Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Composites
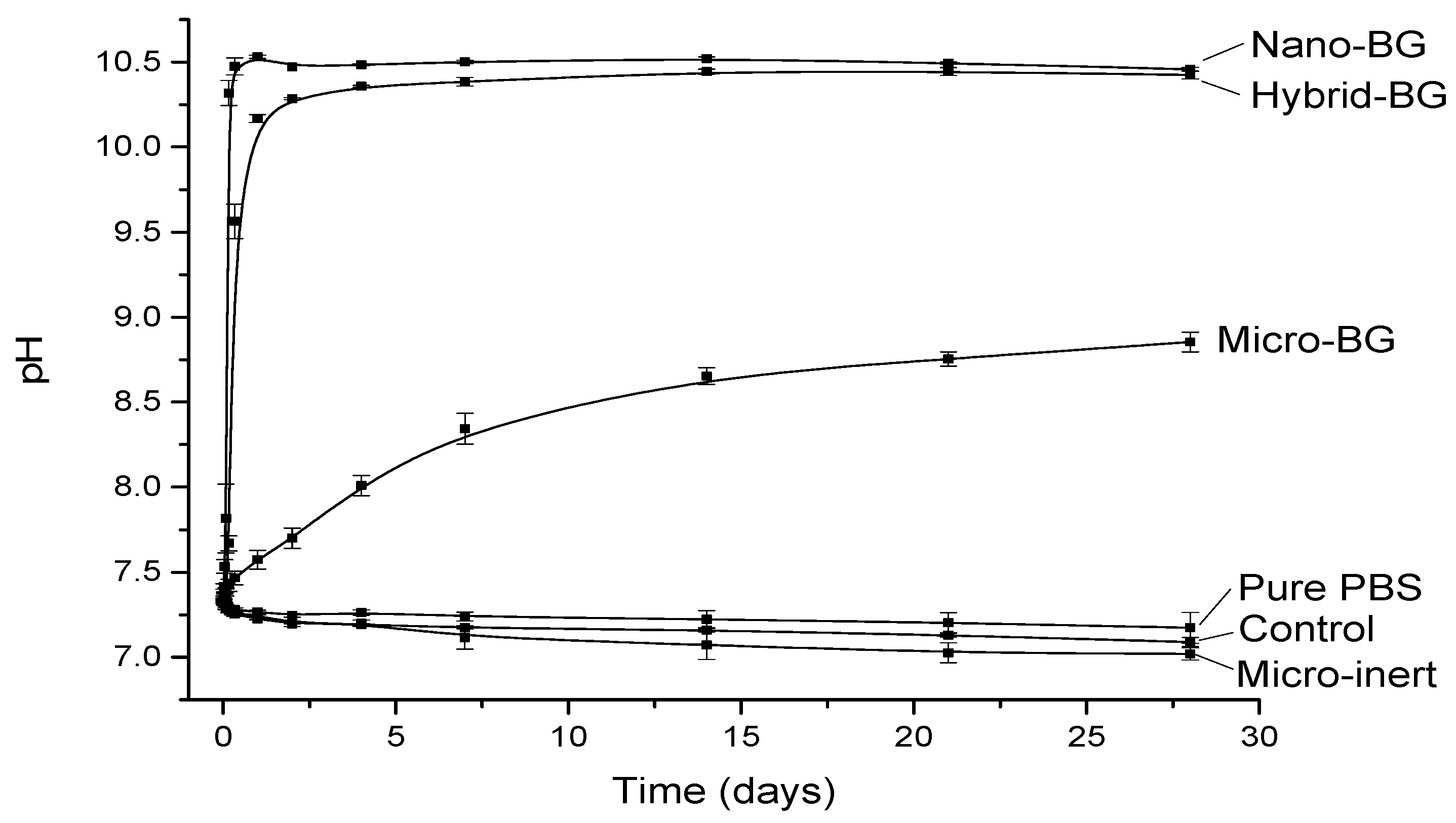
2.2. pH Measurements
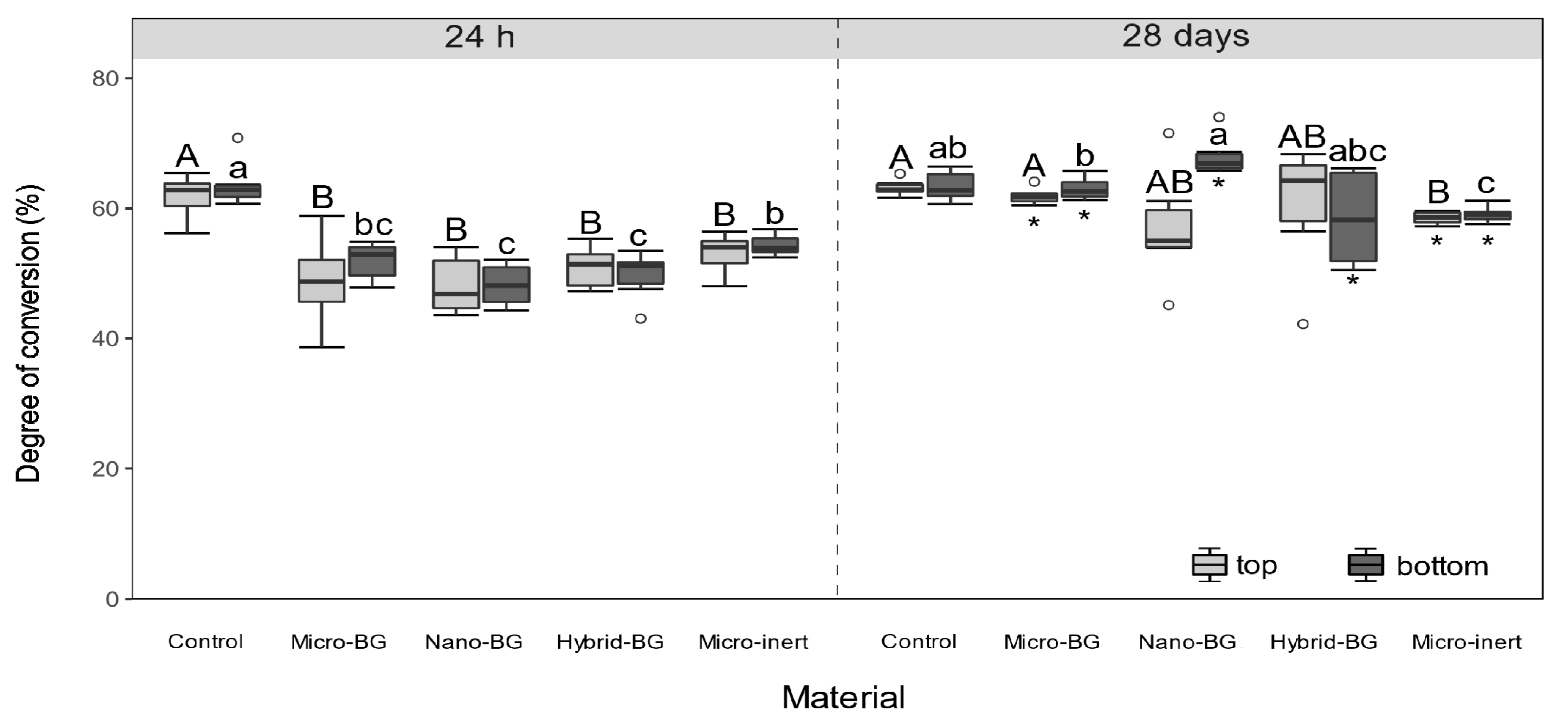
2.3. Degree of Conversion
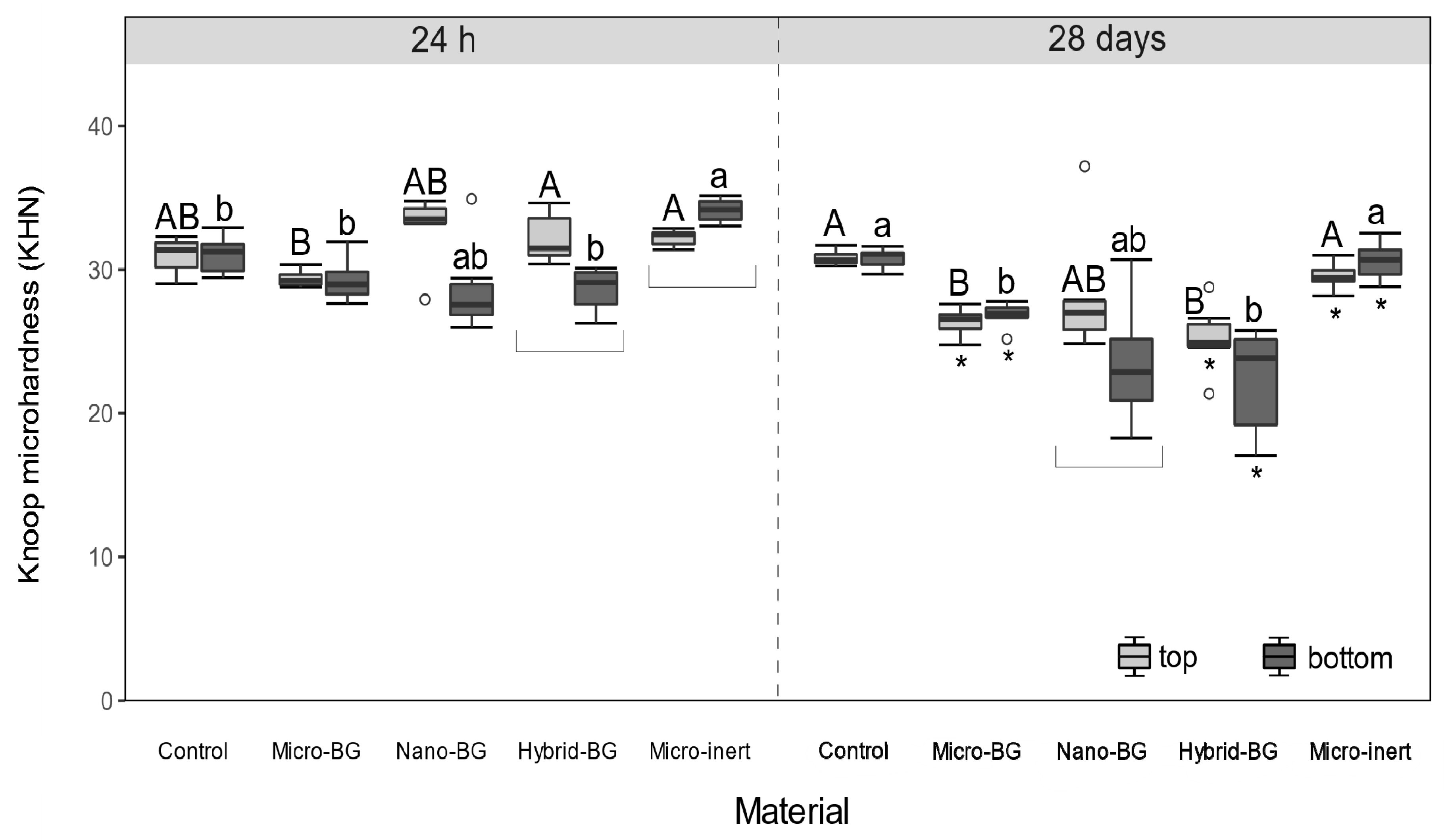
2.4. Knoop Microhardness
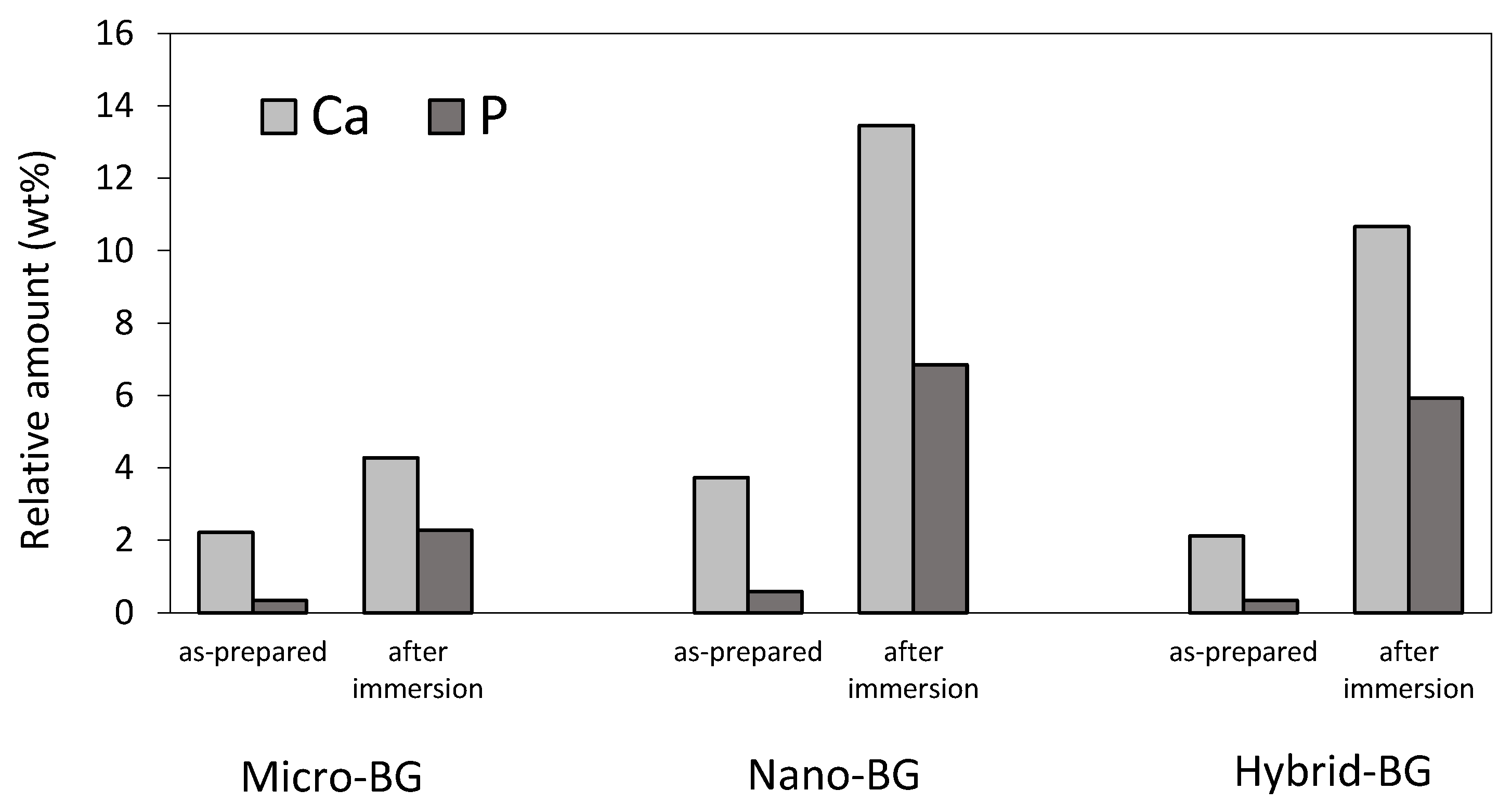
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tauböck, T.T.; Marovic, D.; Zeljezic, D.; Steingruber, A.D.; Attin, T.; Tarle, Z. Genotoxic potential of dental bulk-fill resin composites. Dent. Mater. 2017, 33, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Tauböck, T.T.; Jäger, F.; Attin, T. Polymerization shrinkage and shrinkage force kinetics of high- and low-viscosity dimethacrylate- and ormocer-based bulk-fill resin composites. Odontology 2019, 107, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Flury, S.; Pfefferkorn, F.; Lussi, A.; Noack, M.J. Restorative dentistry and restorative materials over the next 20 years: A Delphi survey. Dent. Mater. 2014, 30, 442–448. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D.; Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141–1150. [Google Scholar] [CrossRef]
