Health in Preconception, Pregnancy and Postpartum Global Alliance: International Network Pregnancy Priorities for the Prevention of Maternal Obesity and Related Pregnancy and Long-Term Complications
Abstract
1. Introduction
2. Method
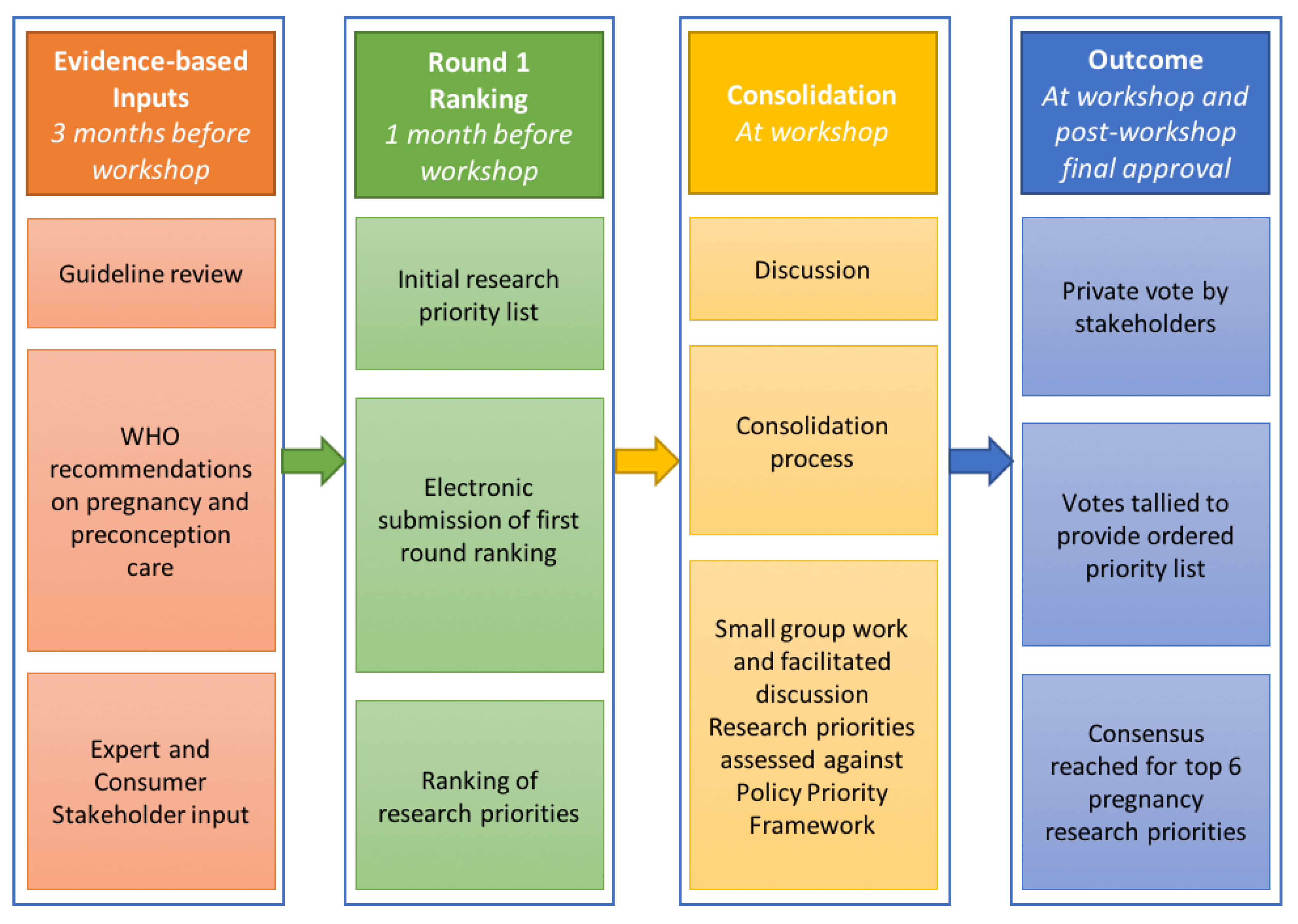
2.1. Research Priority Setting Process
2.1.1. Inputs
2.1.2. Pre-Workshop Ranking (Round 1)
2.1.3. Workshop Processes
First Group Discussion, Vote and Sense-Making (Round 2)
Second Group Discussion and Vote (Round 3)
Consensus Development of Research Priorities
3. Results
3.1. Research Priority Setting Process
3.1.1. Inputs
3.1.2. Pre-Workshop Ranking (Round 1)
3.1.3. Workshop Processes
First Group Discussion, Vote and Sense-Making (Round 2)
Second Group Discussion and Vote (Round 3)
Consensus Development of Research Priorities
- Promoting healthy diet and nutrition, including
- Supplementation
- Optimising gestational weight gain management
- Screening for pregnancy complications and pre-existing conditions, including
- Gestational diabetes mellitus and diabetes, hypertension, fetal growth monitoring
- Risk profiling (e.g., deep vein thrombosis, sleep apnoea)
- Medications
- Optimising physical activity
- Optimising mental health
- Postpartum (including intrapartum) care, including
- Breastfeeding support
- Postnatal depression screening and management
- Optimising sleep
- Primary studies are needed to improve basic understanding of mechanisms, pathways, biological drivers, and impacts on outcomes, including offspring outcomes. This includes exploring dietary components that contribute specifically to weight gain during pregnancy and the physiological mechanisms associated with diet and weight gain in pregnancy.
