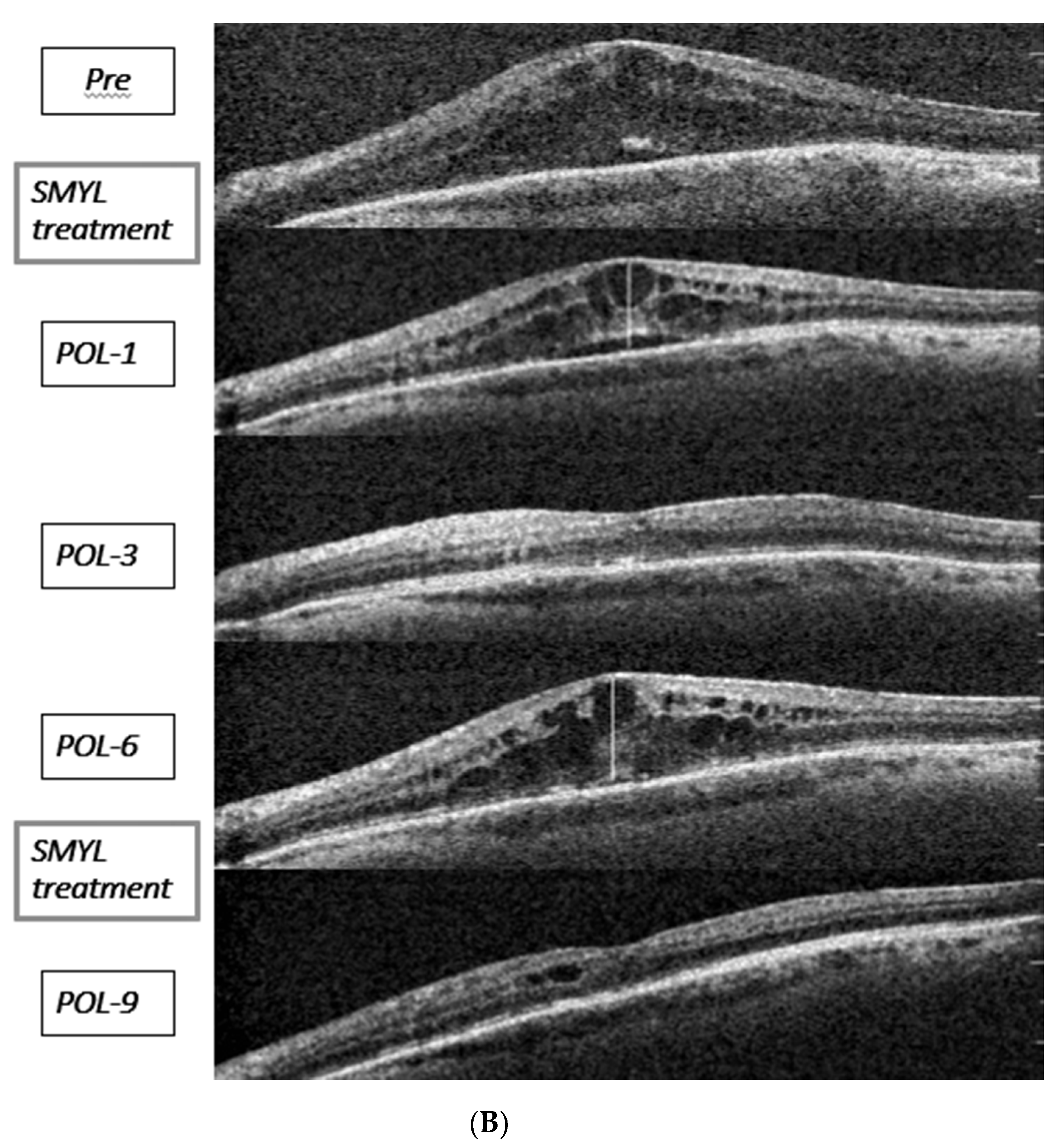
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grzybowski, A.; Sikorski, B.L.; Ascaso, F.J.; Huerva, V. Pseudophakic cystoid macular edema: Update 2016. Clin. Interv. Aging. 2016, 11, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Irvine, S.R. A newly defined vitreous syndrome following cataract surgery. Am. J. Ophthalmol. 1953, 36, 599–619. [Google Scholar] [CrossRef] [PubMed]
- Gass, J.D.M.; Norton, E.W.D. Fluorescein studies of patients with macular edema and papilledema following cataract extraction. Trans. Am. Ophthalmol. Soc. 1966, 64, 232–249. [Google Scholar] [PubMed]
- Flach, A.J. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans. Am. Ophthalmol. Soc. 1998, 96, 557–634. [Google Scholar] [PubMed]
- Yonekawa, Y.; Kim, I.K. Pseudophakic cystoid macular edema. Curr. Opin. Ophthalmol. 2012, 23, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; D’Aloisio, R.; Mastropasqua, R.; Di Antonio, L.; Di Nicola, M.; Di Martino, G.; Evangelista, F.; Erroi, E.; Doronzo, E.; Mariotti, C. Anatomical and Functional Changes of the Retina and the Choroid after Resolved Chronic CSCR. J. Clin. Med. 2019, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Martin, D.F.; Hubbard, G.B.; Srivastava, S.K.; Yan, J.; Bergstrom, C.S.; Aaberg, T.M. Incidence of post vitrectomy macular edema using optical coherence tomography. Ophthalmology 2009, 16, 1531–1537. [Google Scholar] [CrossRef]
- Chatziralli, I.; Theodossiadis, G.; Dimitriou, E.; Kazantzis, D.; Theodossiadis, P. Macular Edema after Successful Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment: Factors Affecting Edema Development and Considerations for Treatment. Ocul. Immunol. Inflamm. 2019, 1–6. [Google Scholar] [CrossRef]
- Sholz, P.; Altay, L.; Fauser, S. A review of subthreshold micropulse laser for treatment of macular disorders. Adv. Ther. 2017, 34, 1528–1555. [Google Scholar] [CrossRef]
- Demirel, S.; Batioğlu, F.; Özmert, E. Intravitreal ranibizumab for the treatment of cystoid macular edema in Irvine-Gass syndrome. J. Ocul. Pharmacol. Ther. 2012, 28, 636–639. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Maia, M.; Garcia-Amaris, R.A.; Roca, J.A.; Sanchez, J.G.; Berrocal, M.H.; Wu, L. Pan-American Collaborative Retina Study Group. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: The Pan-American Collaborative Retina Study Group results. Ophthalmology 2009, 116, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Gawecki, M. Micropulse Laser treatment of Retinal Diseases. J. Clin. Med. 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Erichev, V.P.; Kozlova, I.V.; Kosova, J.V. Frequency and type of macular edema after cataract surgery in patients with glaucoma. Vestn. Oftalmol. 2019, 135, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.J.; Johnston, R.L.; Buscombe, C.; Sallam, A.B.; Moamed, Q.; Yang, Y.C.; United LKidom Pseudophakic Macular Edema Study Group. Risk factors and incidence of macular edema after cataract surgery: A database study of 81984 eyes. Ophthalmology 2016, 123, 316–323. [Google Scholar] [CrossRef]
