Anatomical Considerations of Intramedullary Humeral Nailing and Lengthening
Abstract
1. Introduction
2. Material and Methods
- Group 1—patients with CT imaging of the humerus (n = 10)
- o
- 3 female humeri, 7 male
- Group 2—anatomical specimens (n = 20)
- o
- 12 female humeri, 8 male
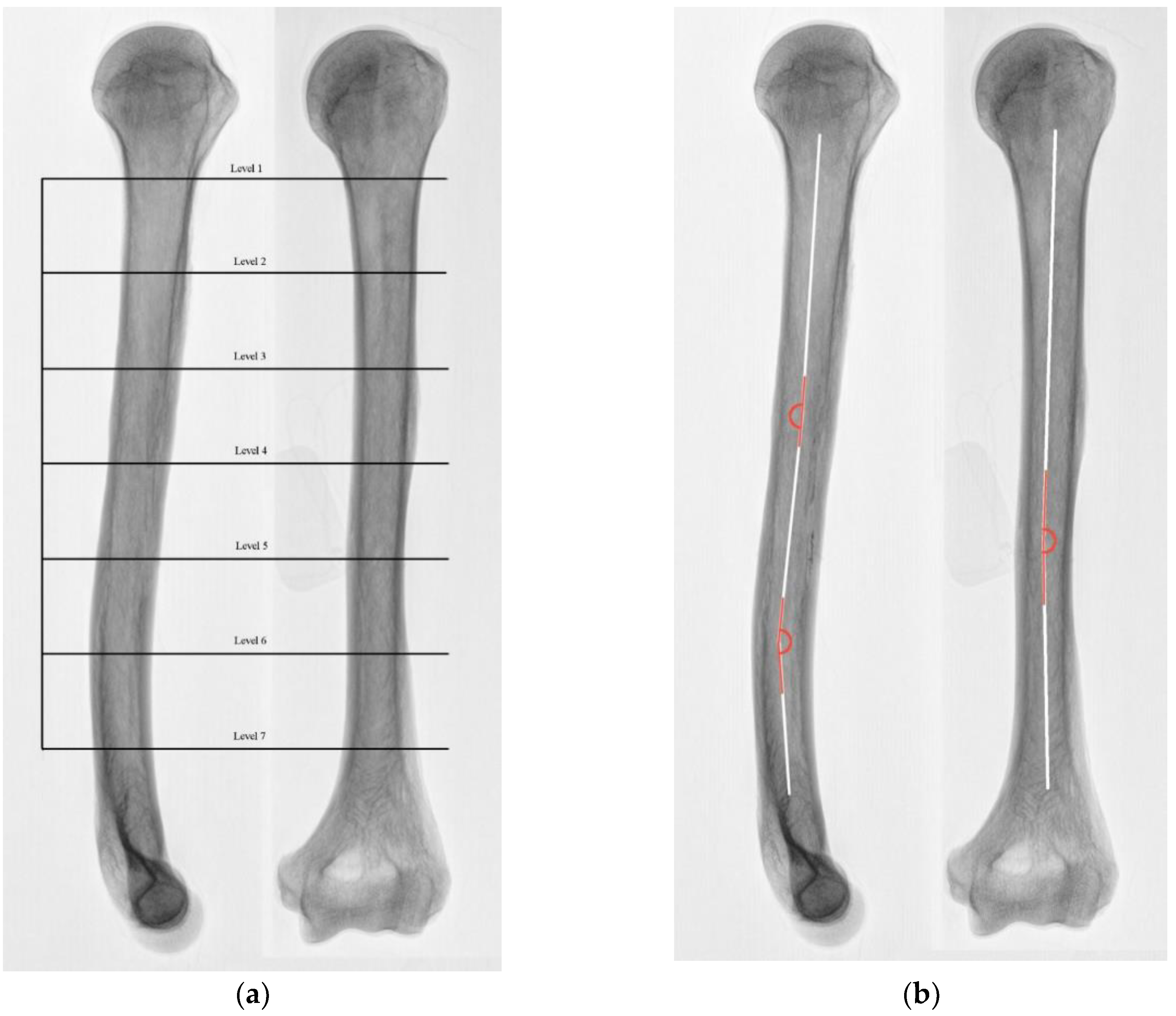
2.1. Group 1 CT Evaluation (10 Humeri)
2.2. Group 2 Anatomical Evaluation (20 Humeri)
2.3. Statistical Analysis
3. Results
3.1. Medullary Cavity Width (MCW)
3.2. Cortical Thickness (CoT)
3.3. Course of the Humeral Cavity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gubin, A.V.; Borzunov, D.Y.; Malkova, T.A. The Ilizarov paradigm: Thirty years with the Ilizarov method, current concerns and future research. Int. Orthop. 2013, 37, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Paley, D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin. Orthop. Relat. Res. 1990, 250, 81–104. [Google Scholar] [CrossRef]
- Paley, D. PRECICE intramedullary limb lengthening system. Expert Rev. Med. Devices 2015, 12, 231–249. [Google Scholar] [CrossRef] [PubMed]
- Wozasek, G.E.; Zak, L. Intramedullary upper arm lengthening. Unfallchirurg 2018, 121, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Tiefenboeck, T.M.; Zak, L.; Wozasek, G.E. Intramedullary magnetically actuated limb lengthening in a patient with congenital humeral limb shortening. Injury 2016, 47, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, A.I.; Standard, S.C.; Robert Rozbruch, S.; Herzenberg, J.E. Humeral Lengthening with the PRECICE Magnetic