- Factors that impact lifestyle change throughout pregnancy, such as social support, and mental and physical health, as well as taking a life course approach (including transitions from preconception and to postpartum) need further understanding of how they can be applied or addressed in lifestyle interventions in pregnancy.
- Synthesis is required for all levels of evidence including observational studies, randomised controlled trials and pragmatic implementation trials. Evidence synthesis of intervention studies are imperative, rather than additional randomised controlled trials. Here, secondary research should be prioritised to understand the effective components of lifestyle intervention in pregnancy and establish cost-effectiveness.
- Implementation research is vital to drive evidence of efficacy into broader effectiveness and practice. This will include incorporating systems level approaches, health professional training, and adaptation of policies. A biopsychosocial lens must be applied amid multidisciplinary, population approaches to target the issues at hand.
- Consumer engagement is essential early in the research process and across the implementation and scale up pathway. Consumer engagement refers to an active partnership between the researchers and the individuals affected or impacted by the research (e.g., recipients of a health service), rather than the traditional mode of having research conducted to or for them [26]. It may take many forms during all stages of the research cycle, from research participant to partner in research design [27].
- Co-design techniques are paramount to the design of interventions that are based on stakeholder partnership, and that can be implemented in a cost-effective manner at scale.
- Once the body of evidence is sufficient, risk or score cards for pregnancy lifestyle health can be developed and used to cost-effectively and efficiently triage individuals for appropriate screening, treatment or models of care to improve maternal, fetal and long-term outcomes at an individual and public health level.
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australian Institute of Health and Welfare. Australia’s Mother’s and Babies 2016—In Brief; Australian Institute of Health and Welfare: Canberra, Australia, 2018. [Google Scholar]
- Institute of Medicine. Weight Gain during Pregnancy: Reexamining the Guidelines—Report Brief; Institute of Medicine, National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- National Services Scotland. Births in Scottish Hospitals—Year Ending 31 March 2018; Information Services Division: Edinburgh, Scotland, 2018. [Google Scholar]
- World Health Organization. The Double Burden of Malnutritition—Policy Brief; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Gunderson, E.P. Childbearing and obesity in women: Weight before, during, and after pregnancy. Obstet. Gynecol. Clin. N. Am. 2009, 36, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Sabounchi, N.S.; Hovmand, P.S.; Osgood, N.D.; Dyck, R.F.; Jungheim, E.S. A novel system dynamics model of female obesity and fertility. Am. J. Public Health 2014, 7, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, N.M.; Nicholson, W.K.; Schmitt, J. The association of pregnancy and the development of obesity—Results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int. J. Obes. 2007, 31, 1642. [Google Scholar] [CrossRef]
- Lindsay, A.C.; Greaney, M.L.; Wallington, S.F.; Mesa, T.; Salas, C.F. A review of early influences on physical activity and sedentary behaviors of preschool-age children in high-income countries. J. Spec. Pediatric Nurs. 2017, 22, e12182. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Dwi Putra, S.E.; Li, J.; Hocher, B. Developmental Origins of Disease—Crisis Precipitates Change. Cell. Physiol. Biochem. 2016, 39, 919–938. [Google Scholar] [CrossRef]
- World Health Organization. Meeting to Develop a Global Consensus on Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. WHO Recommendations on Antenatal Care for a Positive Ppregnancy Experience; World Health Organization: Luxembourg, 2016. [Google Scholar]
- National Institute for Health and Care Excellence. Weight Management before, during, and after Pregnancy (PH27); National Institute for Health and Care Excellence: Manchester, UK, 2010. [Google Scholar]
- Health Canada. Prenatal Nutrition Guidelines for Health Professionals: Gestational Weight Gain; Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Public Health Agency of Canada. Family-Centred Maternity and Newborn Care: National Guidelines. Chapter 2: Preconception Care; Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/maternity-newborn-care-guidelines-chapter-2.html (accessed on 18 March 2020).