- Liu, F.; Wang, R.; Shi, Y.; Jiang, X.; Sun, B.; Zhu, M. Novel Ag nanocrystals based dental resin composites with enhanced mechanical and antibacterial properties. Prog. Nat. Sci. 2013, 23, 573–587. [Google Scholar] [CrossRef][Green Version]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent. Mater. 2012, 28, 573–583. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Lombardo, G.; Marinucci, L.; Kenny, J.M.; Torre, L.; Cianetti, S. Effect of nanohydroxyapatite, antibiotic, and mucosal defensive agent on the mechanical and thermal properties of glass ionomer cements for special needs patients. J. Mater. Res. 2018, 33, 638–649. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Davis, H.B.; Gwinner, F.; Mitchell, J.C.; Ferracane, J.L. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent. Mater. 2014, 30, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Wong, F.S.L.; Johal, A.; Hill, R.G. Fluoride containing bioactive glass composite for orthodontic adhesives—Ion release properties. Dent. Mater. 2017, 33, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Kwon, J.S.; Kim, K.N.; Kim, K.M. Enamel surface with pit and fissure sealant containing 45S5 bioactive glass. J. Dent. Res. 2016, 95, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Feitosa, V.P.; Cama, G.; Brauer, D.S.; Sauro, S. Effects of composites