Lengthening Nail. HSS J. 2017, 13, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Shadi, M.; Musielak, B.; Koczewski, P.; Janusz, P. Humeral lengthening in patients with achondroplasia and in patients with post-septic shortening: Comparison of procedure efficiency and safety. Int. Orthop. 2018, 42, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Furmetz, J.; Kold, S.; Schuster, N.; Wolf, F.; Thaller, P.H. Lengthening of the humerus with intramedullary lengthening nails-preliminary report. Strategies Trauma Limb Reconstr. 2017, 12, 99–106. [Google Scholar] [PubMed]
- Rommens, P.M.; Kuechle, R.; Bord, T.; Lewens, T.; Engelmann, R.; Blum, J. Humeral nailing revisited. Injury 2008, 39, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Böhler, L. Die Technik der Knochenbruchbehandlung im Frieden und Kriege. Die Marknagelung nach Küntscher; Wilhelm Maudrich Wien: Vienna, Austria, 1944; Volume 9. [Google Scholar]
- Ndou, R.; Maharaj, S.; Schepartz, L.A. A radiographic investigation of the relationships between humeral cortical bone thickness, medullary canal width and the supratrochlear aperture (STA). Surg. Radiol. Anat. 2017, 39, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Pospula, W.; Abu Al Noor, T.; Roshdy, T.; Al Rowaih, A. Radioanatomical measurements of the medullary cavity of the humerus in Kuwait: Ethnic differences and clinical implications for fracture fixation. Med. Princ. Pract. 2004, 13, 206–210. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://imagej.nih.gov/ij/ (accessed on 31 December 2019).
