Differences in the Sub-Metatarsal Fat Pad Atrophy Symptoms between Patients with Metatarsal Head Resection and Those without Metatarsal Head Resection: A Cross-Sectional Study
Abstract
1. Introduction
2. Experimental Section
Statistical Analysis
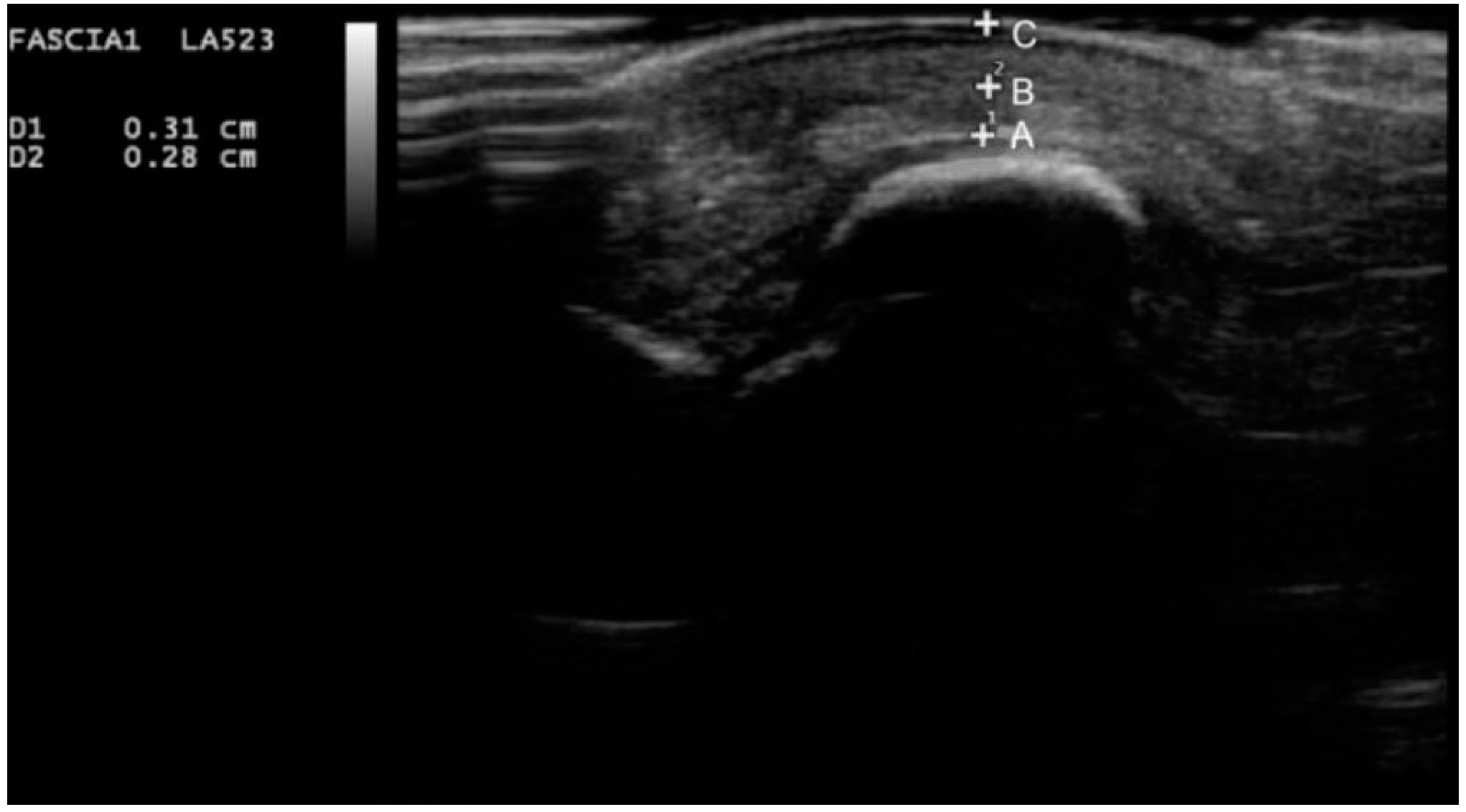
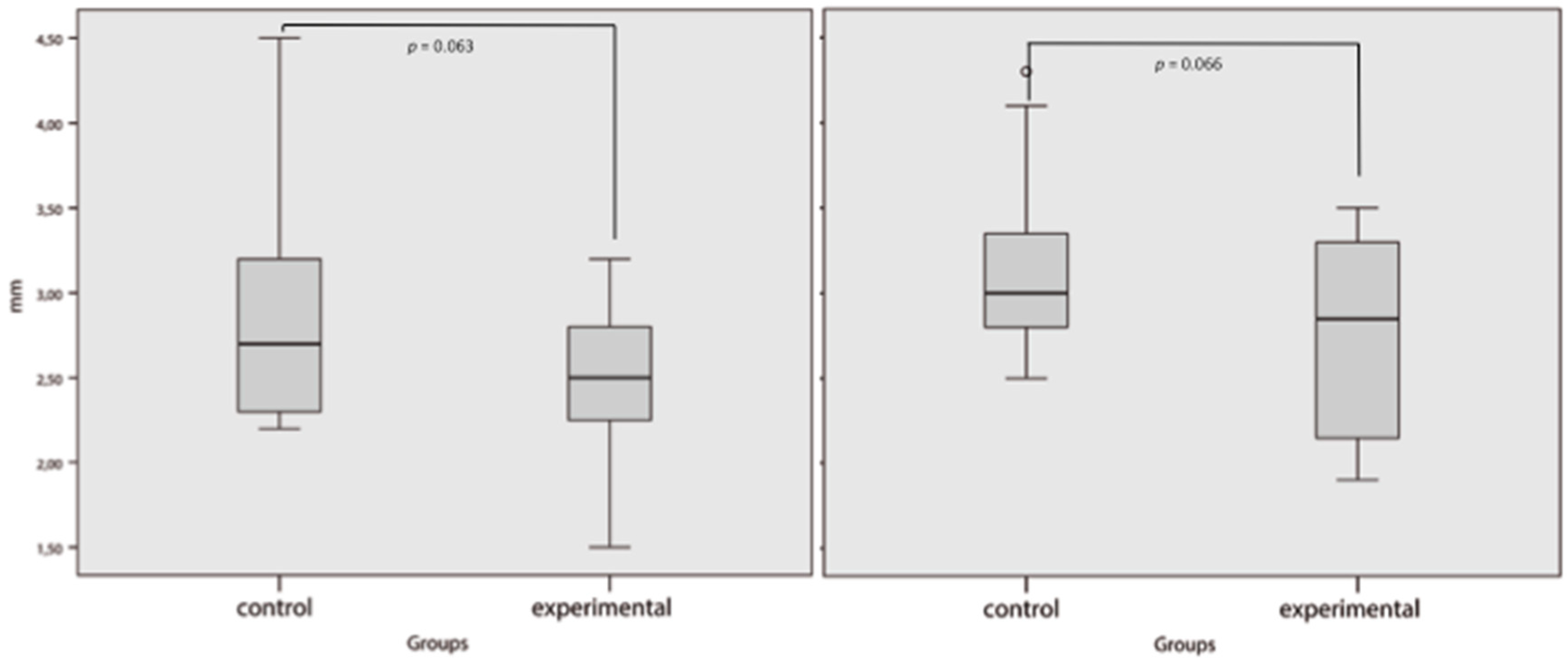
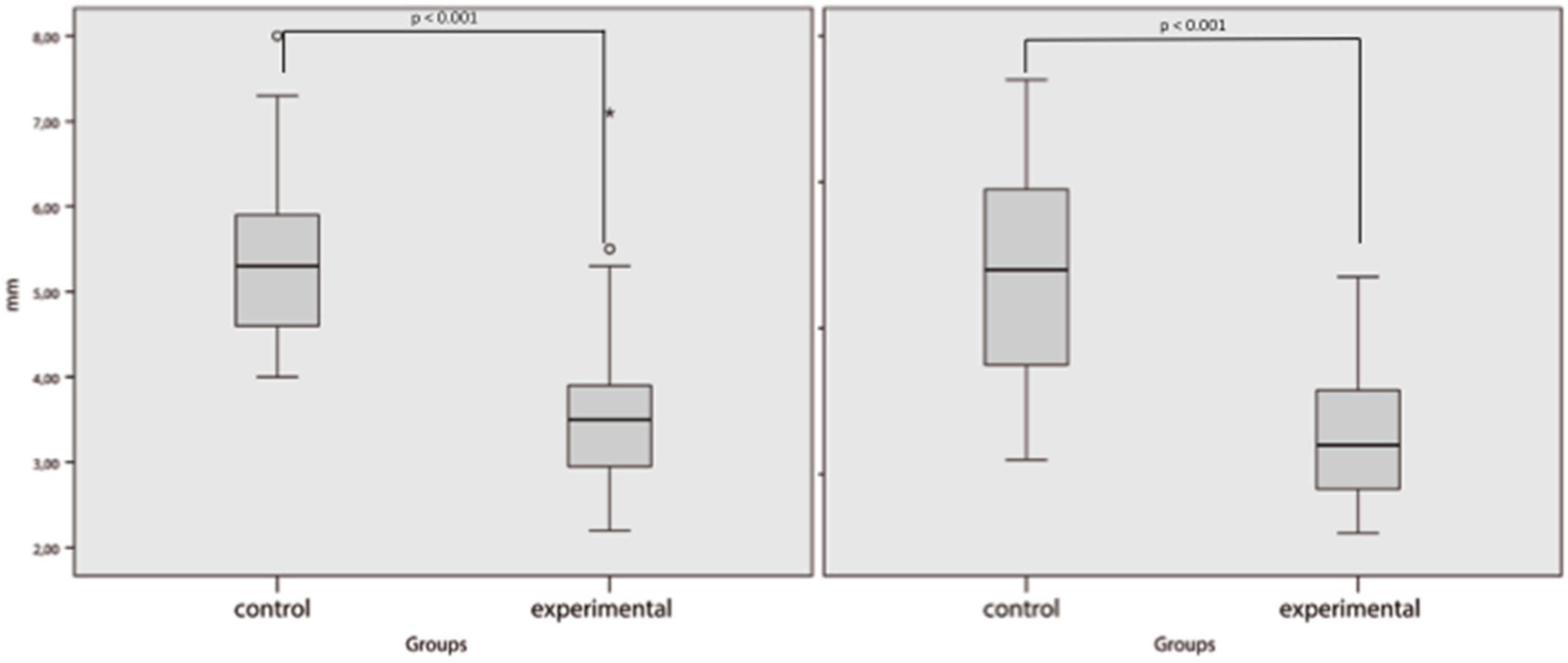
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Boulton, A.J.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The global burden of diabetic foot disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic foot ulcers and their recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Walsh, J.W.; Hoffstad, O.J.; Sullivan, M.O.; Margolis, D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet. Med. 2016, 33, 1493–1498. [Google Scholar] [CrossRef]
- Pickwell, K.; Siersma, V.; Kars, M.; Apelqvist, J.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovská, A.; Jude, E.; Mauricio, D.; et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care 2015, 38, 852–857. [Google Scholar] [CrossRef]