containing bioactive glasses on demineralized dentin. J. Dent. Res. 2017, 96, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Johal, A.; Hill, R.G.; Wong, F.S.L. Fluoride containing bioactive glass composite for orthodontic adhesives—Apatite formation properties. Dent. Mater. 2018, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Velamakanni, S.; DiRenzo, K.; Lefkelidou, A.; Fenno, J.C.; Kasuga, T.; Boccaccini, A.R.; Papagerakis, P. Designing dental composites with bioactive and bactericidal properties. Mater. Sci. Eng. C 2015, 52, 267–272. [Google Scholar] [CrossRef]
- Tiskaya, M.; Al-Eesa, N.A.; Wong, F.S.L.; Hill, R.G. Characterization of the bioactivity of two commercial composites. Dent. Mater. 2019, 35, 1757–1768. [Google Scholar] [CrossRef]
- Heid, S.; Stoessel, P.R.; Tauböck, T.T.; Stark, W.J.; Zehnder, M.; Mohn, D. Incorporation of particulate bioactive glasses into a dental root canal sealer. Biomed. Glasses 2016, 2, 29–37. [Google Scholar] [CrossRef]
- Simila, H.O.; Karpukhina, N.; Hill, R.G. Bioactivity and fluoride release of strontium and fluoride modified Biodentine. Dent. Mater. 2018, 34, e1–e7. [Google Scholar] [CrossRef]