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Acan, A.E.; Basci, O.; Havitcioglu, H. Aneurysmal bone cyst healing response with intramedullary lengthening nail. Acta Orthop. Traumatol. Turc. 2018, 52, 232–235. [Google Scholar] [CrossRef]
- Garcia-Coiradas, J.; Rodriguez-Niedenführ, M.; Lopiz, Y.; Garcia-Fernandez, C.; Sanudo, J.R.; Vazquez, T.; Marco, F. A new straigt proximal humeral nail: A cadaveric study of its anatomical relationships. Eur. J. Anat. 2012, 16, 184–189. [Google Scholar]
- Lee, F.Y.; Schoeb, J.S.; Yu, J.; Christiansen, B.D.; Dick, H.M. Operative lengthening of the humerus: Indications, benefits, and complications. J. Pediatr. Orthop. 2005, 25, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, A.H.; Mathias, K.J.; Smith, F.W. Measurement of the bony anatomy of the humerus using magnetic resonance imaging. Proc. Inst. Mech. Eng. H 2002, 216, 31–35. [Google Scholar] [CrossRef] [PubMed]
- NUVASIVE. PRECICE UNYTE Humeral Nail Sales Sheet; NuVasie Inc., Ed.; NuVasie Inc.: Aliso Viejo, CA, USA, 2016. [Google Scholar]
- WITTENSTEIN. Smarter Limb Lengthening; Wittenstein, Ed.; WITTENSTEIN: Igersheim, Germany, 2016. [Google Scholar]

| MCW (Overall, n = 30) mm | CoT (Overall, n = 30) mm | MCW (Group 1, n = 10) mm | CoT (Group 1, n = 10) mm | |
|---|---|---|---|---|
| Level 1 sag | 21.31 ± 2.92 | 2.30 ± 0.43 ant 2.76 ± 0.44 post | 19.19 ± 3.14 | 2.39 ± 0.64 ant 2.53 ± 0.92 post |
| Level 1 cor | 21.51 ± 3.12 | 2.67 ± 0.40 med 2.23 ± 0.24 lat | 21.76 ± 3.05 | 2.50 ± 0.71 med 2.03 ± 0.67 lat |
| Level 2 sag | 14.94 ± 3.07 | 3.91 ± 0.76 ant 3.51 ± 0.71 post | 14.93 ± 2.47 | 3.48 ± 1.02 ant 2.72 ± 0.83 post |
| Level 2 cor | 15.05 ± 2.84 | 3.75 ± 0.73 med 3.29 ± 0.50 lat | 15.84 ± 2.65 | 2.92 ± 0.92 med 2.58 ± 0.76 lat |
| Level 3 sag | 12.82 ± 2.38 | 4.68 ± 1.13 ant 3.88 ± 0.64 post | 13.86 ± 2.18 | 4.06 ± 1.30 ant 3.15 ± 1.09 post |
| Level 3 cor | 11.54 ± 2.38 | 3.99 ± 0.75 med 4.52 ± 0.92 lat | 13.92 ± 2.74 | 3.47 ± 0.93 med 3.98 ± 0.99 lat |
| Level 4 sag | 12.30 ± 2.86 | 5.08 ± 1.20 ant 4.55 ± 0.67 post | 13.36 ± 2.28 | 4.27 ± 1.43 ant 3.49 ± 1.04 post |
| Level 4 cor | 9.91 ± 2.61 | 4.18 ± 1.09 med 4.08 ± 1.00 lat | 12.50 ± 2.59 | 3.53 ± 0.91 med 3.94 ± 1.16 lat |
| Level 5 sag | 10.37 ± 2.08 | 4.43 ± 0.68 ant 4.33 ± 0.49 post | 12.80 ± 2.38 | 3.94 ± 1.02 ant 3.69 ± 1.05 post |
| Level 5 cor | 9.32 ± 2.15 | 4.72 ± 0.72 med 4.75 ± 0.52 lat | 11.32 ± 1.89 | 3.93 ± 1.15 med 3.58 ± 1.00 lat |
| Level 6 sag | 9.25 ± 1.48 | 4.14 ± 0.41 ant 4.04 ± 0.51 post | 12.01 ± 1.96 | 3.78 ± 0.99 ant 3.43 ± 1.03 post |
| Level 6 cor | 9.64 ± 2.28 | 4.15 ± 0.42 med 4.04 ± 0.51 lat | 11.35 ± 1.88 | 3.55 ± 0.96 med 3.68 ± 1.03 lat |