- Harbour, J.W.; Smiddy, W.E.; Rubsamen, P.E.; Murray, T.G.; Davis, J.L.; Flynn, H.W., Jr. Pars plana vitrectomy for chronic pseudophakic cystoid macular edema. Am. J. Ophthalmol. 1995, 120, 302–307. [Google Scholar] [CrossRef]
- Teja, S.; Sawatzky, L.; Wiens, T.; Maberley, D.; Ma, P. Ozurdex for refractory macular edema secondary to diabetes, vein occlusion, uveitis and pseudophakia. Can. J. Ophthalmol. 2019, 54, 540–547. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye (Lond) 2013, 27, 787–794. [Google Scholar] [CrossRef]
- Neffendorf, J.E.; Gupta, B.; Williamson, T.H. Intraoperative complications of patients undergoing small-gauge and 20-gauge vitrectomy: A database study of 4274 procedures. Eur. J. Ophthalmol. 2017, 27, 226–230. [Google Scholar] [CrossRef]
- Ogata, N.; Tombran-Tink, J.; Jo, N.; Mrazek, D.; Matsumura, M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am. J. Ophthalmol. 2001, 132, 427–429. [Google Scholar] [CrossRef]
- Flaxel, C.; Bradle, J.; Acott, T.; Samples, J.R. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina 2007, 27, 629–634. [Google Scholar] [CrossRef]
- Inagaki, K.; Shuo, T.; Katakura, K.; Ebihara, N.; Murakami, A.; Ohkoshi, K. Sublethal Photothermal Stimulation with a Micropulse Laser Induces Heat Shock Protein Expression in ARPE-19 Cells. J. Ophthalmol. 2015, 2015, 729792. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.R.; Johnson, D.; Hollands, H.; Smallman, D.; Baxter, S.; Eng, K.T.; Kratky, V.; ten Hove, M.W.; Sharma, S.; El-Defrawy, S. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. J. Cataract. Refract. Surg. 2008, 34, 64–96. [Google Scholar] [CrossRef]
- Cho, H.; Madu, A. Etiology and treatment of the inflammatory causes of cystoid macular edema. J. Inflamm. Res. 2009, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.R.; Dowell, N.G.; Jackson, T.L.; Tofts, P.S.; Hughes, E.H. Measuring the Effect of Pars Plana Vitrectomy on Vitreous Oxygenation Using Magnetic Resonance Imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, E.; Novack, R.L.; Hatchell, D.L. Vitrectomy prevents retinal hypoxia in branch retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 1990, 31, 284–289. [Google Scholar]
- Stefansson, E.; Landers, M.B., III; Wolbarsht, M.L. Vitrectomy, lensectomy, and ocular oxygenation. Retina 1982, 2, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M.; Shui, Y.B.; Beebe, D.C. Vitrectomy surgery increases oxygen exposure to the lens: A possible mechanism for nuclear cataract formation. Am. J. Ophthalmol. 2005, 139, 302–310. [Google Scholar] [CrossRef]