- Waltimo, T.; Mohn, D.; Paqué, F.; Brunner, T.J.; Stark, W.J.; Imfeld, T.; Schätzle, M.; Zehnder, M. Fine-tuning of bioactive glass for root canal disinfection. J. Dent. Res. 2009, 88, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Profeta, C.A.; Mannocci, F.; Foxton, R.M.; Thompson, I.; Watson, T.F.; Sauro, S. Bioactive effects of a calcium/sodium phosphosilicate on the resin-dentine interface: A microtensile bond strength, scanning electron microscopy, and confocal microscopy study. Eur. J. Oral Sci. 2012, 120, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sauro, S.; Osorio, R.; Fulgêncio, R.; Watson, T.F.; Cama, G.; Thompson, I.; Toledano, M. Remineralisation properties of innovative light-curable resin-based dental materials containing bioactive micro-fillers. J. Mater. Chem. B 2013, 1, 2624–2638. [Google Scholar] [CrossRef]
- Jun, S.K.; Yang, S.A.; Kim, Y.J.; El-Fiqi, A.; Mandakhbayar, N.; Kim, D.S.; Roh, J.; Sauro, S.; Kim, H.W.; Lee, J.H.; et al. Multi-functional nano-adhesive releasing therapeutic ions for MMP-deactivation and remineralization. Sci. Rep. 2018, 8, 5663. [Google Scholar] [CrossRef]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Mechanical properties of experimental composites containing bioactive glass after artificial aging in water and ethanol. Clin. Oral Investig. 2019, 23, 2733–2741. [Google Scholar] [CrossRef]