| Level 7 sag | 8.93 ± 1.10 | 4.13 ± 0.45 ant 4.07 ± 0.45 post | 10.15 ± 1.96 | 3.84 ± 1.02 ant 3.93 ± 1.15 post |
| Level 7 cor | 9.93 ± 2.00 | 3.83 ± 0.58 med 4.25 ± 0.63 lat | 11.66 ± 2.03 | 3.79 ± 1.09 med 4.29 ± 1.38 lat |
| MCW (Anatomical, n = 20) mm | MCW (Radiographs, n = 20) mm | Intraclass Correlation κ | Difference in % | |
|---|---|---|---|---|
| Level 1 sag | 17.59 ± 2.52 | 19.73 ± 3.12 | 0.778 | 12 |
| Level 1 cor | 20.50 ± 2.82 | 23.14 ± 2.78 | 0.754 | 12 |
| Level 2 sag | 14.77 ± 2.27 | 15.08 ± 2.45 | 0.961 | 2 |
| Level 2 cor | 15.68 ± 2.01 | 16.40 ± 3.09 | 0.768 | 4 |
| Level 3 sag | 13.98 ± 1.97 | 14.27 ± 2.21 | 0.915 | 2 |
| Level 3 cor | 13.48 ± 2.45 | 15.56 ± 2.17 | 0.761 | 15 |
| Level 4 sag | 13.05 ± 1.75 | 14.21 ± 2.24 | 0.763 | 8 |
| Level 4 cor | 12.14 ± 1.90 | 14.15 ± 1.98 | 0.649 | 16 |
| Level 5 sag | 12.52 ± 1.18 | 14.30 ± 2.36 | 0.590 | 14 |
| Level 5 cor | 10.97 ± 1.14 | 12.66 ± 1.23 | 0.565 | 15 |
| Level 6 sag | 11.79 ± 1.34 | 13.60 ± 1.52 | 0.618 | 15 |
| Level 6 cor | 11.31 ± 1.38 | 12.25 ± 1.55 | 0.849 | 8 |
| Level 7 sag | 9.00 ± 1.03 | 11.88 ± 1.72 | 0.231 | 32 |
| Level 7 cor | 11.13 ± 1.45 | 13.07 ± 1.65 | 0.506 | 17 |
| CoT (Anatomical, n = 20) mm | CoT (Radiographs, n = 20) mm | Intraclass Correlation κ | Difference in % | |
|---|---|---|---|---|
| Level 1 | 2.35 ± 0.82 lat | 1.60 ± 0.36 lat | 0.113 | 46 |
| Level 2 | 2.59 ± 0.63 post | 2.11 ± 0.64 lat | 0.616 | 22 |
| Level 3 | 3.16 ± 1.05 post | 2.77 ± 1.17 post | 0.672 | 14 |
| Level 4 | 3.16 ± 1.05 post | 2.89 ± 0.92 post | 0.722 | 9 |
| Level 5 | 3.79 ± 1.07 post | 3.03 ± 0.70 lat | 0.683 | 25 |
| Level 6 | 3.26 ± 0.99 post | 3.25 ± 1.19 post | 0.918 | 0 |
| Level 7 | 3.74 ± 1.40 med | 3.41 ± 0.89 ant | 0.718 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, G.M.; Zak, L.; Hirtler, L.; Wozasek, G.E. Anatomical Considerations of Intramedullary Humeral Nailing and Lengthening. J. Clin. Med. 2020, 9, 806. https://doi.org/10.3390/jcm9030806
Schwarz GM, Zak L, Hirtler L, Wozasek GE. Anatomical Considerations of Intramedullary Humeral Nailing and Lengthening. Journal of Clinical Medicine. 2020; 9(3):806. https://doi.org/10.3390/jcm9030806
Chicago/Turabian StyleSchwarz, Gilbert Manuel, Lukas Zak, Lena Hirtler, and Gerald Eliot Wozasek. 2020. "Anatomical Considerations of Intramedullary Humeral Nailing and Lengthening" Journal of Clinical Medicine 9, no. 3: 806. https://doi.org/10.3390/jcm9030806
APA StyleSchwarz, G. M., Zak, L., Hirtler, L., & Wozasek, G. E. (2020). Anatomical Considerations of Intramedullary Humeral Nailing and Lengthening. Journal of Clinical Medicine, 9(3), 806. https://doi.org/10.3390/jcm9030806