- Nabuurs-Franssen, M.H.; Huijberts, M.S.; Nieuwenhuijzen Kruseman, A.C.; Willems, J.; Schaper, N.C. Health-related quality of life of diabetic foot ulcer patients and their caregivers. Diabetologia 2005, 48, 1906–1910. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Barn, R.; Waaijman, R.; Nollet, F.; Woodburn, J.; Bus, S.A. Predictors of barefoot plantar pressure during walking in patients with diabetes, peripheral neuropathy and a history of ulceration. PLoS ONE 2015, 10, e0117443. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, J.; Ribeiro, I.; Dinis-Ribeiro, M. Predictive factors for diabetic foot ulceration: A systematic review. Diabetes Metab. Res. Rev. 2012, 28, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Naemi, R.; Chatzistergos, P.; Suresh, S.; Sundar, L.; Chockalingam, N.; Ramachandran, A. Can plantar soft tissue mechanics enhance prognosis of diabetic foot ulcer? Diabetes Res. Clin. Pract. 2017, 126, 182–191. [Google Scholar] [CrossRef]
- Chao, C.Y.; Zheng, Y.P.; Cheing, G.L. Epidermal thickness and biomechanical properties of plantar tissues in diabetic foot. Ultrasound Med. Biol. 2011, 37, 1029–1038. [Google Scholar] [CrossRef]