- Hashimoto, M.; Iijima, M.; Nagano, F.; Ohno, H.; Endo, K. Effect of monomer composition on crystal growth by resin containing bioglass. J. Biomed. Mater. Res. B 2010, 94, 127–133. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Tauböck, T.T.; Attin, T.; Tarle, Z. Degree of conversion of experimental resin composites containing bioactive glass 45S5: The effect of post-cure heating. Sci. Rep. 2019, 9, 17245. [Google Scholar] [CrossRef]
- Mohn, D.; Bruhin, C.; Luechinger, N.A.; Stark, W.J.; Imfeld, T.; Zehnder, M. Composites made of flame-sprayed bioactive glass 45S5 and polymers: Bioactivity and immediate sealing properties: Bioactive root fillers. Int. Endod. J. 2010, 43, 1037–1046. [Google Scholar] [CrossRef]
- Waltimo, T.; Brunner, T.J.; Vollenweider, M.; Stark, W.J.; Zehnder, M. Antimicrobial effect of nanometric bioactive glass 45S5. J. Dent. Res. 2007, 86, 754–757. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of nanoscale and microscale bioactive glass on the properties of P(3HB)/Bioglass® composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Misra, S.K.; Ansari, T.; Mohn, D.; Valappil, S.P.; Brunner, T.J.; Stark, W.J.; Roy, I.; Knowles, J.C.; Sibbons, P.D.; Jones, E.V.; et al. Effect of nanoparticulate bioactive glass particles on bioactivity and cytocompatibility of poly(3-hydroxybutyrate) composites. J. R. Soc. Interface 2010, 7, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, M.; Brunner, T.J.; Knecht, S.; Grass, R.N.; Zehnder, M.; Imfeld, T.; Stark, W.J. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007, 3, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Grass, R.N.; Stark, W.J. Glass and bioglass nanopowders by flame synthesis. Chem. Commun. 2006, 13, 1384–1386. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, P.; Mohn, D.; Zehnder, M.; Attin, T.; Tauböck, T.T. Light transmittance and polymerization of bulk-fill composite materials doped with bioactive micro-fillers. Materials 2019, 12, 4087. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A.; Hashinger, D.T.; Fairhurst, C.W. Calibration of FTIR conversion analysis of contemporary dental resin composites. Dent. Mater. 1990, 6, 241–249. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 2 January 2020).
- Wickham, H. Tidyverse: Easily Install and Load the ‘tidyverse’. R Package Version 1.2.1. 2017. Available online: https://CRAN.R-project.org/package=tidyverse (accessed on 2 January 2020).