- Teede, H.; Harrison, C.; Lombard, C.; Boyle, J.; East, C.; Brown, W. NH&MRC Case for Action: Proposal: Obesity Prevention through Preventing Excess Weight Gain during Pregnancy and Postpartum; National Health and Medical Research Council: Canberra, Australia, 2014. [Google Scholar]
- The International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: Meta-analysis of individual participant data from randomised trials. BMJ 2017, 358. [Google Scholar] [CrossRef]
- Skouteris, H.; Teede, H.; Thangaratinam, S.; Bailey, C.; Baxter, J.-A.; Bergmeier, H.; Harrison, C.; Hill, B.; Jack, B.; Jorgensen, L.; et al. Commentary: Addressing healthy lifestyle during preconception and pregnancy: Time for urgent action. Front. Endocrinol. 2019, 10, 163. [Google Scholar] [CrossRef]
- Skouteris, H.; Huang, T.; Millar, L.; Kuhlberg, J.; Dodd, J.; Callaway, L.; Forster, D.; Collins, C.; Hills, A.; Harrison, P.; et al. A systems approach to reducing maternal obesity: The Health in Preconception, Pregnancy and Post-Birth (HIPPP) Collaborative. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 397–400. [Google Scholar] [CrossRef]
- Hill, B.; Skouteris, H.; Teede, H.J.; Cailey, C.; Baxter, J.; Bergmeier, H.J.; Borges, A.L.V.; Harrison, C.; Jack, B.; Jorgensen, L.; et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: International network preconception research priorities for the prevention of maternal obesity and related pregnancy and long-term complications. J. Clin. Med. 2019, 8, 2119. [Google Scholar] [CrossRef]
- Delbecq, A.L.; Van de Ven, A.H.; Gustafson, D.H. Group Techniques for Program Planning: A Guide to Nominal Group and Delphi Procesess; Scott-Foresman: Dallas, TX, USA, 1975. [Google Scholar]
- Rankin, N.M.; McGregor, D.; Butow, P.N.; White, K.; Phillips, J.L.; Young, J.M.; Pearson, S.A.; York, S.; Shaw, T. Adapting the nominal group technique for priority setting of evidence-practice gaps in implementation science. BMC Med Res. Methodol. 2016, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.; Hill, B.; Boyle, J.; Arnott, L.; Baber, R.; Byles, J.; Butera, R.; DeFazio, A.; Fourer, M.; Frayne, J.; et al. Women’s Health: Research and translation activities needed. In InSight+; Medical Journal of Australia: Australia; Available online: https://insightplus.mja.com.au/2019/21/womens-health-research-and-translation-activities-needed/ (accessed on 18 March 2020).
- Teede, H.; Johnson, A.; Buttery, J.; Jones, C.; Boyle, D.; Jennings, G.L.; Shaw, T. Australian Health Research Alliance: National priorities in data driven healthcare improvement. Med. J. Aust. 2019, 211, 494–497.e1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Policy Brief Preconception Care: Maximizing the Gains for Maternal and Child Health; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- National Institute for Health Research (NIHR). Involve Strategy 2012–2015. Putting People First in Research; INVOLVE: Southampton, UK, 2012. [Google Scholar]
- Amirav, I.; Vandall-Walker, V.; Rasiah, J.; Saunders, L. Patient and researcher engagement in health research: A parent’s perspective. Pediatrics 2017, 140, e20164127. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.J.; Sui, Z.; Cramp, C.S.; Dodd, J.M. A decrease in diet quality occurs during pregnancy in overweight and obese women which is maintained post-partum. Int. J. Obes. 2012, 37, 704. [Google Scholar] [CrossRef] [PubMed]
- Pick, M.E.; Edwards, M.; Moreau, D.; Ryan, E.A. Assessment of diet quality in pregnant women using the Healthy Eating Index. J. Am. Diet. Assoc. 2005, 105, 240–246. [Google Scholar] [CrossRef]
- Nehring, I.; Schmoll, S.; Beyerlein, A.; Hauner, H.; von Kries, R. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1225–1231. [Google Scholar] [CrossRef]
- Kothe, E.; Bailey, C.; Weiner, C.; Nagle, C.; Nowson, C.; Hill, B.; McPhie, S.; Savaglio, M.; Skouteris, H. An investigation of Australian midwifery curricula for obesity management and health behaviour change training. Nurse Educ. Pract. 2019, 36, 54–57. [Google Scholar] [CrossRef]