- Lopez-Lopez, D.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Soriano-Medrano, A.; Palomo-Lopez, P.; Morales-Ponce, A.; Rodriguez-Sanz, D.; Calvo-Lobo, C. Relationship Between Decreased Subcalcaneal Fat Pad Thickness and Plantar Heel Pain. A Case Control Study. Pain Physician 2019, 22, 109–116. [Google Scholar] [PubMed]
- Dalal, S.; Widgerow, A.D.; Evans, G.R. The plantar fat pad and the diabetic foot—A review. Int. Wound J. 2015, 12, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Gooding, G.A.; Stess, R.M.; Graf, P.M.; Moss, K.M.; Louie, K.S.; Grunfeld, C. Sonography of the sole of the foot. Evidence for loss of foot pad thickness in diabetes and its relationship to ulceration of the foot. Investig. Radiol 1986, 21, 45–48. [Google Scholar] [CrossRef]
- Waldecker, U.; Lehr, H.A. Is there histomorphological evidence of plantar metatarsal fat pad atrophy in patients with diabetes? J. Foot Ankle Surg 2009, 48, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.D.; Mueller, M.J.; Smith, K.E.; Commean, P.K.; Pilgram, T.; Johnson, J.E. Structural changes in the forefoot of individuals with diabetes and a prior plantar ulcer. J. Bone Joint Surg. Am. 2002, 84, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Aragon-Sanchez, J.; Lazaro-Martinez, J.L.; Alvaro-Afonso, F.J.; Molines-Barroso, R. Conservative surgery of diabetic forefoot osteomyelitis: How can i operate on this patient without amputation? Int. J. Low Extrem. Wounds 2015, 14, 108–131. [Google Scholar] [CrossRef]
- Molines-Barroso, R.J.; Lazaro-Martinez, J.L.; Aragon-Sanchez, J.; Garcia-Morales, E.; Beneit-Montesinos, J.V.; Alvaro-Afonso, F.J. Analysis of transfer lesions in patients who underwent surgery for diabetic foot ulcers located on the plantar aspect of the metatarsal heads. Diabet. Med. 2013, 30, 973–976. [Google Scholar] [CrossRef]
- Bus, S.A.; Monteiro-Soares, M.; Ramussen, A.; Raspovic, A.; Sacco, I.; van Netten, J.J. IWGDF Guideline on the prevention of foot ulcers in persons with diabetes. Diabetes Metab. Res. Rev. 2019, in press. Available online: https://iwgdfguidelines.org/prevention-guideline/ (accessed on 2 March 2020).