- Moreau, J.L.; Weir, M.D.; Giuseppetti, A.A.; Chow, L.C.; Antonucci, J.M.; Xu, H.H. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1264–1273. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef]
- Yang, S.Y.; Piao, Y.Z.; Kim, S.M.; Lee, Y.K.; Kim, K.N.; Kim, K.M. Acid neutralizing, mechanical and physical properties of pit and fissure sealants containing melt-derived 45S5 bioactive glass. Dent. Mater. 2013, 29, 1228–1235. [Google Scholar] [CrossRef]
- Par, M.; Lapas-Barisic, M.; Gamulin, O.; Panduric, V.; Spanovic, N.; Tarle, Z. Long term degree of conversion of two bulk-fill composites. Acta Stomatol. Croat. 2016, 50, 292–300. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef]
- Kim, K.-H.; Ong, J.L.; Okuno, O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. J. Prosthet. Dent. 2002, 87, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hickel, R. Investigations on mechanical behaviour of dental composites. Clin. Oral Investig. 2009, 13, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K.; McDonough, W.G. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Oral, O.; Lassila, L.V.; Kumbuloglu, O.; Vallittu, P.K. Bioactive glass particulate filler composite: Effect of coupling of fillers and filler loading on some physical properties. Dent. Mater. 2014, 30, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M.; Vouvoudi, E.C. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dent. Mater. 2011, 27, 598–607. [Google Scholar] [CrossRef]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Dentin bond strength of experimental composites containing bioactive glass: Changes during aging for up to 1 year. J. Adhes. Dent. 2018, 20, 325–334. [Google Scholar]
- Schmalz, G.; Hickel, R.; van Landuyt, K.L.; Reichl, F.-X. Nanoparticles in dentistry. Dent. Mater. 2017, 33, 1298–1314. [Google Scholar] [CrossRef]
- Bouschlicher, M.R.; Rueggeberg, F.A.; Wilson, B.M. Correlation of bottom-to-top surface microhardness and conversion ratios for a variety of resin composite compositions. Oper. Dent. 2004, 29, 698–704. [Google Scholar]
- Asmussen, E.; Peutzfeldt, A. Influence of specimen diameter on the relationship between subsurface depth and hardness of a light-cured resin composite. Eur. J. Oral Sci. 2003, 111, 543–546. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Coniglio, M.; Valenti, C.; Negri, P.; Lombardo, G.; Costanzi, E.; Cianetti, S.; Montaseri, A.; Marinucci, L. Biological effects of resin monomers on oral cell populations: Descriptive analysis of literature. Eur. J. Paediatr. Dent. 2019, 20, 224–232. [Google Scholar] [PubMed]

| Micro-Sized Bioactive Glass 45S5 | Nano-Sized Bioactive Glass 45S5 | Micro-Sized Inert Barium Glass | |
|---|---|---|---|
| Mean Particle Size (µm) | 5.6 | 0.07 | 6.7 |
| Specific Surface Area (m2/g) | 1.1 | 35.3 | 1.7 |
| Composition (wt%) | 45% SiO2 | 45% SiO2 | 55% SiO2 |
| 24.5% CaO | 24.5% CaO | 25% BaO | |
| 24.5% Na2O | 24.5% Na2O | 10% Al2O3 | |
| 6% P2O5 | 6% P2O5 | 10% B2O3 | |
| Product Name/LOT | G018-144/SM528 | experimental batch | GM27884/M92605 |
| Material Designation | Composition (wt%) |
|---|---|
| Control | 100% Heliomolar Flow |
| Micro-BG | 85% Heliomolar Flow + 15% micro-sized BG |
| Nano-BG | 85% Heliomolar Flow + 15% nano-sized BG |
| Hybrid-BG | 85% Heliomolar Flow + 7.5% micro-sized BG + 7.5% nano-sized BG |
| Micro-inert | 85% Heliomolar Flow + 15% micro-sized inert barium glass |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odermatt, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass. J. Clin. Med. 2020, 9, 772. https://doi.org/10.3390/jcm9030772
Odermatt R, Par M, Mohn D, Wiedemeier DB, Attin T, Tauböck TT. Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass. Journal of Clinical Medicine. 2020; 9(3):772. https://doi.org/10.3390/jcm9030772
Chicago/Turabian StyleOdermatt, Reto, Matej Par, Dirk Mohn, Daniel B. Wiedemeier, Thomas Attin, and Tobias T. Tauböck. 2020. "Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass" Journal of Clinical Medicine 9, no. 3: 772. https://doi.org/10.3390/jcm9030772
APA StyleOdermatt, R., Par, M., Mohn, D., Wiedemeier, D. B., Attin, T., & Tauböck, T. T. (2020). Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass. Journal of Clinical Medicine, 9(3), 772. https://doi.org/10.3390/jcm9030772