- Aquino, M.R.; Olander, E.K.; Needle, J.J.; Bryar, R.M. Midwives’ and health visitors’ collaborative relationships: A systematic review of qualitative and quantitative studies. Int. J. Nurs. Stud. 2016, 62, 193–206. [Google Scholar] [CrossRef]
- Gafoor, R.; Booth, H.P.; Gulliford, M.C. Antidepressant utilisation and incidence of weight gain during 10 years’ follow-up: Population based cohort study. BMJ 2018, 361, k1951. [Google Scholar] [CrossRef]
- Harrison, C.L.; Brown, W.J.; Hayman, M.; Moran, L.J.; Redman, L.M. The role of physical activity in preconception, pregnancy and postpartum health. Semin. Reprod. Med. 2016, 34, e28–e37. [Google Scholar] [CrossRef]
- Harrison, C.L.; Lombard, C.B.; Strauss, B.J.; Teede, H.J. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: A randomized controlled trial. Obesity 2013, 21, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Moran, L.J.; Dodd, J.M. Physical activity levels during pregnancy and gestational weight gain among women who are overweight or obese. Health Promot. J. Aust. 2013, 24, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Dervis, S.; Haman, F.; Adamo, K.B.; Redman, L.M. Energy intake requirements in pregnancy. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.M.; Harper, B.D.; Arms-Chavez, C.J.; LoBello, S.G. Estimated prevalence of antenatal depression in the US population. Arch. Women’s Ment. Health 2016, 19, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.-L.; Falah-Hassani, K.; Shiri, R. Prevalence of antenatal and postnatal anxiety: Systematic review and meta-analysis. Br. J. Psychiatry 2017, 210, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Falah-Hassani, K.; Shiri, R.; Dennis, C.L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol. Med. 2017, 47, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Hartley, E.; Hill, B.; McPhie, S.; Skouteris, H. The associations between depressive and anxiety symptoms, body image, and weight in the first year postpartum: A rapid systematic review. J. Reprod. Infant Psychol. 2018, 36, 81–101. [Google Scholar] [CrossRef]
- Milgrom, J.; Gemmill, A.W.; Bilszta, J.L.; Hayes, B.; Barnett, B.; Brooks, J.; Ericksen, J.; Ellwood, D.; Buist, A. Antenatal risk factors for postnatal depression: A large prospective study. J. Affect. Disord. 2008, 108, 147–157. [Google Scholar] [CrossRef]
- O’Neil, A.; Itsiopoulos, C.; Skouteris, H.; Opie, R.S.; McPhie, S.; Hill, B.; Jacka, F.N. Preventing mental health problems in offspring by targeting dietary intake of pregnant women. BMC Med. 2014, 12, 208. [Google Scholar] [CrossRef]
- Hill, B.; McPhie, S.; Fuller-Tyszkiewicz, M.; Gillman, M.W.; Skouteris, H. Psychological health and lifestyle management preconception and in pregnancy. Semin. Reprod. Med. 2016, 34, 121–128. [Google Scholar] [CrossRef]
- Daley, A.; Foster, L.; Long, G.; Palmer, C.; Robinson, O.; Walmsley, H.; Ward, R. The effectiveness of exercise for the prevention and treatment of antenatal depression: Systematic review with meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Mammen, G.; Faulkner, G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Mulherin, K.; Miller, Y.D.; Barlow, F.K.; Diedrichs, P.C.; Thompson, R. Weight stigma in maternity care: Women’s experiences and care providers’ attitudes. BMC Pregnancy Childbirth 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Kumar, A.; Blumfield, M.; Truby, H. Maternal nutrition and weight management in pregnancy: A nudge in the right direction. Nutr. Bull. 2018, 43, 69–78. [Google Scholar] [CrossRef]
- Johnson, K.A.; Gee, R.E. Interpregnancy care. Semin. Perinatol. 2015, 39, 310–315. [Google Scholar] [CrossRef]
- Gu, V.; Feeley, N.; Gold, I.; Hayton, B.; Robins, S.; Mackinnon, A.; Samuel, S.; Carter, C.S.; Zelkowitz, P. Intrapartum synthetic oxytocin and its effects on maternal well-being at 2 months postpartum. Birth 2016, 43, 28–35. [Google Scholar] [CrossRef]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Lawson, A.; Murphy, K.E.; Sloan, E.; Uleryk, E.; Dalfen, A. The relationship between sleep and postpartum mental disorders: A systematic review. J. Affect. Disord. 2015, 176, 65–77. [Google Scholar] [CrossRef]