- Dorin, G. Subthreshold and micropulse diode laser photocoagulation. Semin Ophthalmol. 2003, 18, 147–153. [Google Scholar] [CrossRef] [PubMed]

| ID | Age | Eye | PCME | Surgical Intervention | Duration PCME (Months) | Treatments Prior to SMYL | Baseline BCVA (LogMAR) | Baseline CMT (µm) | n of SMYL |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 86 | OD | UC | Phaco/IOL | 4 | NSAIDs | 0.40 | 644 | 1 |
| P2 | 80 | OD | UC | Phaco/IOL | 11 | 3 TA, 1 Ozurdex | 0.40 | 303 | 1 |
| P3 | 89 | OD | UC | Phaco/IOL | 15 | NSAIDs | 0.15 | 301 | 1 |
| P4 | 74 | OS | UC | Phaco/IOL | 8 | 2 TA | 0.15 | 290 | 1 |
| P5 | 79 | OS | UC | Phaco/IOL | 10 | 3 Lucentis | 0.52 | 770 | 1 |
| P6 | 79 | OD | CC | Phaco/IOL and PC-R +Scleral fixation same IOL | 16 | NSAIDs, 3 TA | 0.52 | 532 | 3 |
| P7 | 67 | OS | CC | Phaco/IOL and PC-R + IC-IOL posterior | 9 | NSAIDs 2 TA | 0.40 | 493 | 1 |
| P8 | 73 | OS | RD (m-Off) | PPV + SO | 13 | 1 TA, 1 Ozurdex | 1.30 | 600 | 2 |
| P9 | 36 | OD | RD (m-On) | PPV + SO | 10 | NSAIDs | 0.70 | 890 | 1 |
| P10 | 53 | OD | RD (m-On) | PPV + SO | 5 | NSAIDs | 0.70 | 550 | 1 |
| BCVA (LogMAR) | p Value (pre vs post) | CMT (µm) | p Value (pre vs post) | |
|---|---|---|---|---|
| Baseline | 0.52 ± 0.34 | 537.30 ± 202.18 | ||
| M1 | 0.30 ± 0.27 | <0.001 | 374.60 ± 163.75 | <0.001 |
| M2 | 0.25 ± 0.18 | <0.001 | 353.60 ± 125.30 | <0.001 |
| M3 | 0.22 ± 0.19 | <0.001 | 342.40 ± 108.37 | <0.001 |
| M6 | 0.22 ± 0.19 | <0.001 | 336.00 ± 100.30 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdina, T.; D’Aloisio, R.; Lazzerini, A.; Ferrari, C.; Valerio, E.; Mastropasqua, R.; Cavallini, G.M. The Role of Subthreshold Micropulse Yellow Laser as an Alternative Option for the Treatment of Refractory Postoperative Cystoid Macular Edema. J. Clin. Med. 2020, 9, 1066. https://doi.org/10.3390/jcm9041066
Verdina T, D’Aloisio R, Lazzerini A, Ferrari C, Valerio E, Mastropasqua R, Cavallini GM. The Role of Subthreshold Micropulse Yellow Laser as an Alternative Option for the Treatment of Refractory Postoperative Cystoid Macular Edema. Journal of Clinical Medicine. 2020; 9(4):1066. https://doi.org/10.3390/jcm9041066
Chicago/Turabian StyleVerdina, Tommaso, Rossella D’Aloisio, Andrea Lazzerini, Cecilia Ferrari, Edoardo Valerio, Rodolfo Mastropasqua, and Gian Maria Cavallini. 2020. "The Role of Subthreshold Micropulse Yellow Laser as an Alternative Option for the Treatment of Refractory Postoperative Cystoid Macular Edema" Journal of Clinical Medicine 9, no. 4: 1066. https://doi.org/10.3390/jcm9041066
APA StyleVerdina, T., D’Aloisio, R., Lazzerini, A., Ferrari, C., Valerio, E., Mastropasqua, R., & Cavallini, G. M. (2020). The Role of Subthreshold Micropulse Yellow Laser as an Alternative Option for the Treatment of Refractory Postoperative Cystoid Macular Edema. Journal of Clinical Medicine, 9(4), 1066. https://doi.org/10.3390/jcm9041066