- Hinchliffe, R.J.; Brownrigg, J.R.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.; Zierler, R.; Schaper, N. International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab. Res. Rev. 2016, 32, 37–44. [Google Scholar] [CrossRef]
- Ince, P.; Abbas, Z.G.; Lutale, J.K.; Basit, A.; Ali, S.M.; Chohan, F.; Morbach, S.; Möllenberg, J.; Game, F.L.; Jeffcoate, W.J. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care 2008, 3, 964–967. [Google Scholar] [CrossRef]
- Schaper, N.C.; Van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. IWGDF Practical Guidelines on the prevention and management of diabetic foot disease. Diabetes Metab. Res. Rev. 2019, in press. [Google Scholar]
- Chicharro-Luna, E.; Pomares-Gomez, F.J.; Ortega-Avila, A.B.; Marchena-Rodriguez, A.; Blanquer-Gregori, J.F.J.; Navarro-Flores, E. Predictive model to identify the risk of losing protective sensibility of the foot in patients with diabetes mellitus. Int. Wound J. 2020, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.; Jones, S.; Causby, R.S.; Thoirs, K. Can ultrasound measures of intrinsic foot muscles and plantar soft tissues predict future diabetes-related foot disease? A systematic review. PLoS ONE 2018, 13, e0199055. [Google Scholar] [CrossRef] [PubMed]
- Abouaesha, F.; van Schie, C.H.; Griffths, G.D.; Young, R.J.; Boulton, A.J. Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care 2001, 24, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Rajagopal, K.V.; Hande, H.M.; Maiya, A.G.; Mayya, S.S. Intrinsic foot muscle and plantar tissue changes in type 2 diabetes mellitus. J. Diabetes 2015, 7, 850–857. [Google Scholar] [CrossRef]
- Ou, H.; Zhan, P.; Kang, L.; Su, J.; Hu, X.; Johnson, S. Region-specific constitutive modeling of the plantar soft tissue. Biomech. Model Mechanobiol. 2018, 17, 1373–1388. [Google Scholar] [CrossRef]
- Thomas, V.J.; Patil, K.M.; Radhakrishnan, S.; Narayanamurthy, V.B.; Parivalavan, R. The role of skin hardness, thickness, and sensory loss on standing foot power in the development of plantar ulcers in patients with diabetes mellitus—A preliminary study. Int. J. Low Extrem. Wounds 2003, 2, 132–139. [Google Scholar] [CrossRef]
- Bus, S.A.; Maas, M.; Cavanagh, P.R.; Michels, R.P.; Levi, M. Plantar fat-pad displacement in neuropathic diabetic patients with toe deformity: A magnetic resonance imaging study. Diabetes Care 2004, 27, 2376–2381. [Google Scholar] [CrossRef]
- Patel, V.G.; Wieman, T.J. Effect of metatarsal head resection for diabetic foot ulcers on the dynamic plantar pressure distribution. Am. J. Surg. 1994, 167, 297–301. [Google Scholar] [CrossRef]
- Aragon-Sanchez, J.; Lazaro-Martinez, J.L.; Hernandez-Herrero, C.; Campillo-Vilorio, N.; Quintana-Marrero, Y.; Garcia-Morales, E.; Hernández-Herrero, M.J. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabet. Med. 2012, 29, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Munro, B.J.; Lord, S.R.; Menz, H.B.; Steele, J.R. Soft tissue thickness under the metatarsal heads is reduced in older people with toe deformities. J. Orthop. Res. 2011, 29, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Flores, E.; Cauli, O. Quality of life in individuals with diabetic foot syndrome. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Akdeniz, M.; Tomova-Simitchieva, T.; Bobbert, T.; Moga, A.; Lachmann, N.; Blume-Peytavi, U.; Kottner, J. Comparing skin characteristiacs and molecular markers of xerotic foot skin between diabetic and non-diabetic subjects: An exploratory study. J. Tissue Viability 2019, 28, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Bagci, I.S.; Ruini, C.; Niesert, A.C.; Horvath, O.N.; Berking, C.; Ruzicka, T.; von Braunmühl, T. Effects of Short-Term Moisturizer Application in Different Ethnic Skin Types: Noninvasive Assessment with Optical Coherence Tomography and Reflectance Confocal Microscopy. Skin Pharmacol. Physiol. 2018, 31, 125–133. [Google Scholar] [CrossRef]

| n = 38 | Intra-Subject Reliability ICC (95% CI) | Inter-Subject Reliability ICC (95% CI) | |
|---|---|---|---|
| Observer A | Observer B | ||
| 1st Metatarsal Plantar Skin | 958 * (919–978) | 959 * (921–979) | 795 * (606–894) |
| 2nd Metatarsal Plantar Skin | 932 * (855–968) | 929 * (849–967) | 779 * (529–896) |
| 1st Metatarsal Fat Pad | 993 * (986–996) | 993 * (986–996) | 962 * (926–980) |
| 2nd Metatarsal Fat Pad | 994 * (988–997) | 989 * (977–995) | 952 * (898–978) |
| (n = 38 Patients) | Control Group n = 19 | Experimental Group n = 19 | p-Value |
|---|---|---|---|
| Male/Female n (%) | 14 (74)/5 (26) | 13 (68)/6 (32) | 0.721 |
| Type 1/Type 2 DM n (%) | 4 (21)/15 (79) | 1 (5)/18 (95) | 0.150 |
| PAD n (%) | 12 (63) | 10 (53) | 0.511 |
| Retinopathy n (%) | 4 (21) | 5 (26) | 0.703 |
| Nephropathy n (%) | 3 (16) | 2 (11) | 0.631 |
| Mean age ± SD (Years) | 60 ± 11.1 | 65 ± 9.4 | 0.124 |
| Diabetes Mellitus (Years), Mean ± SD | 23 ± 18.4 | 21 ± 12.9 | 0.746 |
| SINBAD Classification Score (Points), Mean ± SD | 2.3 ± 7.5 | 3.1 ± 1.3 | 0.036 |
| Glycated Haemoglobin mmol/mol, Mean ± SD | 53.57 ± 8.83 | 58.61 ± 16.63 | 0.254 |
| Body Mass Index (kg/cm2), Mean ± SD | 29.84 ± 5.91 | 28.69 ± 5.47 | 0.537 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molines-Barroso, R.J.; García-Álvarez, Y.; García-Klepzig, J.L.; García-Morales, E.; Álvaro-Afonso, F.J.; Lázaro-Martínez, J.L. Differences in the Sub-Metatarsal Fat Pad Atrophy Symptoms between Patients with Metatarsal Head Resection and Those without Metatarsal Head Resection: A Cross-Sectional Study. J. Clin. Med. 2020, 9, 794. https://doi.org/10.3390/jcm9030794
Molines-Barroso RJ, García-Álvarez Y, García-Klepzig JL, García-Morales E, Álvaro-Afonso FJ, Lázaro-Martínez JL. Differences in the Sub-Metatarsal Fat Pad Atrophy Symptoms between Patients with Metatarsal Head Resection and Those without Metatarsal Head Resection: A Cross-Sectional Study. Journal of Clinical Medicine. 2020; 9(3):794. https://doi.org/10.3390/jcm9030794
Chicago/Turabian StyleMolines-Barroso, Raúl Juan, Yolanda García-Álvarez, José Luis García-Klepzig, Esther García-Morales, Francisco Javier Álvaro-Afonso, and José Luis Lázaro-Martínez. 2020. "Differences in the Sub-Metatarsal Fat Pad Atrophy Symptoms between Patients with Metatarsal Head Resection and Those without Metatarsal Head Resection: A Cross-Sectional Study" Journal of Clinical Medicine 9, no. 3: 794. https://doi.org/10.3390/jcm9030794
APA StyleMolines-Barroso, R. J., García-Álvarez, Y., García-Klepzig, J. L., García-Morales, E., Álvaro-Afonso, F. J., & Lázaro-Martínez, J. L. (2020). Differences in the Sub-Metatarsal Fat Pad Atrophy Symptoms between Patients with Metatarsal Head Resection and Those without Metatarsal Head Resection: A Cross-Sectional Study. Journal of Clinical Medicine, 9(3), 794. https://doi.org/10.3390/jcm9030794