- Patnode, C.D.; Henninger, M.L.; Senger, C.A.; Perdue, L.A.; Whitlock, E.P. Evidence Report: Primary care interventions to support breastfeeding: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016, 316, 1694–1705. [Google Scholar] [CrossRef]
- Shorey, S.; Chee, C.Y.I.; Ng, E.D.; Chan, Y.H.; Tam, W.W.S.; Chong, Y.S. Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 104, 235–248. [Google Scholar] [CrossRef]
- Lim, S.; Liang, X.; Hill, B.; Teede, H.; Moran, L.J.; O’Reilly, S. A systematic review and meta-analysis of intervention characteristics in postpartum weight management using the TIDieR framework: A summary of evidence to implementation. Obes. Rev. 2019. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Aquino, M.; Olander, E.K.; Bryar, R.M. A focus group study of women’s views and experiences of maternity care as delivered collaboratively by midwives and health visitors in England. BMC Pregnancy Childbirth 2018, 18, 505. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, A.; Hedderson, M.M.; Albright, C.L.; Ehrlich, S.F.; Quesenberry, C.P., Jr.; Peng, T.; Feng, J.; Ching, J.; Crites, Y. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: A feasibility randomized control trial. Diabetes Care 2011, 34, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Olander, E.K.; Hill, B.; Willey, S.; Skouteris, H. Weight management across pregnancy and postpartum care: The need for interprofessional education and collaboration. Nurse Educ. Pract. 2019, 41, 102651. [Google Scholar] [CrossRef]
- Lang, A.Y.; Bartlett, R.; Robinson, T.; Boyle, J.A. Perspectives on preconception health among migrant women in Australia: A qualitative study. Women Birth 2019. Epub ahead of print. [Google Scholar] [CrossRef]
| Pregnancy Research Priority | Round 1 Ranking | Round 2 Ranking | Round 3 Ranking |
|---|---|---|---|
Promoting healthy diet and nutrition
| 1 a, 5 b | 1 | 1 |
| Optimising gestational weight management | 2 | 2 | 2 |
Screening for pregnancy complications and pre-existing medical conditions
| 3 c | 3 | 3 |
| Optimising physical activity | 4 | 4 | 4 |
| Optimising mental health | 6 | 5 | 5 |
Postpartum (including intrapartum) care
| Not rankedd | 6 | 6 |
| Substance use (including alcohol and tobacco) | 7 | 7 | 7 |
Infections
| 8 | 8 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, B.; Skouteris, H.; Boyle, J.A.; Bailey, C.; Walker, R.; Thangaratinam, S.; Sundseth, H.; Stephenson, J.; Steegers, E.; Redman, L.M.; et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: International Network Pregnancy Priorities for the Prevention of Maternal Obesity and Related Pregnancy and Long-Term Complications. J. Clin. Med. 2020, 9, 822. https://doi.org/10.3390/jcm9030822
Hill B, Skouteris H, Boyle JA, Bailey C, Walker R, Thangaratinam S, Sundseth H, Stephenson J, Steegers E, Redman LM, et al. Health in Preconception, Pregnancy and Postpartum Global Alliance: International Network Pregnancy Priorities for the Prevention of Maternal Obesity and Related Pregnancy and Long-Term Complications. Journal of Clinical Medicine. 2020; 9(3):822. https://doi.org/10.3390/jcm9030822
Chicago/Turabian StyleHill, Briony, Helen Skouteris, Jacqueline A. Boyle, Cate Bailey, Ruth Walker, Shakila Thangaratinam, Hildrun Sundseth, Judith Stephenson, Eric Steegers, Leanne M. Redman, and et al. 2020. "Health in Preconception, Pregnancy and Postpartum Global Alliance: International Network Pregnancy Priorities for the Prevention of Maternal Obesity and Related Pregnancy and Long-Term Complications" Journal of Clinical Medicine 9, no. 3: 822. https://doi.org/10.3390/jcm9030822
APA StyleHill, B., Skouteris, H., Boyle, J. A., Bailey, C., Walker, R., Thangaratinam, S., Sundseth, H., Stephenson, J., Steegers, E., Redman, L. M., Montanaro, C., Lim, S., Jorgensen, L., Jack, B., Borges, A. L. V., Bergmeier, H. J., Baxter, J.-A. B., Harrison, C. L., & Teede, H. J. (2020). Health in Preconception, Pregnancy and Postpartum Global Alliance: International Network Pregnancy Priorities for the Prevention of Maternal Obesity and Related Pregnancy and Long-Term Complications. Journal of Clinical Medicine, 9(3), 822. https://doi.org/10.3390/jcm9030822








